Abstract
The present study was carried out to investigate the protective role of Triphala (a combination in equal proportions by weight of fruit powder of Terminalia belerica, Terminalia chebula and Emblica officinalis) against 1,2-dimethylhydrazinedihydrochloride (DMH) induced Endoplasmic reticulum stress (ER stress) in mouse liver. An oral dose of 3 mg/kg body wt in drinking water for 5 weeks significantly (P < 0.001) increased the levels of serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), serum Alkaline phosphatase (ALP) and total bilirubin thus suggesting damage to mouse liver and biliary dysfunction. The DMH administration invariably led to increase in the liver microsomal proteins of molecular weight of about 29 (ERp29) and 53 kDa (ERp53) and decrease in the protein of molecular weight of 36 kDa (ERp36) thereby suggesting the interference of DMH and its metabolites with normal protein biosynthesis and folding, in the reticular membranes of the liver cells thus developing ER stress. Histological studies show necrosis, large sized hepatocytes with increased N:C ratio, aberrant mitotic figures and prominent nucleoli in the liver of DMH treated mice. In animals fed 5% Triphala in diet (w/w) during DMH administration, there was significant decrease in the above changes in the liver suggesting the suppression of DMH induced ER stress in liver. Triphala significantly (P < 0.05) decreased lipid peroxidation and also the activity of lactate dehydrogenase (LDH) in mouse liver. It simultaneously increased the level of reduced glutathione (GSH) and the activity of glutathione-S-transferase (GST) thereby suggesting that it prevents peroxidative damage and also diverts the active metabolites (electrophiles) of DMH from their interactions with critical cellular bio-molecules which could be responsible for its protective action against DMH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
1,2-Dimethylhydrazinedihydrochloride is a highly toxic and carcinogenic and affects number of body organs including liver [1, 2]. It is metabolized in the liver [3, 4] and produces highly reactive electrophiles i.e., carbonium ions and alkyl free radicals which severely damage the liver causing necrosis and fatty infiltration [5], methylate nucleobases and disrupt the polysomal assembly [6, 7]. When administered orally, it alters the composition of intestinal brush border membranes and intestinal absorptive functions [8, 9].
Although such changes have not been studied in humans, the adverse effects of alkyl hydrazine derivatives cannot be overlooked owing to the direct exposure to them through chemical and pharmaceutical regimens [10, 11]. Dimethylhydrazine has been shown to be present in tobacco [12], commercial and wild mushrooms [13] and other food items also [14].
Conventional and synthetic drugs in use in the treatment of liver diseases can have many severe side effects and there is growing interest worldwide to shift to the traditional medicinal plant products. Drug induced hepato-toxicity is a potentially serious adverse effect of the anti-tubercular drug isoniazid (an alkylated hydrazine) and it has been shown in experimental animals that its administration in toxic doses affects liver, cell membranes and other organelles which is supported by the release of aspartate and alanine amino transferases and alkaline phosphatase [15, 16].
Triphala is a combination of fruit powder of three plants namely Terminalia chebula, Terminalia belerica and Emblica officinalis mixed in equal proportions by weight. Terminalia chebula and Terminalia belerica are important constituents of Haritaki and Vibhitaka, respectively, which are important Ayurvedic drugs. Emblica officinalis is used in a number of Ayurvedic formulations used for rejuvenation and has antioxidant activity. Triphala improves digestion and assimilation, reduces serum cholesterol, exerts potent cardio protective effect, improves liver functions, has anti inflammatory and antiviral properties. It also reduces blood sugar, has anti-mutagenic properties and possesses radio protective activities. It inhibits lipid peroxidation and also acts as scavenger of free radicals. It is rich in active ingredients like tannins, carbohydrates, saponins, ellagic acid, sorbitol and ascorbic acid [17–25]. In the present study, an attempt has been made to evaluate the protective role of Triphala on the toxic and carcinogenic damage due to DMH induced ER stress in mouse liver.
Materials and Methods
Chemicals
1,2-Dimethylhydrazinedihydrochloride was purchased from Sigma chemical company, St. Louis, USA. All other chemicals were purchased from local firms and were of high grade purity. Triphala was purchased locally and was mixed in diet as per pre determined concentration of 5% w/w [26] and pellets were prepared and stored in neat and clean bags.
Animals
Adult male mice of Laca strain (24–26 gm each) were used in the study. The animals were assorted in four groups.
Group I (n = 8): These animals were maintained on normal diet and served as controls.
Group II (n = 8): These animals were given DMH (3 mg/kg body wt/day in drinking water) for 35 days [8, 27] and served as DMH treated group.
Group III (n = 8): These animals were given DMH as in group II and were provided 5% Triphala diet from the day of DMH administration and served as DMH+Triphala treated group.
Group IV (n = 8): These animals were maintained on 5% Triphala treated diet only for 2 weeks and served as Triphala treated group. These animals were used for studying the lipid peroxidation, antioxidant status and LDH activity in liver.
Preparation of Serum, Liver Homogenate, Liver Microsomes and the Cytoslic Fraction
The animals were sacrificed after 2 weeks (Group I & IV) and 5 weeks (Group I, II & III) by decapitation. 2 ml of blood was collected. The serum was separated by spinning the clotted blood at 2500 rpm for 15 min and was used for estimation of biochemical parameters like SGPT, SGOT [28], alkalinephosphatase [29] and total bilirubin [30]. The livers of the animals were thoroughly perfused immediately with cold normal saline and were then removed. They were blotted dry, weighed quickly and homogenized in ice cold 0.15 M Tris–KCl buffer (0.15 M KCl + 10 m M Tris–HCl, pH 7.4) to yield 10% (w/v) homogenate. An aliquot of the homogenate (0.5 ml) each was used for the assay of reduced glutathione [31] and the levels of lipid peroxidation [32]. For measuring lipid peroxidation, to 0.5 ml of the homogenate, 3.0 ml of 1% H3PO4 and 1.0 ml of 0.6% thiobarbituric acid were added. The contents were stirred thoroughly and were then heated on boiling water bath for 45 min. The contents were then cooled. 4.0 ml of N butanol was then added and the contents were shaken thoroughly and then centrifuged to separate the butanol layer whose OD was taken at 535 and 520 nm. Tetramethoxy propane standard was also run simultaneously. The difference of the value at the two wave lengths corresponded to the value for MDA concentration. The rest of the homogenate was centrifuged at 10,000 rpm for 60 min in Beckman cold centrifuge. An aliquot of the supernatant, after appropriate dilution, was used for the estimation of GST [33] and LDH [34] whereas the microsomal pellet was obtained by spinning the remaining supernatant at 1,05000×g for 30 min in an ultracentrifuge. The microsomal pellet was re-suspended in appropriate amount of homogenizing buffer and was subjected to SDS-PAGE by applying 75 μg microsomal proteins in each well of 10% slab gels for separating microsomal proteins [35]. Standard proteins of known molecular weights were also simultaneously subjected to electrophoresis. The electrophoretograms obtained were then scanned with the help of LKB 2202 Laser Beam Densitometer at 550 nm. Proteins were estimated by the method of Lowry et al. [36] using Bovine Serum Albumin as standard at 660 nm.
Statistical analyses of the data were done using student’s t-test.
Results
The mice treated with DMH showed significant liver damage as observed from elevated levels of hepato-specific parameters. Activities of SGPT, SGOT, ALP and total bilirubin in the serum were increased significantly (P < 0.001) i.e., 6.96-, 12.85-, 9.16- and 7.13-folds, respectively, in DMH treated mice in comparison to control animals (Table 1). However, 5% dietary Triphala administration along with DMH showed a significant (P < 0.001) decrease in SGPT (83.5%), SGOT (77.35%), ALP (83.7%) and total bilirubin (86.92%) from DMH treated animals thus indicating the protective role of Triphala against DMH induced hepatic damage.
Histopathological examination revealed cell necrosis, fatty infiltration, hepatocytes with increased N:C ratio, pleomorphic nuclei, aberrant mitotic figures and prominent nucleoli in DMH treated mice indicating hepatic damage and appearance of pre-neoplastic lesions due to DMH (Fig. 1a). However, the administration of Triphala to DMH treated animals (Fig. 1b) seems to prevent such hepatic changes when compared with normal animals (Fig. 1c).
Analyses of protein profiles of the liver microsomal membranes (endoplasmic reticulum) of DMH treated, DMH+Triphala treated and control mice are presented in Fig. 2a–c.
It was interesting to note from Fig. 2a that DMH administration invariably leads to increase in the level of proteins of M. Wt of 29 kDa (ERp29) in peak X and 53 kDa (ERp53) in peak Y and decrease in the level of protein of M. Wt. of 36 kDa (ER36) in peak A (Fig. 2b) thus indicating the ER stress and activation of UFRs due to DMH. However, the administration of Triphala to DMH treated animals (Fig. 2b) seems to bring the protein pattern to normal as in Fig. 2c thus preventing ER stress.
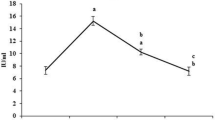
Figure 3 shows the antioxidant status and LDH activity of the control and Triphala treated animals. The level of lipid peroxidation measured as MDA was decreased significantly (P < 0.05) by 50% and the activity of LDH was decreased by 40% in Triphala treated animals as compared to control mice. The glutathione level was enhanced by 50% and the activity of GST was elevated by 37% due to Triphala treatment as compared to controls.
Antioxidant status and LDH activity in control and Triphala treated mouse liver. LP n mole malondialdehyde formed/mg protein; LDH activity μ mole NADH/min/mg protein; GSH μ mole GSH/mg protein and GST activity μ mole CDNB-GSH conjugate formed/min/mg protein (Values were mean ± SD of 4 independent observations)
Discussion
The levels of hepatic enzymes are used as diagnostic indicators of hepatic injury. SGPT, SGOT and serum Bilirubin are the most sensitive tests employed in the diagnosis of hepatic diseases [37–41]. Elevated levels of serum enzymes are indicative of cellular leakage and loss of functional integrity of cell membranes in liver [42, 43]. Administration of DMH in our study significantly increased serum levels of SGPT, SGOT in DMH treated mice. However, Triphala administration to DMH treated mice led to decrease in the activities of marker enzymes in serum showing the stabilization of the plasma membranes as well as the repair of hepatic tissue damage due to DMH. Serum ALP was also raised in DMH treated animals. ALP is excreted via bile by the liver. In liver damage due to DMH, there is defective excretion of bile which is reflected in increased level of ALP in the serum [44].
Elevated level of serum bilirubin is a very sensitive test to substantiate loss of functional integrity of liver and severity of necrosis [45]. Depletion of elevated bilirubin level along with decrease of ALP activity in the serum of mice treated with DMH+Triphala suggests that Triphala stabilizes the biliary dysfunction due to DMH. Results obtained from histo-pathological study also show signs of recovery from DMH hepato-toxicity and carcinogenicity (Fig. 1b).
The DMH treatment leads to changes in the liver microsomal proteins as shown in Fig. 2 a which came back to the normal protein patterns (Fig. 2c) when treated with Triphala (Fig. 2b).The author of this communication during his doctoral research in 1980’s had shown the appearance of almost similar protein peak changes in the microsomes of the mouse liver during carcinogenic stress after treating the animals with DMH for 35 days [11].
The DMH treatment causes mutations and leads to the accumulation of mutated proteins [46] causing activation of ER stress signalling pathways (UPRs). ERp53 has now been well characterised as tumor suppressor gene product [46] and a latest study has shown ERp29 also to possess tumor suppressor role [47].The decrease in the ERp36 suggests that DMH suppresses the expression of the gene responsible for the synthesis of this protein possibly due to hyper-methylation. However, detailed studies are underway in our laboratory to find out the exact role of such microsomal protein changes in the ER stress signalling pathways (UPRs). The normal protein patterns of microsomal membranes of hepatocytes in DMH+Triphala treated animals (Fig. 2b) suggests the inhibitory effects of the active ingredients of Triphala on the formation of active metabolites of DMH and/or their neutralization, leading to their paucity for interactions with critical cellular bio-molecules thus preventing mutations and the accumulation of unfolded proteins in the ER thereby inhibiting the development of ER stress and the UPRs.
Oxidative stress is an important element of mutagenesis and carcinogenesis [48]. Free radicals produced as a result of oxidative stress initiate chain reactions that lead to the process of lipid peroxidation which causes damage to cell membranes. So any agent inhibiting free radical processes contributes to chemo-protection of cellular damage and also the carcinogenesis.
Figure 3 shows the effect of Triphala on lipid per-oxidation and LDH activity in mouse liver. LDH is also an important biochemical indicator of cellular damage. Free radical mediated membrane damage could release LDH into the cytosol and prevention of free radical formation avoids the release of LDH. It is important to note that specific activity of LDH was low in Triphala treated mice as compared to control mice (Fig. 3). Also significantly low levels of per-oxidation were observed in Triphala treated animals as compared to controls (Fig. 3). Thus, it is quite clear that Triphala has a potent ability to prevent oxidative stress which property, therefore, might be linked to its chemo-preventive potential [26]. Figure 3 also shows the elevation in the level of GSH and the Sp. Activity of GST. GSH helps in maintaining the reduced milieu of the cell and is involved in the detoxification of various xenobiotics including carcinogens [49]. It behaves as an antioxidant and protects biological systems [50]. It catalyzes antioxidant processes of thiol compounds and in turn protects the cells from electrophiles and free radical induced oxidative damage [51]. It belongs to phase II enzymes that inactivate the ultimate carcinogens and divert them from reactions with critical cellular biomolecules [52]. Thus, it could be inferred from increased levels of glutathione-S-transferase that Triphala has a potent ability to inhibit electrophilic damage to cell which otherwise could lead to initiation and promotion of cancer.
The present study has demonstrated the protective potential of Triphala against DMH induced early neoplastic alterations coupled to ER stress in mouse liver. The protective effect of Triphala could result due to stimulation of hepatic regeneration by preventing damage by alkyl free radicals as well as by neutralization and excretion of electrophiles generated due to DMH metabolism, by the active ingredients of Triphala.
References
Toth B. Morphological studies of angiosarcinomas induced by 1,2-dimethylhydrazine in syrian golden hamsters. Cancer Res. 1972;32(12):2818–27.
Nomura K, Schlake W, Grundman E. New aspects of intestinal carcinogenesis by DMH and the influence of antilymphocyte globulin on it’s progress. Cancer Res. 1978;92(1):17–73.
Druckery H, Preusmann R, Schmahl D, Blum G. Topics in chemical carcinogenesis. In: Nakahara M, editor, Tokyo: University of Tokyo Press; 1972. p. 73–103.
Nagasawa HT, Shirota FN, Matsumoto H. Decomposition of methylazoxy methanol, the aglycone of cycansin in D2O. Nature. 1972;236:234–5.
Comstok CC, Lawson LH, Greene EA, Oberst FW. AMA Arch Ind Health. 1954;10:476–9.
Hawks A, Magee PN. The alkylation of nucleic acids of rat and mouse in vivo by the carcinogen 1,2-dimethylhydrazine. Br J Cancer. 1974;30:440–7.
Swenberg JA, Cooper HLN, Bucheler J, Kleihues P. 1,2-Dimethylhydrazine induced methylation of DNA bases in various rat organs and the effect of pre treatment with disulfiram. Cancer Res. 1979;32:146–52.
Sharma KK, Pathak RM, ShramaV, Dani HM. Effects of orally administered 1,2-dimethylhydrazine on lipid and protein composition of mouse small intestinal brush border membranes. Res Bull PU Chandigarh, India 1995;45(1–4): 1–9
Sharma KK, Sharma V, Dani HM. Effects of orally administered 1,2-Dimethylhydrazine on the absorptive activities of small intestine of mouse. Res Bull PU, Chandigarh, India 1995;45(I–IV): 11–17
Toth B. Synthetic and naturally occurring hydrazines as possible cancer causative agents. Cancer Res. 1975;35(12):3693–7.
Sharma KK. Biochemical studies on the structural and functional aspects of cell membranes during chemical carcinogenesis PhD thesis. Chandigarh: Panjab University; 1991. p. 5–7.
Liu YY. Chemical studies on tobacco smoke. quantitative analysis of hydrazine in tobacco and cigarette smoke. Anal Chem. 1974;46:885–9.
Kostela JG, Lawrence BH. Hydrocarbon constituents from white strains of the mushroom Agaricus bisporus. J Agr Food Chem. 1981;20(1):185–6.
Wilbert S, Steinbrecher K, Gunderson E. Prevalence of hydrazine derivatives in food and food products. J Agr Food Chem. 1990;52:214.
Parthasarthy R, Raghupati Sarma G, Janardhanam B, Ramachandran P, Santha T, Sibasubramaniam S. Hepatic toxicity in South Indian patients during treatment of tuberculosis with short-course regimens containing isoniazide, aflatoxin and pyrazinamide. Tubercle. 1986;67:99–106.
Shakun NP, Tabachuk OP. The comparative action of isoniazide, aflatoxin and ethambutol on liver function. Eksp Klin Farmakol. 1992;55:45.
Suvarajan VV. Ayurvedic drugs and their plant sources. Lebanon, NH: International Science Publisher; 1994.
Suresh K, Vasudevan DM. Augmentation of murine natural killer cell and antibody dependent cellular cytotoxicity activities by Phyllanthus emblica, a new immuno modulator. J Ethnopharmacol. 1994;44:55–60.
Sabu MC, Kuttan R. Anti-diabetic activity of medicinal plants and it’s relationship with their antioxidant property. J Ethno pharmacol. 2002;81(2):155–62.
Tokura K, Kagawa S. Anti cancer agents containing chebulanin from Terminalia chebula. Jpn Kokai Tokyo Koho JP. 1995;7:138–65.
Kaur S, Arora S, Kaur K, Kumar S. The in vitro antimutagenic activity of Triphala—an Indian herbal drug. Food Chem Toxicol. 2002;40(4):527–34.
Jagetia GC, Baliga MS, Malagi KJ, Kamath MS. The evaluation of the protective effect of Triphala (an ayurvedic rejuvenating drug) in mice exposed to y radiation. Phytomedicine. 2002;9:99–108.
Reddy VRC. Cardiotonic activity of the fruits of Terminalia chebula. Fitoterapia. 1990;61:517–25.
Ghosal S, Tripathi VK, Chauhan S. Active constituents of Emblica officinalis, Part I. The chemistry and antioxidative effects of two new hydrolysable tannins, emblicanin a and b. Ind J Chem Section B-Organic Chemistry including Medicinal Chemistry. 1996;35:941–8.
Bhattacharya A. Antioxidant activity of active tannoid principles of Emblica officinalis (amla). Ind J Expt Biol. 1999;37:676–80.
Deep G, Dhiman M, Rao AR, Kale RK. Chemopreventive potential of Triphala (s composite Indian drug) on Benzo (a) Pyrene induced Forestomach Tumorigenesis in murine tumor model. J Exp Clin Cancer Res. 2005;24(4):555–63.
Bedell MA, Lewis JG, Billing KC, Swenberg JA. Cell specificity in hepatocarcinogenesis: preferential accumulation of O6 methylguanine in target cell DNA during continuous exposure to rats to 1,2-dimethylhydrazine. Cancer Res. 1982;42(8):3079–83.
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:53–6.
Kind PRN, King EJJ. Estimation of plasma phosphatase by determination of hydrolyzed phenol with anti-pyrine. J Clin Pathol. 1957;7:322–30.
Malloy HT, Evelyn KA. The determination of bilirubin with the photometric colorimeter. J Biol Chem. 1937;119:481–90.
Moron MA, Depierre JW, Mannervick B. Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78.
Mihira M, Uchiyama M. Determination of malonaldehyde precursors in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–8.
Habig WH, Pabst MJ, Jokoby WB. The first step in mercapturic acid formation. J Biol Chem. 1994;249:7130–9.
Bergmeyer HU, Bernt E. Methods in enzymatic analysis, vol. II. New York and London: Verlag Academic Pres; 1974. p. 5574–9.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5.
Lowry OH, Rosenbrough NJ, Farr A, Randalll RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75.
Harper HA. The functions and tests of liver, In: Review of physiological chemistry. Los Altos, California: Lange Medical Publishers; 1961. p. 271–283.
Sathiyanaryanan L, Arulmozhi S, Chdiambarnathan N. Anticholesterlemic, hepatoprotective and antioxidant activity of Gilnus lotoides Linn. against ethanol induced liver damage in rats. Phcog Mag. 2006;2:160–2.
Dortman RB, Lawhorn GT. Serum enzymes as indicators of chemical induced liver damage. Drug Chem Toxicol. 1978;1:163–71.
Abul K, Najmi KK, Pillai SN, Aqil PM. Free radical scavenging and hepatoprotective activity of jigrine against galactosamine induced liver damage. Drug Chem Toxicol. 1978;1:163–71.
Roa RR. Mechanism of drug induced hepatotoxicity. Ind J Pharamcol. 1973;5:313–8.
Kim MK, Hyun SH, Choung SY. Effect of herbal extract mixtures on serum and liver lipid metabolism in chronic ethanol administered rats. J Health Sci. 2006;52:344–51.
Suresh Kumar SV, Sujatha C, Syamala J, Nagasudha B, Mishra SH. Protective effect of root extract of operculina terpethum Linn. Against paracetamol induced hepatotoxicity in rats. Ind J Pharma Sci. 2006;68:32–5.
Singh B, Saxena AK, Chandan BK, Anand KK, Suri OP, Suri KA, Satti NK. Hepatoprotective activity of verbenalin on experimental liver damage in rodents. Fitoterapia. 1989;69:135–40.
Meshkibaf MH, Ebeahimi A, Ghodsi R, Ahmadi A. Chronic effects of lamotrigine on liver function in adult male rats. Ind J Clin Biochem. 2006;21:161–264.
Hainaut P, Hollstein M. p53 and human cancer: the first ten thousand mutations. Adv Cancer Res. 2000;77:81–137.
Zhang D, Richardson DR. Endoplasmic reticulum protein29 (ERp29): an emerging role in cancer. Int J Biochem Cell Biol. 2011;43(1):33–6.
Ames BN, Shigenoga MK, Hagen TM. Oxidants, antioxidants and degenerative diseases of ageing. Proc Nat Acad Sci USA. 1993;90:7915–22.
Ketterer B. Protective role of glutathione and glutathione-S-transferases in mutagenesis and carcinogenesis. Mutat Res. 1988;202:343.
Aggarwal A, Choudhary D, Uperti M. Rath:II studies in liver as a distant organ of tumour bearing mice. Mol Cell Biochem. 2001;224:9–17.
Dixon DP, Cummins I, Cle DJ, Edwards R. Glutathione mediated detoxification system in plants. Curr Opin Plant Biol. 1998;1:256.
Talalay P, Delong MJ, Prochaska HJ. Molecular mechanism in protection against carcinogensis. In: Cory JG, Szentivani Plenum A, editors. Cancer biology and therapeutics. New York: Plenum Press; 1987. p. 187.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, A., Sharma, K.K. Chemoprotective Role of Triphala Against 1,2-Dimethylhydrazine Dihydrochloride Induced Carcinogenic Damage to Mouse Liver. Ind J Clin Biochem 26, 290–295 (2011). https://doi.org/10.1007/s12291-011-0138-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-011-0138-y