Abstract
Mucositis is a very painful unavoidable and common side effect in head and neck cancer patients undergoing curative radiotherapy and can affect the planned treatment. In this study, attempt is made at understanding the efficacy of Emblica officinalis Linn (amla) when combined with providone iodine in mitigating radiation-induced mucositis, weight loss and tumor control. This was a retrospective chart based study and was carried out by extracting the data from the files of patients with cancer of head and neck who used amla in combination with iodine or iodine alone during the course of the curative radiotherapy (> 60 Gy). The data was entered in to Microsoft excel and subjected to statistical analysis using SPSS 17 software. The results indicate that when compared with iodine alone, the group where iodine and amla gargling were used was very effective in delaying mucositis, reduced incidence of intolerable mucositis (P = 0.027), quantitative grade of weight loss (P = 0.016), incidence of severe weight loss (P = 0.03) without affecting tumor response. The results suggest that when compared with iodine alone, amla when combined with iodine was more effective in mitigating radiation mucositis and by not interfering with the tumor cell kill. As far as the authors are aware of this is the first study that shows the usefulness of combining iodine with Amla in mitigating radiation-induced mucositis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiation-induced mucositis across the epithelial lining of the digestive tract is a major non hematological side effect in the Head and Neck cancer (HNC). The curative radiation treatment, which usually goes on for 6 to 7 consecutive weeks, causes inflammatory reactions in the oral and pharyngeal mucosa, which at times can be very serious and life-threatening [1]. Factors like the location of the cancer growth, use of chemotherapy (chemo-irradiation), the radiation dose, field of radiation, immunological and comorbidities status of patients are some of the important factors influencing the severity and the development of oral mucositis [2]. The adverse symptoms of oral mucositis include pain, infections, reduced food intake, and associated weight loss in the patients [1, 2]. In severe cases, oral mucositis compels treatment breaks or discontinuation or altering of the initially planned regimen, affecting the outcome [1, 2].
In clinics, clinical management of oral mucositis is done by judicious antibacterial, antifungal, and analgesic medications [1, 2]. In severe conditions, patients develop infection and will require admission into the intensive care unit to reduce the chances of systemic infection, septicemia, and complications due to metabolic crises. All these conditions together can precipitate and lead to the death of the individual [1, 2]. Clinically, the currently available treatment options for oral mucositis are limited to certain drugs like Mucotrol and MuGard [1, 2]. Additionally, colloidal silver solutions, topical antiseptics, antimicrobial agents, salt and soda rinses or hydrogen peroxide rinses and parenteral administration of amifostine are used for treating oral mucositis [1, 2]. Biological agents such as recombinant human KGF-1 (palifermin) have shown some beneficial effects against oral mucositis; however, its high cost prevents its widespread usage [3]. So it is a need of the hour to identify novel agents that are beneficial, with wide acceptability, affordable and ease of application.
Humans use plants and their bioactive products as therapeutic agents to improve health and mitigate different ailments [4]. Studies in the recent past have shown that Aloe vera [5, 6], Zataria multiflora [7], black mulberry molasses [8], Silymarin [9], Plantago major [10], Glycyrrhiza glabra [11], Turmeric [12], date palm pollen [13], Isatis indigotica [14], Calendula officinalis [15], cystus® tea [16], lapacho-based medication [17] are effective against radiation-induced mucositis. Additionally, Hangeshashinto (TJ-14) a Japanese traditional herbal medicine containing Pinellia Tuber, Scutellariae Radix, Glycyrrhizae Radix, Zizyphi Fructus, Ginseng Radix, Zingiberis Processum Rhizoma, and Coptidis Rhizoma [18]; a polyherbal formulation containing propolis, Aloe vera, calendula, and chamomile [19] and a gargle containing 2 drops of a 1:1 mix of the essential oils of Leptospermum scoparium and Kunzea ericoides in water [20] are also reported to mitigate radiation-induced mucositis in HNC patients. These plant products are non or less toxic, relatively cheap, and widely accepted than synthetic drugs [4]. The radio-protective roles of plant products are investigated widely for the last 3 decades [21]. Some studies suggest free radical scavenging, antioxidant properties, metal chelating properties, and immunostimulatory effects of plant products are useful in protecting laboratory animals against radiation-induced sickness and mortality. In addition, it reduces oxidative stress, enhances wound healing, and improves immune functioning [21]. Cumulatively all these events decrease the cellular damage caused by radiation at the sublethal doses and help the animals recuperate [21].
The fruits of Emblica Officinalis Gaertn or Phyllanthus Emblica Linn, commonly known as Indian gooseberry or amla or amlaki are some of the popular dietary agents in India [4]. The fruits are light greenish-yellow in color and nearly spherical in shape. It looks hard on appearance with six vertical stripes or furrows. The seeds in the fruit are centrally placed and with six vertical stripes or furrows [4]. The seeds are centrally placed and 4–5 mm long. Phytochemically, they contain a higher level of ascorbic acid than in oranges or lemons [4].
Amla also contains gallic acid, ellagic acid, chebulinic acid, chebulagic acid, emblicanin-A, emblicanin-B, punigluconin, pedunculagin, ellagotannin, trigallayl glucose, chebulagic acid, corilagin and isostrictiniin. Amla also has a high level of flavonoids like quercetin, kaempferol 3 O alpha L (6″ methyl) rhamnopyranoside and kaempferol 3 O alpha L (6″ ethyl) rhamnopyranoside [4]. In Indian subcontinents, amla fruits are used as important dietary, culinary, and medicinal constituents. The fully ripe fruits are used to prepare murabba, burfi, ladu, fresh juice, pickle, chutneys, and curries in India. In addition, the juice prepared from the ripe fruits is used as a coolant in the hot summer season. Most importantly, amla is an essential medicinal component in the traditional Indian Ayurvedic system and various other folk systems of medicine [4].
Scientific investigations have shown that amla possesses antibacterial, antifungal, and antiviral properties. Furthermore, the antioxidant properties of amla contribute to the free-radical scavenging, anti-mutagenic and immunomodulatory properties. Additionally, antipyretic and analgesic effects of amla have been demonstrated in various pre-clinical investigations. Amla also has anti-tussive, anti-atherogenic, hypolipidemic, hepatoprotective, renoprotective, and neuroprotective properties [4]. In the current study, the efficacy of amla in mitigating radiation-induced mucositis and its interference with tumor growth are analyzed.
Materials and Methods
Treatment Protocol
The study is based on the retrospective chart and was conducted at—from March 2014 to June 2014. The institutional ethics committee approves the study (MIO/IEC/2019/01/07). The hospital had the services of a senior Ayurvedic physician with expertise in Rasayana Shastra (rejuvenation) from 2013 to 2014. In association with radiation oncologists, a supportive protocol was employed without altering the standard treatment. The care was taken to improve the quality of life in patients undergoing curative radiation treatment and using amla gargle as an adjunct treatment to the standard povidone-iodine. The standard protocol for preventing radiation mucositis was to use a povidone-iodine mouthwash twice a day [22]. The Ayurvedic physician had made a slight change in the routine protocol by incorporating two more gargles with 1% amla water (one after lunch and another in the evening after tea, while retaining the povidone-iodine mouthwash). An oncology nurse carefully monitored the patients medication, diet schedule and care during the treatment in the hospital.
Radiation Therapy
All patients who participated in this study received external irradiation from a linear accelerator (Varian, Model Unique Performance, Palo Alto, CA, USA) at a maximum energy level of 6 MV at a dose rate of 300 MU/min. All planned fields were treated every day with no more than one fraction of 2 Gy per day, five times a week, at the same period of the day without any intended gaps. The final planned target dose is 60 to 70 Gy (in seven consecutive weeks). Whenever chemo-irradiation was planned, carboplatin infusion (70 to 150 mg/m2/day intravenous) [23] or cisplatin infusion (40 to 50 mg/m2/day IV) [24] was administered every week before exposure to the first weekly radiation. The care of patients during and after radiotherapy was in accordance with the hospital guidelines. All the patients received standard oral, dental, medical, and supportive care from a qualified dental, general physician, psychologist, and nutritionist. The diet plan for the patient was followed as per the individual requirement and considering the local dietary habits. The breakfast consisted of soft food like idli, dosa, upma, millet/rice stew, and soft bread. Additionally, they were also provided with either vegetable-lentil or chicken soup once a day. The lunch and dinner consisted of rice stew seasoned with cooked vegetables and lentils. Boiled eggs were also provided during breakfast to add up as an additional protein source.
Preparation of Amla
The Ayurveda unit of the hospital recommended the use of 1% amla. Briefly, 1% amla mouthwash was prepared by dissolving 1 g of dried amla powder in 100 ml of hot water with vigorous stirring. The solution was further cooled to room temperature and filtered through a sterile cloth mesh to remove the particulate matter. The filtration procedure was repeated twice to obtain a filtrate devoid of any particulate matter and used for gargling.
Oral Care in Patients
Patients used soft-bristled oral brushes and were taught to swish their mouth with 10 ml of the 1% povidone-iodine mouthwash once in the morning and once at night. The patients who volunteered to use 1% amla water were instructed to gargle once after lunch and once in the evening. Each time holding the solution in the mouth for 60 s and then to expectorate it. Oral intake of food and drink was prohibited for 30 min after iodine or amla rinse. Patients were treated with lidocaine gel and NSAIDs and/or opioids whenever they experienced severe pain due to mucositis.
Grading of Mucositis
The severity of mucositis was assessed in all the patients by a senior orodental pathologist. The observation was done in the upper and lower lips, right and left checks, right and left ventral and lateral tongue, the floor of the mouth, soft palate/ fauces, hard palate, and oropharyngeal areas. It was done carefully before the start of the radiation treatment during the first dental checkup and at weekly intervals during radiation therapy according to the RTOG guidelines as described earlier [25]. In brief, the grading is scaled from 0 to 4, depending on the severity of oral mucositis. Grades 1 and 2 were ‘tolerable’, and grades 3 and 4 were ‘intolerable’ forms of mucositis [25]. The grades for oral mucositis are defined as follows: grade 0 = no mucositis and no change over baseline; grade 1 (mild) = irritation, the experience of slight pain; grade 2 (moderate) = patchy mucositis that may produce inflammatory sero sanguinitis discharge and moderate pain; grade 3 (severe) = confluent, fibrinous mucositis associated with severe pain; grade 4 (life-threatening) = ulceration, hemorrhage, or necrosis that is seen and usually life-threatening.
The response to radiotherapy was assessed during the first follow-up (i.e., 4 to 6 weeks after completion of treatment) clinically and radiologically. A senior radiation oncologist did the clinical assessment according to the guidelines prescribed by World Health Organization [26]. The degree of tumor volume shrinkage was considered an index of radio responsiveness. Patients with 100% regression of tumor at the primary site were considered complete responders (CR). In contrast, partial responders (PR) had a higher than 50% regression, and non-responders (NR) had a lower than 50% regression [26].
Statistical Analysis
The demographic, pathological, and clinical details were recorded from the patient’s treatment file. The incidence and severity of mucositis, weight loss, treatment breaks, and completion rates of treatments without interruption were noted from the patient files. The data were entered into Microsoft excel and subjected to statistical analysis. Unpaired “t-test” was used to compare the extent of severe mucositis score weekly, testing equality of proportion for the delay in incidence and the number of tolerable and intolerable mucositis. In contrast, the X2 test was used to compare the total incidence of worst-ever grades of ulceration, the number of treatment days lost due to intolerable mucositis, and weight loss. An accurate note of the number of treatment days lost during the course of the treatment and weight loss at the end of the treatment was also made.
Results
Records of 50 patients treated for HNC from March 2014 to June 2014 on an inpatient basis were reviewed retrospectively. The files of patients who were treated with curative intent using the conventional fractioned radiation of 2 Gy per day and 5 days per week with or without cisplatin (60 mg/m2 intravenous infusion once a week during RT on the first day of the week) and admitted to the inpatient facility throughout the treatment period were selected. The group with only povidone-iodine gargle twice a day was considered to control cohorts, while those who used povidone-iodine and amla were considered the test group. The details of the patients' age, sex, location of cancer, stage of cancer are all represented in Table 1, while treatment type, dose, the incidence of breaks, use of opioids, loss of days due to mucositis are shown in Table 2.
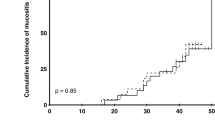
The study population of control cohorts consisted of 30 patients (20 males and 10 females), while for amla, 20 (16 men and 4 females). The mean age was 53.97 ± 11.33 in the control while it was 53.12 ± 11.04 in the amla cohort. Regarding the tumor site, the majority of the patients in both cohorts had tumors of the tongue (16.67 vs. 15%). Radiation exposure caused mucositis in both the cohorts, and the incidence and mean of mucositis increased in both cohorts with time (Figs. 1, 2, 3). However, when compared to the control, in the amla cohort, the onset of both tolerable and intolerable mucositis was delayed and was significant at most time points (Fig. 3).
The incidence of development of intolerable mucositis was more in the iodine group than in the amla + iodine group (93.33 vs. 70) and was significant (P = 0.027) (Table 2). The analysis also showed that the incidence of development of early intolerable mucositis (before week 4) was more in the control group than in the amla group (30 vs. 10%) and was significant (P = 0.043) (Table 2). With respect to the number of patients who had a treatment break, the incidence (33.33 vs. 15%), as well as number of days (5.80 ± 1.75 vs 5.50 ± 1.53), were less in the amla group (Table 2). Additionally, it was also observed that the weight loss was less in the amla cohorts (2.57 ± 1.25 vs 1.78 ± 1.17; P = 0.016) (Table 2). The incidence of severe weight loss (More than 2 kg) was also more in the controls (P = 0.03) (Table 2). The treatment response performed 4 to 6 weeks after the last fraction of radiation showed that there was no significant difference in the observed cases of the complete response (CR) and no response (NR) (Table 2).
Discussion
This study observed that compared to the control (iodine alone), the test group where iodine and amla gargling was used was very effective in delaying the development of radiation-induced mucositis during most of the radiation treatment period (Fig. 1). However, the most important observation is that amla rinse was more effective in delaying and mitigating the development of intolerable mucositis, a dreaded side effect responsible for increasing morbidity, prolonged hospitalization, and escalates the treatment cost [1, 2]. As far as the authors are aware, this is the first study that addresses the beneficial effects of combining povidone-iodine with amla in mitigating radiation-induced mucositis. However, previous studies do suggest amla to be effective as a radio-protective agent and to protect mice against the whole body exposed to radiation-induced sickness and mortality [27], to mitigate radiation-induced hematopoietic damage [28]; gastrointestinal damage [29] and to reduce the radiation-induced oxidative stress in mice [30]. Additionally, the phytochemical geraniin is shown to prevent radiation-induced apoptosis and oxidative stress in Chinese hamster lung fibroblast (V79-4) cells [31], while corilagin is shown to reduce radiation-Induced brain Injury in mice [32].
The other most important observation of our study was that gargling with amla did not interfere with the radiation treatment response. Previous studies have shown that amla induced cytotoxicity in the tumor cells [A549 (lung), HepG2 (liver), HeLa (cervical), MDA-MB-231 (breast), SK-OV3 (ovarian), and SW620 (colorectal)] while sparing the normal cells [MRC5 (normal lung fibroblast)] [33]. Amla also possess chemopreventive effects against DMBA initiated-TPA promoted skin carcinogenesis [33]; 7,12-dimethyabenz(a)anthrecene and croton oil promoted skin carcinogenesis [34]; diethylnitrosoamine-induced hepatocarcinogenesis [35]; mitigate thioacetamide-induced oxidative stress and reduce the promotional events primary hepatocarcinogenesis [36]; benzopyrene-Induced lung Lesion [37]; dimethylbenz (a) anthracene-induced oral carcinogenesis [38]. Together all these observations suggest that the amla could have had a role in enhancing tumor cell kill while protecting the normal cells and needs to be investigated in both preclinical and clinical models of study.
Amla has been scientifically investigated extensively, and observations published attest to its usefulness in curing various maladies affecting the oral cavity. For example, amla is reported to be effective in mitigating microbial growth of S mutans, involved in the development of dental plaque and caries [39]. Gargling with the aqueous extract of amla is more effective than chlorhexidine in preventing caries [40] and chewing on an amla-containing gum is also reported to improve oral health [41]. Amla possess potent antimicrobial effects including against the fastidious oral pathogen S mutans.Cumulatively all these results indicate that the benifecial effects of amla may add to that of the iodine and together both these agents would be effective in keeping the growth of oral pathogens in check and mediate the beneficial effects.
Exposure to radiation delays the healing process. Laboratory studies have shown that topical application of amla was effective in enhancing the closure of open wound by increasing cellular proliferation and cross-linking of collagen at the wound site, by increasing DNA, type III collagen, acid-soluble collagen, aldehyde content, shrinkage temperature and tensile strength and increasing tissue antioxidants like ascorbic acid, alpha-tocopherol, reduced glutathione, superoxide dismutase, catalase, and glutathione peroxidase [42]. Additionally, cell culture studies have also shown that amla enhanced the growth of human keratinocytes, indicating it promotes wound healing [43]. Quick healing of cuts and ulcers is a priority as open wound can facilitate the chances of microbial infection and growth. Worse, systemic infection can lead to septicemia and lead to metabolic crisis and death of the immune compromised individual. The observation that amla heals open wound is of great significance as a similar mechanism may be operating and needs to be scientifically validate in animal/cell culture models of radiation induced wound healing delay assays to affirm the underlying mechanism/s in detail.
From a mechanistic viewpoint, exposure to low linear energy transfer ionizing radiation like X-ray predominantly mediates the cytotoxic effects principally by generating free radicals (indirect effect) than from the DNA damage (direct effects). Amla has been investigated for its free radical scavenging and antioxidant effects in myriad validated assay systems and reported to be effective against a range of free radicals [4, 44]. Amla also possesses anti-inflammatory effects [44–46] and analgesic effects [47]. Together, all these observations indicate that amla gargle might have scavenged the free radicals, mitigated inflammatory reactions, and possibly halted the perpetuation of the cyclical oxidative stress-inflammatory feedback pathways that aggravate the pathogenesis. Also, the analgesic effects might have also contributed to the ensuing beneficial effects and negated/reduced the pain impulses. Studies with cell culture and laboratory animals considering multiple end points will be very useful in establishing the mechanism/s operating to mediate the protective effects and are required.
Conclusions
The result of the present study indicates that amla, when combined with povidone-iodine, possesses better protective effects against radiation-induced mucositis and that the effect was better than the use of standard povidone-iodine gargle alone. Amla has an excellent safety profile, wide acceptability and is effective in delaying and reducing radiation-induced mucositis. The biggest drawback of our study was that this was a non-randomized study and the amla arm consisted of people who were eager to use it. Further, the control arm rinsed their mouth only twice per day with povidone-iodine solution. At the same time, in the test group, the volunteers swished their mouth with the povidone-iodine solution as in the control group and two more times with amla. This increase in swish in the test may have contributed to the observed protection. Our study shows for the first time that gargling with amla along with the regular povidone-iodine swish was effective in mitigating radiation-induced mucositis. The authors suggest that future studies should be focused on ascertaining the effectiveness of amla in combination with standard agent/s and also as a single agent in randomized, double-blinded clinical trials. Endeavors along these lines will be useful for cancer patients undergoing treatment.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Davy C, Heathcote S (2021) A systematic review of interventions to mitigate radiotherapy-induced oral mucositis in head and neck cancer patients. Support Care Cancer 29(4):2187–2202
Sunaga T, Nagatani A, Fujii N, Hashimoto T, Watanabe T, Sasaki T (2021) The association between cumulative radiation dose and the incidence of severe oral mucositis in head and neck cancers during radiotherapy. Cancer Rep 4:e1317
Daugėlaitė G, Užkuraitytė K, Jagelaviciene E, Filipauskas A (2019) Prevention and treatment of chemotherapy and radiotherapy induced oral mucositis. Medicina 55:25
Baliga M, Dsouza J (2011) Amla (Emblica officinalis Gaertn), a wonder berry in the treatment and prevention of cancer. Eur J Cancer Prev 20(225):39
Ahmadi A (2012) Potential prevention: Aloe vera mouthwash may reduce radiation-induced oral mucositis in head and neck cancer patients. Chin J Integr Med 18(8):635–640
Sahebjamee M, Mansourian A, Hajimirzamohammad M, Zadeh MT, Bekhradi R, Kazemian A et al (2015) Comparative efficacy of aloe vera and benzydamine mouthwashes on radiation-induced oral mucositis: a triple-blind, randomised, controlled clinical trial. Oral Health Prev Dent 13(4):309–315
Aghamohammadi A, Moslemi D, Akbari J, Ghasemi A, Azadbakht M, Asgharpour A et al (2018) The effectiveness of Zataria extract mouthwash for the management of radiation-induced oral mucositis in patients: a randomized placebo-controlled double-blind study. Clin Oral Investig 22(6):2263–2272
Demir Dogan M, Can G, Meral R (2017) Effectiveness of black mulberry molasses in prevention of radiotherapy-induced oral mucositis: a randomized controlled study in head and neck cancer patients. J Altern Complement Med 23(12):971–979
Elyasi S, Hosseini S, Niazi Moghadam MR, Aledavood SA, Karimi G (2016) Effect of oral silymarin administration on prevention of radiotherapy induced mucositis: a randomized, double-blinded. Placebo-Controlled Clin Trial Phytother Res 30(11):1879–1885
Soltani GM, Hemati S, Sarvizadeh M, Kamalinejad M, Tafazoli V, Latifi SA (2020) Efficacy of the Plantago major L. syrup on radiation induced oral mucositis in head and neck cancer patients: a randomized, double blind, placebo-controlled clinical trial. Complement Ther Med 51:102397
Mamgain RK, Gupta M, Mamgain P, Verma SK, Pruthi DS, Kandwal A et al (2020) The efficacy of an ayurvedic preparation of yashtimadhu (Glycyrrhiza glabra) on radiation-induced mucositis in head-and-neck cancer patients: a pilot study. J Cancer Res Ther 16(3):458–462
Rao S, Dinkar C, Vaishnav LK, Rao P, Rai MP, Fayad R et al (2014) The Indian spice turmeric delays and mitigates radiation-induced oral mucositis in patients undergoing treatment for head and neck cancer: an investigational study. Integr Cancer Ther 13(3):201–210
Elkerm Y, Tawashi R (2014) Date palm pollen as a preventative intervention in radiation- and chemotherapy-induced oral mucositis: a pilot study. Integr Cancer Ther 13(6):468–472
You W, Hsieh C-C, Huang J (2009) Effect of extracts from indigowood root (Isatis indigotica Fort.) on immune responses in radiation-induced mucositis. J Altern Complement Med (New York, NY) 15:771–778
Babaee N, Moslemi D, Khalilpour M, Vejdani F, Moghadamnia Y, Bijani A et al (2013) Antioxidant capacity of calendula officinalis flowers extract and prevention of radiation induced oropharyngeal mucositis in patients with head and neck cancers: a randomized controlled clinical study. Daru 21(1):18
Ebert N, Kensche A, Lock S, Hadiwikarta WW, Hansch A, Dorr W et al (2021) Results of a randomized controlled phase III trial: efficacy of polyphenol-containing cystus(R) tea mouthwash solution for the reduction of mucositis in head and neck cancer patients undergoing external beam radiotherapy. Strahlenther Onkol 197(1):63–73
Giacomelli I, Scartoni D, Fiammetta M, Baki M, Zei G, Muntoni C et al (2015) Oral lapacho-based medication: an easy, safe, and feasible support to prevent and/or reduce oral mucositis during radiotherapy for head and neck cancer. Nutr Cancer 67(8):1247–1253
Matsumoto C, Sekine-Suzuki E, Nyui M, Ueno M, Nakanishi I, Omiya Y et al (2015) Analysis of the antioxidative function of the radioprotective Japanese traditional (Kampo) medicine, hangeshashinto, in an aqueous phase. J Radiat Res 56(4):669–677
Marucci L, Farneti A, Di Ridolfi P, Pinnaro P, Pellini R, Giannarelli D et al (2017) Double-blind randomized phase III study comparing a mixture of natural agents versus placebo in the prevention of acute mucositis during chemoradiotherapy for head and neck cancer. Head Neck 39(9):1761–1769
Maddocks-Jennings W, Wilkinson JM, Cavanagh HM, Shillington D (2009) Evaluating the effects of the essential oils Leptospermum scoparium (manuka) and Kunzea ericoides (kanuka) on radiotherapy induced mucositis: a randomized, placebo controlled feasibility study. Eur J Oncol Nurs 13(2):87–93
Dowlath MJH, Karuppannan SK, Sinha P, Dowlath NS, Arunachalam KD, Ravindran B et al (2021) Effects of radiation and role of plants in radioprotection: a critical review. Sci Total Environ 779:146431
Kumar PM, Sequeira PS, Shenoy K, Shetty J (2008) The effect of three mouthwashes on radiation-induced oral mucositis in patients with head and neck malignancies: a randomized control trial. J Cancer Res Ther 4:3–8
Pazdur R, Coia L, Hoskins W, Wagman L (eds) (2007) Cancer management: a multidisciplinary approach. FA Davis Company, Philadelphia
Homma A, Inamura N, Oridate N, Suzuki S, Hatakeyama H, Mizumachi T et al (2011) Concomitant weekly cisplatin and radiotherapy for head and neck cancer. Jpn J Clin Oncol 41(8):980–986
Khanal B, Baliga M, Uppal N (2010) Effect of topical honey on limitation of radiation-induced oral mucositis: an intervention study. Int J Oral Maxillofac Surg 39(12):1181–1185
World Health O (1979) WHO handbook for reporting results of cancer treatment. World Health Organization, Geneva
Singh I, Sharma A, Nunia V, Goyal PK (2005) Radioprotection of Swiss albino mice by Emblica officinalis. Phytother Res 19(5):444–446
Hari Kumar KB, Sabu MC, Lima PS, Kuttan R (2004) Modulation of haematopoetic system and antioxidant enzymes by Emblica Officinalis Gaertn and its protective role against γ-radiation induced damages in mice. J Radiat Res 45(4):549–555
Jindal A, Soyal D, Sharma A, Goyal PK (2009) Protective effect of an extract of Emblica officinalis against radiation-induced damage in mice. Integr Cancer Ther 8(1):98–105
Sandhya T, Lathika KM, Pandey BN, Bhilwade HN, Chaubey RC, Priyadarsini KI et al (2006) Protection against radiation oxidative damage in mice by Triphala. Mutat Res/Genet Toxicol Environ Mutagenes 609(1):17–25
Kang KA, Lee IK, Zhang R, Piao MJ, Kim KC, Kim SY et al (2011) Radioprotective effect of geraniin via the inhibition of apoptosis triggered by γ-radiation-induced oxidative stress. Cell Biol Toxicol 27(2):83–94
Tong F, Zhang J, Liu L, Gao X, Cai Q, Wei C et al (2016) Corilagin attenuates radiation-induced brain injury in mice. Mol Neurobiol 53(10):6982–6996
Ngamkitidechakul C, Jaijoy K, Hansakul P, Soonthornchareonnon N, Sireeratawong S (2010) Antitumour effects of Phyllanthus emblica L.: induction of cancer cell apoptosis and Inhibition of in vivo tumour promotion and in vitro invasion of human cancer cells. Phytother Res 24(9):1405–1413
Sancheti G, Jindal A, Kumari R, Goyal P (2005) Chemopreventive action of Emblica officinalis on skin carcinogenesis in mice. Asian Pac J Cancer Prev: APJCP 6:197–201
Sultana S, Ahmed S, Jahangir T (2008) Emblica officinalis and hepatocarcinogenesis: a chemopreventive study in Wistar rats. J Ethnopharmacol 118(1):1–6
Sultana S, Ahmed S, Sharma S, Jahangir T (2004) Emblica officinalis reverses thioacetamide-induced oxidative stress and early promotional events of primary hepatocarcinogenesis. J Pharm Pharmacol 56(12):1573–1579
Wang CC, Yuan JR, Wang CF, Yang N, Chen J, Liu D et al (2016) Anti-inflammatory effects of Phyllanthus emblica L. on benzopyrene-induced precancerous lung lesion by regulating the IL-1β/miR-101/Lin28B signaling pathway. Integr Cancer Ther 16(4):505–515
Krishnaveni M, Mirunalini S (2012) Chemopreventive efficacy of Phyllanthus emblica L. (amla) fruit extract on 7,12-dimethylbenz(a)anthracene induced oral carcinogenesis—a dose–response study. Environ Toxicol Pharmacol 34(3):801–810
Jain I, Jain P, Bisht D, Sharma A, Srivastava B, Gupta N (2015) Use of traditional Indian plants in the inhibition of caries-causing bacteria—Streptococcus mutans. Braz Dent J 26(2):110–115
Velmurugan A, Madhubala MM, Bhavani S, Satheesh Kumar KS, Sathyanarayana SS, Gurucharan N (2013) An in-vivo comparative evaluation of two herbal extracts Emblica officinalis and Terminalia Chebula with chlorhexidine as an anticaries agent: A preliminary study. J Conserv Dent 16(6):546–549
Gao Q, Li X, Huang H, Guan Y, Mi Q, Yao J (2018) The efficacy of a chewing gum containing Phyllanthus emblica fruit extract in improving oral health. Curr Microbiol 75(5):604–610
Sumitra M, Manikandan P, Gayathri VS, Mahendran P, Suguna L (2009) Emblica officinalis exerts wound healing action through up-regulation of collagen and extracellular signal-regulated kinases (ERK1/2). Wound Repair Regener 17(1):99–107
Yamakami Y, Morino K, Takauji Y, Kasukabe R, Miki K, Hossain MN et al (2019) Extract of Emblica officinalis enhances the growth of human keratinocytes in culture. J Integr Med 17(2):141–146
Middha SK, Goyal AK, Lokesh P, Yardi V, Mojamdar L, Keni DS et al (2015) Toxicological evaluation of Emblica officinalis fruit extract and its anti-inflammatory and free radical scavenging properties. Pharmacogn Mag 11(Suppl 3):S427–S433
Li W, Zhu HW, Chen Y, Xiao H, Ge YZ, Hu HE et al (2020) Bioactivity guided isolation of ant‰ inflammatory components from Phyllanthus emblica. Food Sci Nutr 8:2670–2679
Kalaiselvan S, Rasool MK (2015) The anti-inflammatory effect of triphala in arthritic-induced rats. Pharm Biol 53(1):51–60
Lim DW, Kim JG, Kim YT (2016) Analgesic effect of Indian gooseberry (Emblica officinalis fruit) extracts on postoperative and neuropathic pain in rats. Nutrients 8(12):760
Acknowledgements
The authors are grateful to Dr. L.K. Vaishnav, the Ayurvedic physician for initiating of combining Ayurveda with conventional care. The authors are also grateful to Shri Ananthakrishna, the president of Mangalore Institute of Oncology for supporting the study. SVR is grateful to DBT-Ramalingaswami Fellowship.
Funding
Nil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to disclose with respect to the research, authorship, and/or publication of this article.
Consent for Publication
All authors consented to the submission of the article for publication.
Ethical Statement
This was a retrospective study ad was conducted after obtaining clearance from Institutional Ethics Committee (MIO/IEC/2019/01/07).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hegde, S.K., Rao, S., Rao, P. et al. Aqueous Extract of Emblica officinalis Linn (Indian gooseberry) in Combination with Iodine is More Efficacious than Iodine Alone in Mitigating Mucositis in Head and Neck Cancer Patients Undergoing Curative Radiotherapy: Retrospective Observations. Indian J Otolaryngol Head Neck Surg 74 (Suppl 3), 6330–6338 (2022). https://doi.org/10.1007/s12070-021-03059-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-021-03059-w