Abstract
Purpose
To determine the effect of Cystus® tea (Naturprodukte Dr. Pandalis GmbH & Co. KG) as mouthwash compared to sage tea on oral mucositis in patients undergoing radio(chemo)therapy for head and neck cancer.
Methods
In this randomized, prospective phase III study, 60 head and neck cancer patients with primary or postoperative radio(chemo)therapy were included between 04/2012 and 06/2014. They received either sage or Cystus® tea for daily mouthwash under therapy. Mucositis was scored twice a week following the Radiation Therapy Oncology Group and the European Organization for Research and Treatment Cancer (RTOG/EORTC) scoring system. Dental parameters were also recorded. Statistical evaluation of the primary endpoint was performed using t‑test and log rank test.
Results
Data from 57 patients could be evaluated. Patient characteristics showed no significant difference between the two groups (n = 27 sage; n = 30 Cystus®). A total of 55 patients received the prescribed dose (60–66 Gy postoperative; 70–76.8 Gy primary). Mucositis grade 3 was observed in 23 patients (n = 11 sage; n = 12 Cystus®) and occurred between day 16 and 50 after start of therapy. There was no significant difference between the two groups in latency (p = 0.75) and frequency (p = 0.85) of the occurrence of mucositis grade 3. The self-assessment of the oral mucosa and the tolerability of the tea also showed no significant differences. Occurrence of dental pathologies appeared to increase over time after radiotherapy.
Conclusion
Cystus® and sage tea have a similar effect on the occurrence of radiation-induced mucositis regarding latency and incidence. Cystus® tea mouthwash solution is tolerated well and can be applied in addition to intensive oral care and hygiene along with the application of fluorides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiotherapy is one of the standard treatment options for patients with head and neck cancer. Depending on tumor stage, type, performance status and treatment intent, radiotherapy is performed alone or in combination with chemotherapy as definitive treatment or in the postoperative setting. Treatment with ionizing radiation frequently causes early side effects. One of the most common side effects of head and neck radio(chemo)therapy is oral mucositis which occurs in almost all patients [1]. Generally mucositis manifests within the first weeks after the start of therapy and increases during the following weeks up to the completion of radiation treatment. The mucosal reaction can persist for several weeks after the end of treatment.
Mucositis can be scored using the scoring system developed by the Radiation Therapy Oncology Group and the European Organization for Research and Treatment Cancer (RTOG/EORTC). Oral mucositis varies in severity from slight enanthema up to large areas of epitheliolysis, with severe forms being accompanied by pain, discomfort, reduced ingestion, and decreased quality of life [2]. Furthermore, predamaged mucosa is associated with an increased risk of infections that may result in grave consequences such as radionecrosis of the mandible. Mucositis can also lead to interruption or prolongation of treatment, which jeopardizes local tumor control because of the longer time available for cancer stem cells to repopulate [3, 4]. Along with direct damage of the oral mucosa, further short- or long-term effects of radiation therapy in the oral cavity can include radiation caries as well as rapid caries progression and the occurrence of periodontal diseases [5,6,7].
Timely and ideally pre-emptive management of therapy-associated mucositis is therefore of great medical importance [8]. However, currently there are no established procedures used and generally accepted in the clinical setting. Commercially available mouthwashes frequently contain alcohol and are therefore uncomfortable for patients with mucositis, as they can cause pain and a burning sensation. Instead, herbal mouthwashes are often recommended [9].
Cystus® tea could be suitable for clinical practice. Made from the leaves and small twigs of the cistaceae family of plants, it might qualify as a reasonable mouthwash with similar characteristics as sage tea [10, 11]. Various studies showed anti-inflammatory, antifungal, and antioxidant properties of Cystus® extracts [12]. Cystus® tea is often used for prevention and treatment of infections in the upper respiratory tract [11, 13]. A reduction of the initial bacterial colonization and adherence to enamel in the oral cavity could be demonstrated after mouthrinses with Cystus® tea [10, 14] providing a rationale for its use. In addition, the tea has a mild flavor and it contains no ingredients which could be expected to cause any noteworthy side effects. The existing studies with Cystus® tea show good tolerability [11, 15, 16]. A negative interaction of Cystus® tea with radio(chemo)therapy is not expected due to the local use as mouthwash.
Several studies have been published regarding the use of various substances for the prevention or reduction of oral mucositis, but to our knowledge there are no randomized controlled phase III trials comparing the efficacy of two herbal mouthwashes. In this study, we investigate the effect of Cystus® vs. sage tea on oral mucositis in patients undergoing radio(chemo)therapy for head and neck cancer. Sage tea was used as control group due to the fact that it is the most frequently recommended mouthwash and was also used as control in a similar study [17]. In addition to clinical evaluation of mucositis, patient self-assessments and several common dental examination parameters were recorded during the observation period.
Methods
A prospective, single-center, randomized phase III trial was conducted at the Department of Radiation Oncology in cooperation with the Clinic of Operative Dentistry at the University Medical Center Carl Gustav Carus in Dresden, Germany. The study was approved by the institutional ethics committee (EK 281082011).
All patients with histologically confirmed head and neck cancer treated at our institution between April 2012 and May 2014 were screened for inclusion in this study.
The inclusion criteria were the following: age ≥18 years, performance status WHO 0–2, cumulative dose ≥40 Gy in the oral cavity, prescribed dose ≥60 Gy for postoperative and ≥70 Gy for definitive radiotherapy treatment, written informed consent. The exclusion criteria were as follows: previous irradiation of the oral cavity with >10 Gy, pregnancy, expected lack of compliance, participation in another study 4 weeks before or after radiotherapy.
Once patients signed the informed consent, they were enrolled in the study and randomized to receive either Cystus® or sage tea. Stratified block randomization was performed by random number tables, stratified by radiotherapy treatment, to achieve a similar distribution of the patients over the tee groups.
Patients received a supply of tea sufficient for the whole treatment period, as well as instructions for how to prepare and administer the tea as repeated daily flush of the oral cavity. For tea mouthwash preparation 1 l of hot water was poured over a tea bag (1.5 g Cystus® or sage tea) and it should steep for some minutes (7 min for Cystus® tea and 10 min for sage tea). After that, the tea bag was removed and the tea should cool down before use. The recommendation consisted of regular mouth rinses of at least 1.5 min 3–5 times a day, especially after meals. Cystus® tea was provided by Naturprodukte Dr. Pandalis GmbH & Co. KG and sage tea (commercial product) by the University Medical Center Carl Gustav Carus Dresden.
All patients received either postoperative or definitive radio(chemo)therapy with curative intent. Postoperative patients were treated with fractionated radiotherapy up to a total dose of 60–66 Gy to the tumor bed and the lymph node metastasis, and 50 Gy to the elective lymph node regions. In addition, patients with a high risk for recurrence received simultaneous chemotherapy with cisplatin 100 mg/m2 in weeks 1, 3, and 6. In 82% (18/22 patients), only 1 to 2 of the planned 3 chemotherapy cycles could be applied because of increased renal retention parameters or incomplete recovery of blood cell counts. Patients treated with definitive radio(chemo)therapy received conventionally fractionated radiotherapy up to a total dose of 70–76 Gy. Alternatively, a protocol of conventional and hyperfractionated radiotherapy of up to 72 Gy to the primary tumor and lymph node region and 49.6–50 Gy to the elective lymph node region was used (in analogy to [18]). Patients eligible for concomitant chemotherapy received cisplatin 30 mg/m2 once per week and 5‑fluoruracil (5-FU; 600 mg/m2, 120 h continuous infusion) in the first week. In all patients, either intensity-modulated radiotherapy, 3D conventional radiotherapy, or a combination of both was employed.
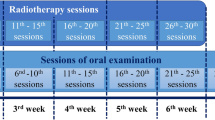
The mucosa was visually evaluated directly before, twice a week during, and 3 months after radiotherapy. Some patients (n = 20) underwent an additional evaluation one week after the end of radiotherapy; this was either due to a scheduled follow-up for early toxicity experienced during treatment, or to the treating physician deeming an additional evaluation necessary. The evaluation was performed by a qualified radiation oncologist in accordance to the RTOG/EORTC criteria [19]. Xerostomia and impairment of taste were also evaluated before, twice a week during, and 3 months after radiotherapy according to the RTOG/EORTC criteria.
In addition to this objective mucositis evaluation, patients performed a self-assessment of the status of the oral cavity and the tolerability of the tea before, once a week during, and 3 months after the end of radiotherapy. This was done with a nongraded visual analogue scale reaching from very bad to very good. For quantitative evaluation, the distance from one end to the area on the scale indicated by the patient was measured (resulting in a value between 0 and 9).
In order to document the dental and periodontal tissue during treatment, several clinical parameters were evaluated by a dentist experienced in assessment of patients undergoing radiotherapy. Examination was performed at baseline and 3 months after radiotherapy.
First, all teeth and existing restorations were examined and decayed, missing and filled teeth/surfaces (DMF S) were documented. Surfaces that showed distinct visual change even when they were wet were recorded as carious lesions (International Caries Detection and Assessment System [ICDAS] score ≥2) [20]. Due to the delayed onset of dental hard tissue defects, this parameter was re-evaluated 3 months after treatment [21]. The periodontal state was assessed using the periodontal screening index (PSI) [17]. In order to detect local inflammatory processes more precisely, the modified Sulcus Bleeding Index (SBI) was applied. A normal SBI should be less than 10%. Finally, the Approximal Plaque Index (API) was determined dichotomously in the first and third quadrant orally and buccally in the second and forth quadrant [22]. An API less than 30% is considered as a desirable condition for the protection against caries.
The primary endpoint of this phase III study was to compare the latency of the occurrence of mucositis grade 3 between the two trial arms. The latency was calculated as time between the start of radiotherapy and the first occurrence of the event. Secondary endpoints were latency for mucositis grade 2, incidence of mucositis grade 3 and 2. A total sample size of 60 patients (30 in each group) was calculated as necessary to detect a difference of 7 days in the latency between the Cystus® and sage tea group by a two-sided t‑test of independent samples. The difference of 7 days was considered as clinically relevant and was therefore used for the sample size calculation. This assumed a significance level of 0.05 and a power of 0.9, as well as a dropout rate of 0.2.
Statistical analysis was performed using R version 3.5.0 (2018-04-23); p-values <0.05 were considered statistically significant. The comparison of the patient characteristics between the two groups was done by Fisher exact test and Mann–Whitney U test for categorical and continuous variables respectively. Two patients who deceased during treatment were excluded from the evaluation of the parameters total treatment time and total radiotherapy dose. Two-sided t‑tests were used for the evaluation of the latency of mucositis grade 3 and 2, and the comparison of the results from the self-assessments between the Cystus® and sage tea group. Only existing data points were included in this analysis; missing values were excluded. In addition, a log-rank test was performed for the latency and frequency evaluation of mucositis grade 3 and 2. Univariate and multivariate Cox regression analyses were performed to investigate parameters which are potentially associated with an incidence of mucositis grade 3. P-values were calculated with the Wald test.
For the evaluation of the prevalence of mucositis grade 3, ≥2, xerostomia, and impairment of taste, to include the effect from time and the two groups, we used linear mixed-effect models (R package lme4).
A statistical analysis of the dental parameters (DMF S, API, SBI) was performed on the matched difference between baseline and 3 months after treatment comparing the two groups using the Mann–Whitney U test.
Data were stored at the Department of Radiation Oncology, except for the dental parameters that were stored at the Clinic of Operative Dentistry at the University Medical Center Carl Gustav Carus in Dresden.
Results
A total of 60 patients were enrolled in this study between 04/2012 and 05/2014. Three patients had to be excluded from analysis: One patient no longer fulfilled the inclusion criteria after discovery of an initially unknown primary tumor. The other two patients could not be evaluated due to worsening of their performance status and death during therapy.
From the remaining 57 patients, two did not complete the course of radiotherapy (because of noncompliance due to alcohol-induced delirium and unexpected death during therapy). These patients were included in the final analysis because they reached the primary endpoint mucositis grade 3.
The patient characteristics are summarized in Table 1. There were no significant differences between the two groups.
Twenty-three patients developed mucositis grade 3 during treatment, of which 11 patients were in the sage tea and 12 in the Cystus® tea group. Latency, measured from the first day of irradiation to the occurrence of mucositis grade 3, ranged between 16 and 50 days for all patients. The mean latency for mucositis grade 3 was 32.2 days (± standard deviation [SD] 9.1 days) vs. 33.4 days (± SD 9.5 days) for the patients with sage and Cystus® tea. There was no statistical difference in mucositis grade 3 latency (t-test, p = 0.75) and frequency (log-rank p = 0.85, Fig. 1).
Incidence of mucositis grade 3 and 2 was 40.4% (n = 23) and 98.2% (n = 56), respectively, in the entire patient cohort (n = 57). With respect to the cumulative incidence of mucositis grade 3 and 2, no significant difference between the two treatment arms was determined (p = 0.85 and p = 0.28). Latency of mucositis grade 2 ranged from 10 to 45 days. The average latency was 22.7 days (± SD 9.1 days) for the patients who received sage tea and 21.2 days (± SD 9.3 days) for Cystus® tea with no statistically significant difference between groups (t-test p = 0.54; log-rank test p = 0.28).
Univariate cox regression analysis evaluated the potential influence of patient and treatment factors on mucositis grade 3. Type of treatment (four-way comparison of postoperative/primary and radiotherapy/radiochemotherapy), postoperative treatment situation, conventional fractionation, and total radiotherapy dose were significantly associated with the occurrence of mucositis grade 3 (p < 0.001; Table 2).
In addition, a multivariate Cox regression analysis including tumor stage, chemotherapy, total treatment time, and total radiotherapy dose was performed. These parameters were selected because an influence on mucositis was likely based on studies in the literature. As other radiotherapy treatment variables (type of treatment, postoperative situation, conventional fractionation, and total radiotherapy dose) were highly correlated and because of the small sample size, total radiotherapy dose was chosen to represent these variables. In this analysis, total radiotherapy dose continued to appear as a significant factor regarding mucositis grade 3 (p = 0.001, HR 1.22 [95%CI 1.08–1.38]), whereas the other three parameters did not (Table 2).
Fig. 2a,b show the prevalence of mucositis grade 3 and ≥2 at baseline, during each treatment week (week 1–8) and 3 months after treatment (week 19). Mucositis recovered completely in all but one patient within 3 months. However, in this patient clinical assessment also showed atrophic mucosa in line with late as opposed to early toxicity.
Prevalence of xerostomia grade 3 and impairment of taste grade 3 were also assessed (Fig. 2c,d) without significant differences.
The results of the weekly self-assessment of the oral cavity and the tolerability of the tea showed no relevant difference (Fig. 3a,b). Only in week 7 was a significant difference observed (p = 0.008); however due to the small number of patients (n = 3 sage, n = 6 Cystus®) at this time, without correction for multiple statistical testing and no difference directly before and at later assessment, this results should be interpreted as an outlier.
Due to the strong heterogeneity of the baseline dental state in comparison to the sample size, evaluation was only possible to a limited extent. Table 3 shows the results of the mean DMF (S) index for all patients as well as the subgroup of patients with conserved teeth at baseline as well as 3 months after radio(chemo)therapy. There was no significant difference between the two teas regarding the changes in the DMF (S) index between the two time points as well as for the single values missing (M) and filled (F) surface. However, the data show a relatively fast increase of caries (decayed (D) surface) from the start to 3 months after the end of treatment in both groups, without a significantly difference between the groups.
The API and SBI showed remarkably high scores at baseline and 3 months after the end of treatment, indicating that the patients’ oral hygiene was consistently insufficient. No differences were observed regarding the changes of SBI between the two groups. However, regarding the API change a significant difference was detected. The patients with Cystus® tea had a slight decrease and the ones with sage tea a slight increase of the API (Table 3). No significant change of PSI scores were observed (data not shown).
Discussion
We conducted a randomized controlled phase III trial comparing sage and Cystus® tea with regard to the latency of mucositis grade 3 as well as several secondary endpoints in a cohort of head and neck cancer patients undergoing primary or postoperative radio(chemo)therapy.
Our results indicate no difference regarding mucositis incidence and latency between the two trial arms, which to the best of our knowledge is the first direct comparison between Cystus® and sage tea in this setting.
The pathophysiology of radiation induced mucositis is a multifactorial process that develops over several weeks including epithelial depletion, inflammation by generation of ROS, activation of transcription factors like NF-kB and several ensuing signaling cascades. In addition, there is an increased risk for secondary bacterial colonization [23]. We did not observe any differences between our two treatment arms. Two potential explanations for this lack of difference could be: First, the pathomechanism of mucositis is complex and influencing a small part of the process might not be sufficient for a measurable effect. Second, the teas used in both control groups are known to have antibacterial [24, 25] and potentially also anti-inflammatory effects [26] as well as impact on cariogenic bacteria [25, 27]. Furthermore, the Cystus® tea has a strong antiviral effect [16].
In this study cohort, we observed a relatively low incidence of mucositis grade 3 of 40% and no occurrence of mucositis grade 4. In comparison, other studies reported an incidence of 34–56% for radiotherapy alone [28]. Additional chemotherapy might lead to further increases [29]. A likely explanation for this low incidence could be that both patient groups benefited from the instructions for regular oral hygiene with either sage or Cystus® tea mouthwashes, respectively.
Both teas can be bought in the pharmacy in good quality or in a drug store, in which the quality depends on the company. The relative costs for both teas are comparable and depend where the tea is bought and whether it is bought loose or already in tea bags. Finally, price and accessibility can be a decision criterion for the choice of tea.
The repeated dental assessments aimed to monitor dental pathologies, which might possibly be associated with the local radiation treatment. However, the high number of incomplete assessments (66%) and the generally poor oral health conditions of the examined patients made it difficult to evaluate the effect of the type of tea on dental status in detail [25]. Incomplete assessments were mainly caused by limited compliance, especially with regard to visit other departments during radiotherapy [30]. This is in line with the general experience that patient compliance in head and neck cancer is often suboptimal. The study team tried to assure adherence to the instructions contained in the study protocol by regular monitoring and reminding if feasible; however, more stringent logistic pathways appear necessary for future studies based on our experience.
Regular mouthrinses neither with sage tea nor with Cystus® tea could prevent the occurrence of carious lesions (DMF S) or gingival inflammation (SBI) during the investigated time period of up to 3 months after radiation treatment, despite Cystus® tea being rich in polyphenols. It has been shown that polyphenols inhibits streptococcus mutans’ adherence and viability as well as to alter the initial salivary protein adsorption on the tooth [14, 31]. However, rinsing alone does not hamper biofilm formation at the tooth surface as is confirmed by the constantly high plaque scores (>65%) in both study groups. Considering the DMF (S) values at baseline it must be concluded that all patients enrolled in the study had a relatively high caries experience compared to others of their age cohort [32]. Limited adherence to regular dental examinations as well as insufficient oral hygiene measures are reasonable explanatory approaches for higher incidence of dental hard tissue defects in head and neck cancer patients. It has been suggested that untreated caries at the time of tumor diagnosis was predictive of poor compliance [30].
The data gathered from the dental examinations in this study emphasize the necessity to further educate head and neck tumor patients about the risks of dental caries- and periodontal inflammation development. Furthermore, short dental recall intervals and consequent instructions in basic oral hygiene procedures are important measures which must accompany and outlast the actual radiotherapy.
Conclusion
We report the outcomes of a randomized controlled phase III trial which to the best of our knowledge is the first to evaluate the efficacy of two different tea-based mouthwashes for the prevention or remedy of treatment-associated mucositis in head and neck cancer patients undergoing radio(chemo)therapy. No statistically significant differences were found between Cystus® and sage tea, with a low incidence of mucositis grade 3 overall and both teas were well tolerated. Sage tea is still recommended and Cystus® tea mouthwash can be applied in addition to intensive oral care and hygiene along with the application of fluorides.
References
Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O, Peterson DE, Raber-Durlacher JE, Sonis ST, Elad S, Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer, International Society of Oral Oncology (MASCC/ISOO) (2014) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120(10):1453–1461. https://doi.org/10.1002/cncr.28592
Epstein JB, Beaumont JL, Gwede CK, Murphy B, Garden AS, Meredith R, Le QT, Brizel D, Isitt J, Cella D (2007) Longitudinal evaluation of the oral mucositis weekly questionnaire-head and neck cancer, a patient-reported outcomes questionnaire. Cancer 109(9):1914–1922. https://doi.org/10.1002/cncr.22620
Withers HR, Maciejewski B, Taylor JM, Hliniak A (1988) Accelerated repopulation in head and neck cancer. Front Radiat Ther Oncol 22:105–110
Bentzen SM, Thames HD (1991) Clinical evidence for tumor clonogen regeneration: interpretations of the data. Radiother Oncol 22(3):161–166
Hong CH, Napenas JJ, Hodgson BD, Stokman MA, Mathers-Stauffer V, Elting LS, Spijkervet FK, Brennan MT, Multi-national Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO) (2010) A systematic review of dental disease in patients undergoing cancer therapy. Support Care Cancer 18(8):1007–1021. https://doi.org/10.1007/s00520-010-0873-2
Gupta N, Pal M, Rawat S, Grewal MS, Garg H, Chauhan D, Ahlawat P, Tandon S, Khurana R, Pahuja AK, Mayank M, Devnani B (2015) Radiation-induced dental caries, prevention and treatment—a systematic review. Natl J Maxillofac Surg 6(2):160–166. https://doi.org/10.4103/0975-5950.183870
Aguiar GP, Jham BC, Magalhaes CS, Sensi LG, Freire AR (2009) A review of the biological and clinical aspects of radiation caries. J Contemp Dent Pract 10(4):83–89
Kufta K, Forman M, Swisher-McClure S, Sollecito TP, Panchal N (2018) Pre-Radiation dental considerations and management for head and neck cancer patients. Oral Oncol 76:42–51. https://doi.org/10.1016/j.oraloncology.2017.11.023
Nagi R, Patil DJ, Rakesh N, Jain S, Sahu S (2018) Natural agents in the management of oral mucositis in cancer patients-systematic review. J Oral Biol Craniofac Res 8(3):245–254. https://doi.org/10.1016/j.jobcr.2017.12.003
Hannig C, Spitzmuller B, Al-Ahmad A, Hannig M (2008) Effects of cistus-tea on bacterial colonization and enzyme activities of the in situ pellicle. J Dent 36(7):540–545. https://doi.org/10.1016/j.jdent.2008.04.002
Kalus U, Grigorov A, Kadecki O, Jansen JP, Kiesewetter H, Radtke H (2009) Cistus incanus (CYSTUS052) for treating patients with infection of the upper respiratory tract. A prospective, randomised, placebo-controlled clinical study. Antiviral Res 84(3):267–271. https://doi.org/10.1016/j.antiviral.2009.10.001
Attaguile G, Russo A, Campisi A, Savoca F, Acquaviva R, Ragusa N, Vanella A (2000) Antioxidant activity and protective effect on DNA cleavage of extracts from cistus incanus L. and cistus monspeliensis L. Cell Biol Toxicol 16(2):83–90
Hannig C, Sorg J, Spitzmuller B, Hannig M, Al-Ahmad A (2009) Polyphenolic beverages reduce initial bacterial adherence to enamel in situ. J Dent 37(7):560–566. https://doi.org/10.1016/j.jdent.2009.03.017
Wittpahl G, Kolling-Speer I, Basche S, Herrmann E, Hannig M, Speer K, Hannig C (2015) The polyphenolic composition of cistus incanus herbal tea and its antibacterial and anti-adherent activity against streptococcus mutans. Planta Med 81(18):1727–1735. https://doi.org/10.1055/s-0035-1557822
Kalus U, Kiesewetter H, Radtke H (2010) Effect of CYSTUS052 and green tea on subjective symptoms in patients with infection of the upper respiratory tract. Phytother Res 24(1):96–100. https://doi.org/10.1002/ptr.2876
Ehrhardt C, Hrincius ER, Korte V, Mazur I, Droebner K, Poetter A, Dreschers S, Schmolke M, Planz O, Ludwig S (2007) A polyphenol rich plant extract, CYSTUS052, exerts anti influenza virus activity in cell culture without toxic side effects or the tendency to induce viral resistance. Antiviral Res 76(1):38–47. https://doi.org/10.1016/j.antiviral.2007.05.002
Steinmann D, Eilers V, Beynenson D, Buhck H, Fink M (2012) Effect of Traumeel S on pain and discomfort in radiation-induced oral mucositis: a preliminary observational study. Altern Ther Health Med 18(4):12–18
Budach V, Stuschke M, Budach W, Baumann M, Geismar D, Grabenbauer G, Lammert I, Jahnke K, Stueben G, Herrmann T, Bamberg M, Wust P, Hinkelbein W, Wernecke KD (2005) Hyperfractionated accelerated chemoradiation with concurrent fluorouracil-mitomycin is more effective than dose-escalated hyperfractionated accelerated radiation therapy alone in locally advanced head and neck cancer: final results of the radiotherapy cooperative clinical trials group of the German cancer society 95-06 prospective randomized trial. J Clin Oncol 23(6):1125–1135. https://doi.org/10.1200/JCO.2005.07.010
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys 31(5):1341–1346. https://doi.org/10.1016/0360-3016(95)00060-C
Pitts NB, Ekstrand KR, Foundation I (2013) International caries detection and assessment system (ICDAS) and its international caries classification and management system (ICCMS)—methods for staging of the caries process and enabling dentists to manage caries. Community Dent Oral Epidemiol 41(1):e41–52. https://doi.org/10.1111/cdoe.12025
Lieshout HF, Bots CP (2014) The effect of radiotherapy on dental hard tissue—a systematic review. Clin Oral Investig 18(1):17–24. https://doi.org/10.1007/s00784-013-1034-z
Lange DE, Plagmann HE, Eenboom A, Promesberger A (1977) Clinical methods for the objective evaluation of oral hygiene. Dtsch Zahnärztl Z 32(1):44–47
Doerr W, Groetz KA, Hartmann JT, Riesenbeck D (2007) Orale Mukositis. Onkologe 13(2):150–157
Beheshti-Rouy M, Azarsina M, Rezaie-Soufi L, Alikhani MY, Roshanaie G, Komaki S (2015) The antibacterial effect of sage extract (salvia officinalis) mouthwash against streptococcus mutans in dental plaque: a randomized clinical trial. Iran J Microbiol 7(3):173–177
Moricz AM, Szeremeta D, Knas M, Dlugosz E, Ott PG, Kowalska T, Sajewicz M (2018) Antibacterial potential of the cistus incanus L. phenolics as studied with use of thin-layer chromatography combined with direct bioautography and in situ hydrolysis. J Chromatogr A 1534:170–178. https://doi.org/10.1016/j.chroma.2017.12.056
Qnais EY, Abu-Dieyeh M, Abdulla FA, Abdalla SS (2010) The antinociceptive and anti-inflammatory effects of salvia officinalis leaf aqueous and butanol extracts. Pharm Biol 48(10):1149–1156. https://doi.org/10.3109/13880200903530763
Gericke S, Lubken T, Wolf D, Kaiser M, Hannig C, Speer K (2018) Identification of new compounds from sage flowers (salvia officinalis L.) as markers for quality control and the influence of the manufacturing technology on the chemical composition and antibacterial activity of sage flower extracts. J Agric Food Chem 66(8):1843–1853. https://doi.org/10.1021/acs.jafc.8b00581
Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, Komaroff E, Nalysnyk L, Zilberberg MD (2003) Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 66(3):253–262
Mallick S, Benson R, Rath GK (2016) Radiation induced oral mucositis: a review of current literature on prevention and management. Eur Arch Otorhinolaryngol 273(9):2285–2293. https://doi.org/10.1007/s00405-015-3694-6
Frydrych AM, Slack-Smith LM, Parsons R (2017) Compliance of post-radiation therapy head and neck cancer patients with caries preventive protocols. Aust Dent J 62(2):192–199. https://doi.org/10.1111/adj.12491
Smullen J, Koutsou GA, Foster HA, Zumbe A, Storey DM (2007) The antibacterial activity of plant extracts containing polyphenols against streptococcus mutans. Caries Res 41(5):342–349. https://doi.org/10.1159/000104791
Carvalho JC, Schiffner U (2018) Dental caries in European adults and senior citizens 1996–2016: ORCA saturday afternoon symposium in Greifswald, Germany—part II. Caries Res 53(3):242–252. https://doi.org/10.1159/000492676
Acknowledgements
We would like to gratefully acknowledge financial support from Naturprodukte Dr. Pandalis GmbH & Co. KG. We thank our participating patients for their willingness to support this study. The personnel of the clinical trials office at OncoRay Dresden, nurses and study nurses in the patient care of the Department of Radiotherapy are gratefully acknowledged. We would like to dedicate this paper to our colleague Prof. Wolfgang Dörr who passed away in October 2019.
Funding
The funders Naturprodukte Dr. Pandalis GmbH & Co. KG of this study had no role in the design, data collection, data analyses, data evaluation, and preparation of the manuscript or decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N. Ebert, A. Kensche, S. Löck, W.W. Hadiwikarta, A. Hänsch, W. Dörr, M. Krause, C. Hannig and M. Baumann received financial support from Naturprodukte Dr. Pandalis GmbH & Co. KG for this study. The funding had no influence on collection, analyses, and data evaluation. N. Ebert was co-principal investigator for funded research projects to the University of Dresden by Merck KGaA (2014–open). N. Ebert confirms that the above funding source was not involved in the design of this study, the preparation of this paper, the materials used, or the collection, analysis, and interpretation of data. M. Krause declares that within the past 5 years she received funding for her research projects and for educational grants to the University of Dresden IBA (2016), Merck KGaA (2016–2030), Medipan GmbH (2014–2018). As chair of OncoRay (Dresden) she signed/s contracts for her institute(s) and for the staff for research funding and collaborations with different companies. In the past 5 years, M. Baumann attended an advisory board meeting of MERCK KGaA (Darmstadt), for which the University of Dresden received a travel grant. He further received funding for his research projects and for educational grants to the University of Dresden by Teutopharma GmbH (2011–2015), IBA (2016), Bayer AG (2016–2018), Merck KGaA (2014–open), Medipan GmbH (2014–2018). He is on the supervisory board of HI-STEM gGmbH (Heidelberg) for the German Cancer Research Center (DKFZ, Heidelberg) and also member of the supervisory body of the Charité University Hospital, Berlin. As former chair of OncoRay (Dresden) and present CEO and Scientific Chair of the German Cancer Research Center (DKFZ, Heidelberg), he has been or is still responsible for collaborations with a multitude of companies and institutions, worldwide. In this capacity, he discussed potential projects with and has signed/signs contracts for his institute(s) and for the staff for research funding and/or collaborations with industry and academia, worldwide, including but not limited to pharmaceutical corporations like Bayer, Boehringer Ingelheim, Bosch, Roche and other corporations like Siemens, IBA, Varian, Elekta, Bruker and others. In this role, he was/is further responsible for commercial technology transfer activities of his institute(s), including the DKFZ-PSMA617 related patent portfolio (WO2015055318 (A1), ANTIGEN (PSMA)) and similar intellectual propterty portfolios. Dr. Baumann confirms that to the best of his knowledge none of the above funding sources was involved in the preparation of this paper.
Ethical standards
The study was approved by the institutional ethics committee of the Technische Universität Dresden (EK 281082011). Only patients who met the inclusion criteria and signed a consent form were enrolled in the study and randomized to receive either Cystus® or sage tea.
Additional information
C. Hannig und M. Baumann share last co-authorship.
Availability of data and material
Data were stored at the Department of Radiation Oncology, except for the dental parameters that were stored at the Clinic of Operative Dentistry at the University Medical Center Carl Gustav Carus in Dresden. The data are not deposited in a repository.
Code availability
Statistical analysis and graph creations were performed using R version 3.5.0 (2018-04-23).
Rights and permissions
About this article
Cite this article
Ebert, N., Kensche, A., Löck, S. et al. Results of a randomized controlled phase III trial: efficacy of polyphenol-containing cystus® tea mouthwash solution for the reduction of mucositis in head and neck cancer patients undergoing external beam radiotherapy. Strahlenther Onkol 197, 63–73 (2021). https://doi.org/10.1007/s00066-020-01684-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-020-01684-y