Abstract
To test whether there are variations in cochlear orientation with respect to age and sex, and its relevance in cochlear implant surgery. Implant otologists rely upon the anatomic landmarks including the facial recess and round window niche and round window membrane for accessibility and placement of electrode array into scala tympani of basal turn of cochlea. Anecdotally, surgeons note variations in cochlear orientation with respect to age. Cochlear orientation studied radiologically by pre-operative CT scan of temporal bone can guide a Surgeon’s approach to cochlear implantation. To investigate the changes in cochlear orientation with respect to age and sex; and its relevance in cochlear implantation. A retrospective analytical study was performed on CT scans of temporal bones in patients (of our hospital from July 2013 to January 2015 i.e. for a period of 18 months) with no congenital or radiological abnormalities of cochlea. The basal turn angulations of cochlea varied with age and majority of change occurred during early age. The basal turn angulations of cochlea in difficult situations during cochlear implantation were correlated with the data. There is a significant variation in cochlear orientation as measured radiologically by basal turn angulations relative to midsagittal plane. The more obtuse and acute basal turn angulations have implications like difficulty in cochleostomy and electrode placement during cochlear implantation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The bony labyrinth inside the petrous part of temporal bone houses the inner ear, including the sense organ of audition in the cochlea, and that of balance in the vestibular apparatus. The modern labyrinth in humans is morphologically different from that of all the primates [1]. The cochlea is shaped individually and different in dimensions (“fingerprint”) [2]. It is considered that the appearance and orientation of the human labyrinth in temporal bone does not change after birth, and that has been the teaching in Otorhinolaryngology. However, much of the data on this subject is contradictory and often flawed [3].
The current opinion in the literature about the developmental changes after birth in the inner ear is not conclusive. Some significant, although minor, morphologic change of inner ear that may occur even after complete ossification of the otic capsule [3]. Although intrauterine growth of the temporal bone is fairly well known, growth of temporal bone after birth is more highly debated [4]. Historically, it was thought that there is no significant change after birth in the morphology and orientation in of the inner ear. According to Bast, the membranous portion of labyrinth grows to its adult size and configuration by 16–20 weeks’ of intrauterine life [5]. Similarly, other studies [6, 7] published around the turn of the previous century have stated that fetal and neonatal labyrinths are morphologically same as compared to those of adults. In contrast to the previous studies that have been published, there are flawed studies stating that there are changes in the labyrinth even into adult life [8, 9].
It is agreed by many that remodelling of the otic capsule is arrested once the labyrinth is fully formed and that is essential for normalcy of auditory mechanism and its maintenance [10]. In contrast, the vault and base of the cranium undergo significant change during extra uterine life of an individual. It now appears that the cranial vault and base undergoes a significant growth curve at ages 1–4 years and also around pubertal age (around 11–12 years) after which is the spheno-occipital synchondrial fusion, which may have an effect on temporal bone morphology [11]. The base of cranium in isolation might grow much linearly, when compared to the rest of the cranium [12]. It has been stated that the growth patterns may have occurred in the squamous, tympanic and mastoid parts of temporal bone and not in the bony labyrinth [13].
Cochlear implant surgery is the currently well accepted modality of treatment in patients with severe to profound sensorineural hearing loss or deafness, who get limited or no benefit from conventional amplification devices. The operation has become a routine surgery in the present era. Expansion of the audiologic criteria for stimulating the cochlea and the preservation of residual hearing has brought in new challenges [14].
The cochlear implant is surgically placed beneath the skin behind the auricle and a very thin electrode array is inserted into the cochlear portion of the labyrinth, where it directly stimulates the existing auditory nerve fibres. The surgical procedure is highly skilled and done under microscope, and requires removal of bone using burrs close to very important structures. Computed tomography (CT) of the temporal bones with thin slice thickness and slice interval i.e. high resolution imaging is a protocol in preoperative diagnostic evaluation prior to cochlear implant surgery. By the above one can visualize and assess the bony structures of the middle and inner ear—to understand the anatomy [15], to diagnose abnormalities or anomalies. Computed tomography (CT) of the temporal bones prior to surgery can detail on anatomic considerations required for surgical management and is very much useful in the preoperative workup of probable candidates for cochlear implantation. Interpretation on CT scan with intra-operative surgical details is partially related to the cause of hearing deficit and the expertise of the cochlear implant surgeon and radiologist [16]. In contrary, few publications suggest that the CT scan of temporal bones is not capable of predicting with authority the small degrees of cochlear ossification, and the auditory pathway [17].
The renewed interest in paediatric cochlear implant surgical procedure made it essential to know how postnatal development of ear will change the surgical outcomes of such devices. In his study, Eby used radiographic and temporal data and found the 3-dimensional postnatal growth pattern of inner ear, middle ear cavity and mastoid process and detailed their effects for cochlear implantation in children [13]. There exists some proved assertion to say that the cochlear basal turn may change its geometric position with respect to the posterior mesotympanic recess as the person grows and this can affect the surgeon’s approach in cochlear implantation [3]. It is not contradictory with other authors [2] who also state that there exists significant difference in the reference range of geometric position of cochlea which will have an effect on the location of electrode arrays and the chance of surgery with preserved hearing. They explained the implant otologist’s hard times or trouble sometimes in insertion of electrode arrays even into the so called “normal” cochleae.
A proper understanding of the possible anatomical changes of the cochlea in humans is required for the knowing the degree of surgical injury caused by inserting different kinds of electrode arrays in cochlear implantation [2]. A three dimensional cochlear organization system easily applies in clinical patients, fulfilling the requirements that are set by an expert group on decision making [18]. Three dimensional volume rendering of the cochlea makes possible to determine the salient features of cochlear morphology and orientation that may fail to be noticed with routine CT imaging. It can direct a surgeon’s way of dealing towards cochleostomy and ultimately introducing the electrode array into scala tympani of basal turn of cochlea to minimize the trauma and thereby preserve remnant neural population and hair cells [19]. Basal turn of cochlea is selected as it is the entitled target site for cochlear implant electrode array placement. The basal turn presents as an indicator en-route to round window and cochlear organization [4]. Escude et al. had published data on the size of the cochlear basal turn using high resolution CT imaging of temporal bones in paediatric and adult population. They found that in normal cochleae, the basal turn tends to vary in size and would affect the depth angles of insertion for electrode array in cochlear implant surgery [20]. In his study, Spoor [1] stated that, on viewing at axial CT scan of temporal bones, there was statistically significant change in the orientation of the prenatal and adult cochlea in relation to the vestibule. In another study using MRI, Spoor firmly established that the labyrinth reaches its adult size between 17 and 19 weeks’ of prenatal life and there may be a variance in the orientation of the cochlea postnatally.
Statistical information related to variations in cochlear orientation with respect to age are very few, and many state that no such changes occur. A few publications do display noteworthy changes in temporal bone anatomy in relevance to cochlear implantation. These orientations are of significance to an otologic surgeon who primarily rely on anatomic landmarks and their relations to estimate surgical anatomy concealed by bone [4]. Postnatal changes in orientation of cochlea have been described by Lloyd et al., of cochlear basal turn angulations being decreased with regard to the sagittal plane with increasing age, and calls into question the earlier analysis regarding cochlear stagnancy [1].
As the rate of pediatric cochlear implantation, is being increased in an impressive manner since early 1990s, although many otologists feel that CI in the pediatric population need much skill to accomplish when compared to the adults, there is hardly any available data to support why this may be the case [4]. Our study helps to test whether there are any significant differences in cochlear orientation with respect to age and sex. Therefore, may have implications to identify the difficulties before hand and guide the surgeon’s approach in cochlear implantation.
Materials and Methods
Criteria
The patients visiting the department of Otorhinolaryngology who were ordered the High Resolution Computed Tomography (HRCT) of temporal bones for various reasons formed the eligible candidates. The candidates with no congenital abnormalities of ear and radiologically detectable cochleovestibular anomalies were included in the study; those with post traumatic event and post operative cases were excluded.
Intervention
A retroprospective analytical study on axial (parallel to lateral semi circular canal) CT scans of temporal bones of patients satisfying inclusion criteria were analysed. All the scans were done with ST 0.67 mm by GE CT scanner at department of Radiology, Christian Medical College, Vellore, India.

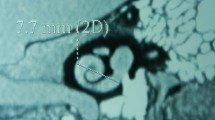
The axial high resolution CT scans showing the basal turn of cochlea were identified and the angulations of former with midsagittal plane were calculated (separately for each side) (Figs. 1 and 2) by the Implant Otology Fellow in association with Radiologist having an experience of 6 years in Head & Neck imaging. The angulations were calculated using software provided by GE Centricity Enterprise Web Version 3.3.
The CT scan of temporal bones of the patients are grouped according to age and sex; and appropriate statistical analysis were used to identify any statistical significance in angulations of basal turn of cochlea. The angulations in difficult cases of cochlear implantation are discussed in relation to the observations made.
Results
Of the patients who visited the Department of Otorhinolaryngology at Christian Medical College, 269 patients were referred to Department of Radiology to undergo HR CT scan of temporal bones for various reasons. After the exclusion criteria were applied, 185 patients (i.e. 370 ears) were included in the study group. Males were 118 and Females were 67 in number (Fig. 3). The mean age of the study group was 32.1 years, range was from 0 to 80 years (Fig. 4).
The mean basal turn angulation at the mean age of 32.1 year was 56.35 degree SE 0.625 (Figs. 5, 6 and 7). The basal turn angulation on the Right side was 56.01 degree SE 0.794 (Fig. 8) and on the Left side was 56.69 degree SE 0.972 (Fig. 9). The basal turn angulations according to age stratification are pictorially depicted below (Fig. 6).
The age groups were made and cochlear orientation (by basal turn angulation with respect to midsagittal line) were calculated and statistically analysed using ANOVA, Windostat Version 9.2 (Table 1). The cochlear orientation across age groups was statistically analysed by Kruskal–Wallis test and across gender by Mann–Whitney—u test (Tables 2 and 3).
The change of basal turn angulation with respect to age was not found to be statistically significant (p = 0.06375) when calculated among all the age groups (Table 2), but was highly significant (p = 0.00122) when the age groups of less than 2 years were compared with the rest (Table 3). The findings on our study reinforce that the most of the cochlear orientation changes occur in the early postnatal life i.e. up to the age of 2 years. The former is highly important in evaluation of a probable pediatric cochlear implant candidate.
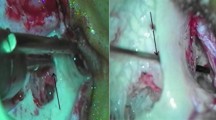
Of the included 185 patients, 23 cochlear implantations were done in 20 patients (3 bilateral, simulataneous) by standard mastoidectomy posteroior tympanotomy (SMPT) approach. In most of the cases, the round window cochleostomy (Figs. 10 and 11) was selected for electrode (Advanced Bionics—precurved electrodes—1 J and Mid-scala) insertion. The basal turn angulations of the 20 patients (Table 4) were compared with the difficult situations (in 4 surgeries) for electrode insertion via Round Window Membrane (RWM) after drilling the niche because of visibility and angulation issues. The difficult situation is defined as inability to visualise the total of RWM after optimal posterior tympanotomy without injuring facial nerve or chorda tympani. We resorted to conventional bony cochleostomy (Fig. 12) anteroinferior to RWM for electrode insertion in difficult cases. The extremes of basal turn angulation was found have difficult access via posterior tympanotomy to RWM for electrode insertions.
Discussion
According to Zehnder et al., remodeling of otic capsule is arrested once labyrinth is fully formed for the normalcy of auditory mechanism [10]. Simon Llyod [3] et al. conducted a similar study on developmental changes in cochlear implantation. The vault and basi cranium grows in a linear fashion (significant at 1–4 and 11–12 years when sphenooccipital synchrondosis occurs) bearing an effect on temporal bone morphology.
We believe that the access to scala tympani of cochlear basal turn for atraumatic cochlear implantation is not just all about orientation. The size of the round window ‘overhang’, the position of the facial nerve and size of the middle ear cavity all have role to play.
Our study is similar to that conducted by Llyod et al., on Indian sub-continental population, which shows significant change of cochlear orientation up to the age of 2 years. Cochlear basal turn may change its geometric position with respect to posterior mesotympanum as person grows and affect CI surgeon’s approach. This is clinically significant when the pediatric cochlear implantation is on a high in our Indian population with many young surgeons adding to the pool of implant otology. We recommend basal turn angulation in preoperative radiological evaluation of probable implant candidates to guide the implant surgeon to plan and proceed accordingly.
Conclusion
In early postnatal life up to 2 years of age, statistically significant changes occur in cochlear orientation in temporal bone measured by basal turn angulations. There are no statistically significant changes in cochlear orientation after 2 years of age and also with respect to sex and side. The extremes of basal turn angulations (approx. <52° and >61°) are found to be difficult for RW electrode insertion in cases of cochlear implantation by standard posterior tympanotomy approach. Preoperative evaluation of imaging and basal turn angulation may guide surgeon’s approach towards atraumatic intracochlear electrode insertion via RW or cochleostomy.
Limitations
Our study is not without limitations as sample size is not large enough to generalize conclusion, interobserver variance is not taken into account, not applicable in cochleovestibular anomalies and observations made is applicable to only rotation in anteroposterior plane as only axial CT sections are studied. Hence we recommend for a similar prospective study on large population taking anomalous cochlea and 3D (3 dimensional) CT scans.
References
Jeffery N, Spoor F (2004) Prenatal growth and development of the modern human labyrinth. J Anat 204(2):71–92
Erixon E, Hogstorp H, Wadin K, Rask-Andersen H (2009) Variational anatomy of the human cochlea: implications for cochlear implantation. Otol Neurotol 30(1):14–22
Lloyd SKW, Kasbekar AV, Kenway B et al (2010) Developmental changes in cochlear orientation—implications for cochlear implantation. Otol Neurotol 31:902–907
Theodore R, Rackan Mc, Fitsum A et al (2012) Comparision of cochlear implant relevant anatomy in children versus adults. Otol Neurotol 33:328–334
Bast TH (1942) Development of the otic capsule. VI. Histological changes and variations in the growing bony capsule of the vestibule and cochlea. Ann Otol Rhinol Laryngol 51:343–357
Siebenmann F (1890) Die Korosions-Anatomie Des Knochernan Labyrinthes Des Menschlichen Ohres. Bergmann, J.F, Wiesbaden
Schonemann A (1906) Schlafenbein und Schadelbasis, eine anatomischotiatrische Studie. N Denkschr algem Schweizer Gesellsch gesamt Naturwiss 40:95–160
Hyrtl J (1845) Vergleichend-Anatomische Untersuchungen Uber das Innere Gehororgan Des Menschen und der Saugethiere. Prague, Ehrlich, F.
Sercer A, Krmpotic J (1958) Further contributions to the development of the labyrinthine capsule. J Laryngol Otol 72:688–698
Zehnder AF, Kristiansen AG, Adams JC et al (2006) Osteoprotegrin knockout mice demonstrate abnormal remodeling of the otic capsule and progressive hearing loss. Laryngoscope 116:201–206
Farkas LG, Posnick JC, Hreczko TM (1992) Anthropometric growth study of the head. Cleft Palate Craniofac J 29:303–308
Richtsmeier JT, Cheverud JM (1986) Finite element scaling analysis of human craniofacial growth. J Craniofac Genet Dev Biol 6:289–323
Eby TL, Nadol JB Jr (1986) Postnatal growth of the human temporal bone. Implications for cochlear implants in children. Ann Otol Rhinol Laryngol 95:356–364
Paprocki A, Biskup B, Kozlowska K et al (2004) The topographical anatomy of the round window and related structures for the purpose of cochlear implant surgery. Folia Morphol (Warsz) 63(3):309–312
Stjernholm C (2003) Aspects of temporal bone anatomy and pathology in conjunction with cochlear implant surgery. Acta Radiol 44(Suppl 430):2–15
Seicshnaydre MA, Johnson MH, Hasenstab MS, Williams GH (1992) Cochlear implants in children: reliability of computed tomography. Otolaryngol Head Neck Surg 107(3):410–417
Nikolopoulos TP, O’Donoghue GM, Robinson KL et al (1997) Preoperative radiologic evaluation in cochlear implantation. Am J Otol 18(6 Suppl):S73–S74
Verbist BM, Joemai RM, Briaire JJ et al (2010) Cochlear coordinates in regard to cochlear implantation: a clinically individually applicable 3 dimensional CT-based method. Otol Neurotol 31(5):738–744
Martinez-Monedero R, Niparko JK et al (2011) Cochlear coiling pattern and orientation differences in cochlear implant candidates. Otol Neurotol 32(7):1086–1093
Escude B, James C, Deguine O et al (2006) The size of the cochlea and predictions of insertion depth angles for cochlear implant electrodes. Audiol Neurotol 11(1 Suppl):27–33
Acknowledgements
The authors thank the subjects and the staff in the department of Radiology and ENT at Christian Medical College, for all their assistance and support. This article was derived during Post doctoral fellowship in Implant Otology at Christian Medical College, Vellore, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The above study was approved by Institutional Review Board (IRB) of Christian Medical College and the consent was waived because the High Resolution CT scans of the temporal bones were done for various reasons (not just for study purpose and did not financially impact the study population), from which the necessary data was extracted from radiology department.
Rights and permissions
About this article
Cite this article
Kiran, A.S., Varghese, A.M., Irodi, A. et al. Radiological Evaluation of Cochlear Orientation and Its Implications in Cochlear Implantation. Indian J Otolaryngol Head Neck Surg 70, 1–9 (2018). https://doi.org/10.1007/s12070-017-1173-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-017-1173-7