Abstract
Research efforts have been centred on creating potential methods for producing green energy. A viable choice in this regard is fuel cells. Air breathing direct methanol fuel cell (AB-DMFC) is one of the many types of fuel cells, that is becoming increasing popular as a source of electricity for recharging portable electronic devices. The current study examined AB-DMFC performance equipped with various open ratio (OR)’s of cathode current collector (CC) such as 38.5%, 45.4%, and 55.4%. The cathode CC in an AB-DMFC is extremely vital to the operation of the fuel cell since it collects electrons, promotes oxygen flow for the cathode reaction and removes water bubbles produced at the cathode side, among other critical functions. For each open ratio of CC, the anodic fuel is supplied by external components like pumps with a concentration range of 1 M to 4 M methanol. When compared to other two open ratios, the experimental findings demonstrated that CC with a 45.4% OR at 2M of methanol concentration generated maximum power density (MPD) of 7.75 mW/cm2. The next stage of experiments continued with the addition of alkali solution (NaOH) to methanol fuel, and it was found that adding alkali solution to methanol fuel enhanced the MPD of 8.5 mW/cm2 and decreased methanol cross over compared with conventional AB-DMFC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Portable electronic gadgets such as computers, laptops, music players, personal digital assistants and cell phones are becoming increasingly popular. Higher power density and quick charging batteries are required for the above gadgets [1, 2]. Fuel cells are an alternative source of electrical energy that might be utilised to power these gadgets. Direct methanol fuel cell (DMFC) is a potential technology and an alternate power source because of its low operating temperature, maximum power density, low emission, high efficiency, noise-free operation, stress-free handling, and less frequent charging.
Based on how the reactants are provided, DMFC may be divided into two types: (1) passive DMFC and (2) active DMFC. In an active DMFC, a pump delivers dilute methanol (water and methanol solution) to the anode, while a compressor or blower delivers the oxidant (oxygen or air) to cathode [3]. There are no auxiliary constituents such as pumps, fans, or blowers for providing reactants in the passive DMFC. Diffusion (concentration gradients), gravity and capillary pressure are used to passively provide reactants, namely, diluted methanol and air/oxygen to catalyst layers.
A great deal of study has gone into determining the process in the fuel cell, as well as the elements that impact DMFC's performance. The term "over potential" refers to a measurement of the cell's losses. Both the anode and the cathode suffer from over potential losses. The following are some of the contributing elements that have a negative effect on AB-DMFC performance;
-
Sluggish Methanol oxidation kinetics.
-
Water crossover and methanol crossover (MCO) through the membrane.
-
Issues with appropriate water management and temperature control inside the cell.
The first element increases the anodic over potential. Moreover, it reduces the cell's voltaic efficiency. Understanding the kinetics of methanol oxidation has been the subject of extensive research, and as a result, effective binary and ternary catalysts for membranes been developed. These catalysts would ensure adequate solution mixing and speed up the reaction. MCO, which occurs at increasing methanol concentrations, is the most difficult challenge in DMFC. The cathode over potential is caused by MCO from anode to cathode, and its interaction with oxygen at cathode inhibits cell performance [4, 5].
Braz et al [6] experimentally studied the influence of perforated current collector (CC)s with OR (open ratio)s of 34%, 42%, and 64.0% on the performance of passive-DMFC. A concentration of methanol 2M is produced by using CC with lower OR (34%) on both the cathode and anode sides. They observed that lower OR of 34% was reported to outperform the other two ORs in cell performance.
Tang et al [7] showed that the performance of DMFC was influenced by OR of the current collector in two different ways. On the one hand, a greater OR expands the area available for mass transfer passage and makes it easier to get rid of gas bubbles that are formed and any leftover water. On the other hand, a greater open ratio typically causes severe methanol crossover and higher methanol rate of permeation, which reduces the cell's performance and the efficiency of methanol consumption.
Calabriso et al [8] analysed the impact of perforated CC with OR of 17% and 35% on the performance of a passive DMFC through experimentation. They found that fuel cell performance was enhanced by a 35% open ratio CC. Similarly, Borello et al [9] investigated the effect of CCs with OR of 36.0% and 38.0% on the performance of passive-DMFC. It was found that higher CC with OR of 38.0% had higher cell performance in the range of methanol concentrations from 1M to 3M.
Kim et al [10] performed research on a passive DMFC by placing a thin metal barrier between two halves of MEA’s. The metal barrier linked DMFC cell architecture was shown to successfully limit methanol crossover. In addition, compared to baseline configuration with no metal barrier, cell performance enhanced by 37.5%.
Yuan et al [11] examined the performance of micro AB-DMFC in relation to operating factors of methanol flow rate, concentration and cell temperature. The findings revealed that the best fuel cell performance was accomplished with a mixture of 1M methanol concentration and 1ml/min flow rate of methanol at 60°C operating temperature.
Mei et al [12] examined the passive-DMFC performance using a (PBI membrane) phosphoric acid doped polybenzimidazole membrane. In comparison to Nafion membrane, it was discovered that PBI membrane decreased methanol crossing.
Yang et al [13] found that in an active DMFC system, the serpentine flow field design is preferred to the parallel on the bipolar plate anode side. There are fewer CO2 bubbles in the single serpentine flow field design than parallel design. The CO2 generated in the anode is carried away by reactant flow since the serpentine profile has just one route.
Kordesch et al [14] discovered that the performance of an active DMFC can be improved by modifying the membrane and introducing a circulating electrolyte. Their research demonstrated that the addition of an electrolyte layer between the Membrane Electrode Assemblies (MEA) helps to decrease MCO. In a separate study, Jung and et al [15] investigated the impact of the microporous layer (MPL) on fuel cell performance. Their findings showed that the use of anode MPL reduced MCO and enhanced cell performance. However, when a cathode MPL was used, it retained water at the cathode side when resulted in reduced cell performance.
Liu et al [16] used a microporous layer-coated cathode to perform research on a passive DMFC (CML). Ammonia and carbon nanotubes were used to make the CML. It was discovered that using CML increased the cell's performance by up to 30.3%. Water removal capability near the cathode also increased, as was the gas transferring characteristic of the gas diffusion layer.
Sabet-Sharghi et al [17] carried out experiments on a DMFC by altering the flowing electrolyte's (FE) concentration, flow rate and channel thickness. Cells with thicker membranes and 0.6 mm FE channels achieved greater peak power density in a 2 M methanol concentration solution.
AA Najmi et al [18] modified Nafion117 membrane with NaOH (Na+ form) solution and used it as a cation exchange membrane (CEM) in a passive DMAFC (Direct Methanol Alkaline Fuel Cell) and compared it to a pristine Nafion117 (H+ form) membrane in a passive DMFC. The concentrations of methanol and NaOH in the anode fuel reservoir had an impact on DMAFC power output, and the ideal value for achieving the highest power output was identified. When the concentrations of methanol and NaOH were 1M and 4M, respectively, the passive DMAFC's maximum power density (MPD) was 3.0 mW/cm2.
In addition, researchers have suggested many ways to improve the cell performance, such as using passive DMAFC through anion exchange membrane (AEM) [6, 19], and [20]. In comparison to DMFCs operating in acidic medium, DMAFC systems may have benefits in basic condition. First, compared to traditional DMFCs, electro-catalytic MOR (Methanol Oxidation Reaction) and ORR (oxygen reduction reaction) are more facile in alkaline medium than in acidic medium [21, 22]. As opposed to conventional DMFCs, it allows the use of non-precious metal catalysts with reduced catalyst loading. Second, the DMAFC works with migrating ions that are no longer proton, which can reduce the amount of methanol that crosses the membrane due to electro-osmosis. The fuel crossover through the membrane from the anode to cathode in the anion exchange membrane in DMAFC is the opposite of the direction of OH- ions from cathode to anode. This results in suppressing the MCO [16, 23].
Based on the literature review, it is clear that most of the research is concerned with the influence of various CC designs on passive DMFCs performance. Some research has been carried out on the effects of methanol flow rate and concentrations on the performance of AB-DMFC. A few researchers have worked on lowering MCO and enhancing the cell performance. Water and methanol crossover are the main issues with AB-DMFC, although they may be reduced by choosing a suitable OR for CCs and concurrently ensuring greater contact between CC and MEA. This problem may be solved by appropriately changing the CC design and adding alkali solution to methanol fuel.
The current section analysed the effect of cathode CC with different ORs on the performance of AB-DMFC was also investigated by adjusting the ORs (38.5%, 45.4%, and 55.4%) and comparing results available in the literature with the current work, as shown in table (1). In addition, experiments were carried out to determine how the methanol flow rate and concentration influenced cell performance. The feasibility study of AB-DMFC using CEM was reported in the current paper. Finally, by adjusting the concentrations of methanol and alkali solutions, the AB-DMFC performance was investigated.
2 Experimental studies
The experiments were carried out the following sub sections.
2.1 Experimental setup and test conditions
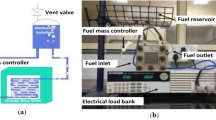
As illustrated in figure 1, a single cell AB-DMFC with an active area of 5 cmx5 cm was developed. The fuel cell is made up of two acrylic end plates, one for anode and the other for cathode. The MEA is a component of a fuel cell that facilitates electrochemical reaction. It comprises two diffusion layers and two catalyst layers.
The membrane used in this particular MEA was Nafion 117, which is a proton exchange membrane. The anode, which is the electrode where oxidation occurs, has a loading of 4 mg cm-2 of Pt-Ru/c catalyst. The cathode, which is the electrode where reduction occurs, has a loading of 4 mg cm-2 of Pt/c catalyst. Perforated CC’s were made of 316L stainless steel having open ratios of 38.5%, 45.40% and 55.40% were used.
In ABDMFC, using a peristaltic pump, the methanol solution was fed to the cell anode side, whereas oxygen from ambient air was naturally carried to reaction sites.
The current-voltage information was recorded using a DC (direct current) electronic load bank. One minute was taken into consideration for every voltage recording in order to get consistent voltage. Figures 1 and 2 show the construction of the experimental setup. For every methanol flow rate and concentration, experiments were carried out at least three times. For the purpose of drawing polarisation curves, average values were taken into account.
Prior to conducting the test, fresh MEA was activated for 12 hours in a solution containing 1 M methanol prior to the experiment, and it was then delivered to the anode flow channel using a peristaltic pump at a methanol flow rate of 1ml/min. The experiment was carried out at room temperature of 25 to 30°C, and at 60 to 65% relative humidity.
2.1a Cell fixture: The AB-DMFC was made up of a Nafion117 membrane with a 5.0 × 5.0 cm active area that was positioned in the midst of two anodes and cathode CCs, which were held in place by two end plates. Polymethylmethacrylate (PMMA) material with an 18mm thickness was used to make a pair of cathode and anode fixture plates.
The anode CC was made of solid 2-mm thick copper plate. As the anode flow field, a graphite plate with a 1-S flow field that had 1mm channel width, 1mm channel depth, and 1mm rib was employed. 5 N-m of manual torque was used to tighten the bolts in the whole assembly of AB-DMFC.
2.2 Experimental procedure
The experiments were conducted in two stages. In the first stage, a perforated current collector (PCC) with three different ORs of 38.5%, 45.4% and 55.40% was considered in the current study, as shown in figure 3. As the anodic fuel, a diluted methanol solution with a range of concentrations from 1 to 4 M was used in the cell for three current collectors. A single serpentine (1-S) flow field channel was carved upon a graphite plate to produce the anode flow field plate. Experiments were carried out to see how the cathode CC design affects the performance of AB-DMFC, as well as altering the flow rate and methanol concentration. The experiments in the second stage examined the effect of the addition of NaOH (concentration of NaOH from 1 to 6 M) to 2 M methanol fuel. The addition of NaOH solution to methanol improved electrochemical kinetic reactions while simultaneously increasing ionic conductivity across the membrane.
2.3 Uncertainty analysis
Uncertainty occurs due to repeated experiments, device and laboratory conditions, human error, instrument calibration, environmental factors etc., [22]. The calculated uncertainty for every component of equipment and the percentage of uncertainty is shown in table 1.
3 Results and discussion
3.1 Influence of experimental parameters on AB-DMFC performance
Figure 4 (a, b, c and d) illustrates the effect of different anode flow rates (1, 2, 3, and 4 ml/min) on the performance of an AB-DMFC operating at room temperature, with different methanol (CH3OH) concentrations (1M, 2M, 3M and 4M). Increasing the methanol concentration from 1 M to 4 M enhances methanol mass transfer to the anode catalyst layer and improves the kinetics of the methanol oxidation process. This characteristic is advantageous as it allows for maximum power density (MPD) of AB-DMFC to be improved at a 2M concentration and extends its operating duration. This improvement occurs because the reaction kinetics of the cell are enhanced, resulting in better output.
However, at higher methanol concentrations (3 M), concentration losses significantly affect the cell performance. It appears that the methanol crossover in a fuel cell is causing a number of issues that affect the cell's performance. Methanol crossover occurs when methanol molecules from the anode side of the fuel cell diffuse through the membrane and reach the cathode side. This can lead to a number of problems, including:
-
1.
Mixed over potential losses: Methanol crossover increases the concentration of methanol at cathode, which can lead to mixed over potential losses. This occurs when the concentration of reactants (oxygen and methanol) at the cathode is not optimal, leading to a decrease in the efficiency of cathode reaction.
-
2.
Cathode catalyst poisoning: Methanol crossover can also cause the cathode catalyst to become poisoned. This occurs when methanol molecules adsorb onto the catalyst surface and block active sites, preventing oxygen from reacting with the catalyst.
-
3.
Cathode water flooding: Methanol crossover can also lead to the production of water at the cathode. This water can accumulate and cause flooding, which blocks the pores of the cathode diffusion layer and reduces the efficiency of the cell.
-
4.
Fuel wastage: Methanol crossover also leads to the loss of fuel from the anode side of the cell, which reduces the overall efficiency of the system.
-
5.
Obstruction of oxygen flow: Methanol crossover can also obstruct the flow of oxygen through the cathode diffusion layer, further reducing the cell's efficiency.
-
6.
Deterioration of membrane quality: Methanol crossover can cause the membrane to degrade over time, reducing its ability to prevent methanol from diffusing through.
To address these issues, researchers are exploring a range of strategies, such as developing new materials with higher methanol-blocking capabilities, optimizing the design of the fuel cell, and using innovative membrane materials that are more resistant to methanol crossover.
When the methanol flow rate was increased from 1 to 2 ml/min, the cell's performance improved, peaked at 2 ml/min, and then declined at 3 ml/min. As previously mentioned, increasing the methanol flow rate for lower rates leads to enhanced cell performance due to increased methanol availability at the anode catalyst layer. However, for higher methanol flow rates, the negative impact of methanol crossover on fuel cell behaviour becomes significant. Thus, the optimal flow rate for enhanced cell performance was determined to be 2 ml/min [24].
3.2 Influence of cathode CC with different OR
3.2a Cathode CC with 38.5% OR: Figure 5 shows the performance characteristics of an AB-DMFC utilising a CC with 38.5% OR at various methanol concentrations. The figure shows that the cell performance improved as the methanol concentration increased. It is obvious that when the methanol concentration improved, the cell's MPD improved. The MPD generated by AB-DMFC at 4 M methanol concentration and a current collector open ratio of 38.5% was 7.36 mW/cm2. Similarly, Figures 6 and 7 show the performance parameters of AB-DMFC using cathode CC with 45.4% and 55.40% ORs, respectively.
3.2b Cathode CC with 45.4% OR: From figure 6, it can be observed that the influence of methanol concentration is not constant, as in the case of CC with 45.4% OR, i.e., the performance of the cell does not continually improve as the methanol concentration rises. The cell performance initially increased with an increase in methanol concentration of up to 2M. However, the methanol concentration was increased further from 2M to 4M, the AB-DMFC performance deteriorated. The cell with a CC of 45.4% OR has an MPD of 7.75 mW/cm2.
3.2c Cathode CC with 55.4% OR: From figure 7, it is clear that the influence of methanol concentration on the performance of the fuel cell is not straightforward and is influenced by various factors, including the current collector open ratio (OR) and the cathode CC.
For the cell with a CC of 55.4% OR, the performance initially improved when methanol concentration was increased from 1M to 3M. However, beyond 3M methanol concentration, the performance began to decline. The fuel cell's maximum power density (MPD) was achieved at a concentration of 3 M methanol, where it was 6.8 mW/cm2.
Furthermore, the effect of methanol concentration on the cell performance was not consistent across different current collector open ratios. Additionally, optimal methanol concentration that provides the best cell performance is influenced by the OR of cathode CC. Therefore, it is important to consider these factors when optimizing the performance of the fuel cell.
The performance of AB-DMFC can be summarized as having a mixed response to an increase in cathode CC with different OR. On the other hand, an increase in CC with OR expands the area for reactant passage and promotes the mass transfer of reactants. As a result, it increases the reaction rate and improves fuel cell performance. It also makes it easier to remove the reaction's by-products, CO2 and H2O, from the reaction sites. This has the advantage of improving fuel cell performance. However, if the CCs of OR increase, there will be more MCO flowing from anode to cathode, which will result in mixed potential on the cathode reaction region. The fuel utilisation rate falls as a result of mixed over potential, and the unreacted methanol prevents oxygen from reaching the cathode reaction sites. The performance of the cell is adversely affected. The CC contact area with reaction sites decreases as OR of the CC increases. This decreases the CC’s capability to conduct more electrons and thus adversely affects the performance of the cell.
Additionally, the OR of CC affects the cell's electrical impedance. However, when methanol concentration rises, it diffuses more quickly between the anode diffusion layer and the anode catalyst layer, increasing the amount of methanol that is accessible for the reaction close to the membrane. Methanol concentration has a negative side effect because it increases the chance of MCO from anode to cathode. This leads to deterioration of cell function and a rise in mixed over potential losses. As a result, the OR of CC and the concentration of methanol have an overall positive and negative influence on the cell performance.
Figures 5, 6 and 7 show the difference of MPD and maximum current density (MCD) with methanol concentration for CC with three different ORs. For a current collector with 38.5% OR, the MPD rises as the methanol concentration improved from 1M to 4M. When methanol concentration increased from 1M to 2M for a current collector with 45.40% OR, the MPD first rises and then falls. When the methanol concentration is increased from 1M to 3M for CC with an OR of 55.5%, the MPD first rises and then decreases.
In the current range of methanol concentrations of 1M to 4M and for the CC with ORs of 38.5%, 45.4% and 55.4%, it can be understood that the fuel cell with CC having 45.4% OR demonstrated the best cell performance with maximum values of MPD and MCD at 2M methanol concentration. The cell with CC of 45.40% OR provided the highest power density values (7.75 mW cm−2) among three current collectors.
3.3 The influence of NaOH concentration on AB-DMFC performance
Figure 8 shows the performance of the cell at various NaOH concentrations with a constant methanol concentration. When the methanol concentration was 2M, the cell performance enhanced noticeably when the NaOH concentration was improved from 1 to 3M, but it dropped noticeably when the NaOH solution concentration was improved from 4 to 6M. In general, the local concentrations of both methanol and hydroxyl ions in the anode catalyst layer impact the power output and performance of an anode catalyst, which in turn causes more reactions to generate electricity. When the methanol or hydroxyl ions concentration changes, on the other hand, will cause a change in the opposite side. Increasing the NaOH solution concentration from 1M to 3M for a 2 M methanol concentration can enhance the hydroxyl ions concentration to a level that corresponds to the methanol concentration at the surfaces of active catalysts. The kinetics of methanol oxidation may be improved by enhancing the NaOH solution concentration to an acceptable level, which improves the MPD and cell performance. However, if the NaOH solution concentration is improved to 4 M, the concentration of hydroxyl ions at the active catalyst surface will be too high, corresponding to 2M concentration of the methanol, causing difficulty in methanol adsorption to the active reaction site, lowering the electrochemical kinetics and reducing performance of the fuel cell [24].
The present experimental results were compared with those available in literature, as shown in table 2. The MPD of present work is better than the values obtained by previous studies. It was found that adding alkali solution to methanol fuel enhanced MPD and decreased MCO.
4 Conclusions
The present work depicts an experimental study on the influence of cathode CC with different OR and the addition of alkali solution (NaOH) to methanol solution on AB-DMFC performance. The experiments were also carried out at cathode CC of three ORs, i.e. 38.5%, 45.40%, and 55.40% respectively. The methanol concentration for each of these three CCs was varied from 1M to 4M. It was revealed that the methanol flow rate and concentrations also influenced the optimum value of CC with OR.
The experimental results led to the following conclusions.
-
The performance of AB-DMFC is affected by methanol concentration. It was discovered that the optimal methanol concentration for the cell was 2 M. The optimal flow rate for methanol was determined to be 2 ml/min, and it was found that this flow rate also affects performance of the cell.
-
The performance of the cell is affected inconsistently by CC open ratio. It was noticed that the optimal OR value varied with methanol concentration. As a result, the CC with an OR of 45.4% shown improved cell performance at 2M concentration of methanol, while the CC with 38.5% of OR provided the highest fuel cell performance at 4M methanol concentration.
-
Within the current experimental range of methanol concentrations of 1M to 4M and three cathode CCs of open ratios 38.5%, 45.4%, and 55.40%, the fuel cell performed best at 2M methanol concentration with CC at 45.4% of OR.
-
It was observed that the anode fuel reservoir's methanol concentration and NaOH concentration both affect the maximum power output of AB-DMFC, and the optimal value for generating the highest power density was discovered.
-
When the concentrations of the methanol and the NaOH were 2M and 3M, respectively, AB-DMFC's MPD was 8.5 mW/cm2. It was found that adding alkali solution to methanol fuel enhanced MPD and decreased MCO. Finally, it was shown from the experimental results that the anode flow rate, the OR of cathode CC, and the addition of alkali solution all significantly affect the AB-DMFC performance.
Abbreviations
- AB-DMFC:
-
Air Breathing Direct Methanol Fuel Cell
- DMFC:
-
Direct Methanol Fuel Cell
- LE:
-
Liquid Electrolyte
- FE:
-
Flowing electrolyte
- CC:
-
Current Collector
- OR:
-
Open Ratio
- MPD:
-
Maximum Power Density
- MCD:
-
Maximum Current Density
- MCO:
-
Methanol Crossover
- MPL:
-
Microporous Layer
- MEA:
-
Membrane Electrode Assemblies
- 1-S:
-
Single serpentine
- DC:
-
Direct Current
References
Rashidi R, Dincer I, Naterer G F and Berg P 2009 Performance evaluation of direct methanol fuel cells for portable applications. Journal of Power Sources 187(2): 509–516. https://doi.org/10.1016/j.jpowsour.2008.11.044
Wang L, He M, Hu Y, Zhang Y, Liu X and Wang G 2015 A “4-cell” modular passive DMFC (direct methanol fuel cell) stack for portable applications. Energy 82: 229–235. https://doi.org/10.1016/j.energy.2015.01.033
“Fuel Cell Handbook (Seventh Edition),” 2004
Ouellette D, Colpan C O, Matida E and Cruickshank C A 2015 A single domain approach to modeling the multiphase flow within a flowing electrolyte–direct methanol fuel cell. International Journal of Hydrogen Energy 40(24): 7817–7828. https://doi.org/10.1016/j.ijhydene.2014.11.103
Ouellette D, Colpan C O, Cruickshank C A and Matida E 2015 Parametric studies on the membrane arrangement and porous properties of the flowing electrolyte channel in a flowing electrolyte–direct methanol fuel cell. International Journal of Hydrogen Energy 40(24): 7732–7742. https://doi.org/10.1016/j.ijhydene.2015.02.001
Braz B A, Moreira C S, Oliveira V B and Pinto A M F R 2019 Effect of the current collector design on the performance of a passive direct methanol fuel cell. Electrochimica Acta 300: 306–315. https://doi.org/10.1016/j.electacta.2019.01.131
Yuan W, Tang Y, Wang Q and Wan Z 2011 Dominance evaluation of structural factors in a passive air-breathing direct methanol fuel cell based on orthogonal array analysis. Applied energy 88(5): 1671–1680. https://doi.org/10.1016/j.apenergy.2010.11.009
Calabriso A, Cedola L, Del Zotto L, Rispoli F and Santori S G 2015 Performance investigation of passive direct methanol fuel cell in different structural configurations. Journal of Cleaner Production 88: 23–28. https://doi.org/10.1016/j.jclepro.2014.06.087
Borello D, Calabriso A, Cedola L, Del Zotto L and Santori S G 2014 Development of improved passive configurations of DMFC with reduced contact resistance. Energy Procedia 61: 2654–2657. https://doi.org/10.1016/j.egypro.2014.12.268
Kim S, Jang S, Kim S M, Ahn C Y, Hwang W, Cho Y H, Sung Y E and Choi M 2017 Reduction of methanol crossover by thin cracked metal barriers at the interface between membrane and electrode in direct methanol fuel cells. Journal of Power Sources 363: 153–160. https://doi.org/10.1016/j.jpowsour.2017.07.071
Yuan Z, Yang J, Zhang Y and Zhang X 2015 The optimization of air-breathing micro direct methanol fuel cell using response surface method. Energy 80: 340–349. https://doi.org/10.1016/j.energy.2014.11.076
Mei R, Xi J, Ma L, An L, Wang F, Sun H, Luo Z and Wu Q 2017 Multi-scaled porous Fe-N/C nanofibrous catalysts for the cathode electrodes of direct methanol fuel cells. Journal of The Electrochemical Society 164(14): F1556. https://doi.org/10.1149/2.0451714jes
Yang H and Zhao T S 2005 Effect of anode flow field design on the performance of liquid feed direct methanol fuel cells. Electrochimica Acta 50(16–17): 3243–3252. https://doi.org/10.1016/j.electacta.2004.11.060
Kordesch K, Hacker V and Bachhiesl U 2001 Direct methanol–air fuel cells with membranes plus circulating electrolyte. Journal of Power Sources 96(1): 200–203. https://doi.org/10.1016/S0378-7753(01)00491-8
Jung S 2013 Non-isothermal multi-dimensional direct methanol fuel cell model with micro-porous layers mitigating water/methanol crossover. Journal of power sources 231: 60–81. https://doi.org/10.1016/j.jpowsour.2012.12.086
Liu G, Ding X, Zhou H, Chen M, Wang M, Zhao Z, Yin Z and Wang X 2015 Structure optimization of cathode microporous layer for direct methanol fuel cells. Applied Energy 147: 396–401. https://doi.org/10.1016/j.apenergy.2015.03.021
Sabet-Sharghi N, Cruickshank C A, Matida E and Hamdullahpur F 2013 Performance measurements of a single cell flowing electrolyte-direct methanol fuel cell (FE-DMFC). Journal of power sources 230: 194–200. https://doi.org/10.1016/j.jpowsour.2012.11.147
Najmi A A, Rowshanzamir S and Parnian M J 2016 Investigation of NaOH concentration effect in injected fuel on the performance of passive direct methanol alkaline fuel cell with modified cation exchange membrane. Energy 94: 589–599. https://doi.org/10.1016/j.energy.2015.11.019
Tang Y, Yuan W, Pan M, Tang B, Li Z and Wan Z 2010 Effects of structural aspects on the performance of a passive air-breathing direct methanol fuel cell. Journal of Power Sources 195(17): 5628–5636. https://doi.org/10.1016/j.jpowsour.2010.03.069
Chen R and Zhao T S 2006 Porous current collectors for passive direct methanol fuel cells. International Conference on Fuel Cell Science, Engineering and Technology 42479: 1155–1161. https://doi.org/10.1016/j.electacta.2006.12.015
Yousefi S, Shakeri M and Sedighi K 2013 The effect of cell orientations and environmental conditions on the performance of a passive DMFC single cell. Ionics 19: 1637–1647. https://doi.org/10.1007/s11581-013-0889-y
Boni M, Rao S S and Srinivasulu G N 2019 Influence of intermediate liquid electrolyte layer on the performance of passive direct methanol fuel cell. International Journal of Green Energy 16(15): 1475–1484. https://doi.org/10.1080/15435075.2019.1671419
Boni M, Srinivasa Rao S and Naga Srinivasulu G 2020 Performance evaluation of an air breathing–direct methanol fuel cell with different cathode current collectors with liquid electrolyte layer. Asia-Pacific Journal of Chemical Engineering 15(4): e2465. https://doi.org/10.1002/apj.2465
Scott K, Yu E, Vlachogiannopoulos G, Shivare M and Duteanu N 2008 Performance of a direct methanol alkaline membrane fuel cell. Journal of Power Sources 175(1): 452–457. https://doi.org/10.1016/j.jpowsour.2007.09.027
Acknowledgements
The authors acknowledge the financial assistance provided by DST-SERB, GoI and TEQIP-II-CoE National Institute of Technology-Warangal, Telangana, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manupati, R., Srinivasulu, G.N. Experimental analysis on the influence of cathode current-collector open ratio on the performance of an air breathing direct methanol fuel cell (AB-DMFC) with the addition of alkali solution. Sādhanā 49, 168 (2024). https://doi.org/10.1007/s12046-024-02477-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12046-024-02477-0