Abstract
Molecularly imprinted polymers of triphenylphosphine oxide (MIPs-TPPO) were synthesized and evaluated for use as a solid scavenger to remove triphenylphosphine oxide from reaction mixtures, a byproduct commonly formed during organic synthesis reactions involving triphenylphosphine (PPh3) reagent. The efficiency of synthesized polymers was initially investigated using a variety of functional monomers and cross-linkers. Precipitation polymerization in acetonitrile was carried out at 80 °C for 6 h with benzoyl peroxide as an initiator, then template removal with dichloromethane. The MIPs-TPPOs affinity was evaluated by rebinding study with template molecule; additionally, the effect of binding time, selectivity, and reusability of synthesized MIPs-TPPOs were investigated. From the results, polymers containing styrene as a functional monomer and ethyleneglycol dimethacrylate as a cross-linker demonstrated the highest performance (86.00%Bound) after only 1 h. Moreover, the selected MIPs-TPPO can be reused at least five times, and this selective material can act as a solid scavenger of triphenylphosphine oxide.
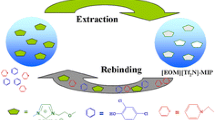
Graphical abstract
Molecularly Imprinted Polymers for triphenylphosphine oxide (MIPs-TPPO) were first synthesized and evaluated. The effects of monomer types, binding time, and reusability were investigated. It was found that MIPs-TPPO showed high efficiency with only one hour of binding time and at least five times reusability.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Triphenylphosphine (PPh3:TPP) is frequently used in organic synthesis for various reactions involving the conversion of carbonyl compounds to alkenes and the activation of various functional groups for nucleophilic substitution, such as alcohols, carbonyl compounds, carboxylic acid, and derivatives. While this reagent is advantageous for well-known reactions such as the Mitsunobu1 and Witting2 reactions, the unavoidable conversion of TPP to triphenylphosphine oxide (TPPO) cannot be avoided. Due to the formation of TPPO byproducts during reactions, it is necessary to use a higher equivalent of TPP to obtain a high yield of products, which complicates product purification.3,4 Chromatography,5,6 precipitation,3,4 and liquid-liquid or solid-phase extraction4 were all investigated for TPPO separation; however, their application was contingent on the uniqueness of the reaction products. Recently, the TPPO byproduct of the reductive cyclization of 4,4'-dibromo-2-nitrophenyl was precipitated using zinc(II)chloride (ZnCl2), resulting in a yield of over 75%.4 While solid-phase extraction is one of the most advantageous separation methods due to its ease of use,7,8 low-cost materials, selectivity, and reusability of the solid sorbent are still required.
Molecularly imprinting polymers (MIPs) have emerged as promising materials for solid-sorbent applications due to their ease of preparation, stability, high selectivity, and reusability.9,10,11,12 The lock and key mechanism of MIPs, which results in high recognition of the target molecule, is generated by the self-assembly process between target molecule (template) and suitable functional monomers by covalent or noncovalent interactions prior to polymerization with cross-linkers. Following template removal to form the selective cavities within the polymers, the target molecule is not only rebound but also removed from MIPs.13,14,15 Numerous applications in separation,15,16,17 sensors,13,18,19 pharmaceutical,20,21 organic synthesis,22 and purification23,24 have been reported based on the advantages of MIPs.
Therefore, this study was the primary to report the synthesis and evaluation of molecularly imprinted polymers using TPPO as a template molecule for use as a solid sorbent in TPPO scavenging. Various functional monomers and cross-linkers were investigated, as well as the effect of binding time, selectivity, and reusability.
2 Experimental
2.1 Synthesis of molecularly imprinted polymer for TPPO (MIPs-TPPO)
All polymers were synthesized in accordance with the literature review.25 In 150 mL round-bottom flask, MIPs-TPPOs were synthesized via precipitation polymerization; 0.25 mmol TPPO and 1 mmol functional monomer were added and dissolved in 25 mL of acetone: acetonitrile (1:1, v/v). After 15 min of incubation, 5 mmol of cross-linker was added, followed by 0.125 mmol of benzoyl peroxide and 25 mL of acetone: acetonitrile (1:1, v/v). The solution was then stirred for 5 min at room temperature before being bubbled with N2 gas for 15 min. Polymerization was carried out at 80 °C for 6 h, followed by acetonitrile washing of the precipitated polymer. The template removal step was carried out by stirring synthesized polymers in 100 mL of dichloromethane for 2 h (5 times) or until no traces of TPPO were detected using thin-layer chromatography. The polymers were then washed with acetonitrile and acetone before being dried overnight in a hot air oven at 80 °C to obtain MIPs-TPPOs. In the absence of TPPO, Non-imprinted polymers (NIPs) were synthesized using the same procedure as MIPs. The yield of synthesized MIPs was calculated using equation (1), and the scanning electron microscope (SEM) was used to examine the morphology of synthesized TPPO.
where WMIP is weight of synthesized MIP after template removal (mg), Wm is weight of functional monomer used in synthesis (mg), Wc is weight of cross-linker used in synthesis (mg).
2.2 Binding study of MIPs-TPPO
By rebinding polymers with TPPO solution (template molecule), the binding of synthesized MIPs-TPPO and its corresponding NIPs was studied in triplicate. 5 mg polymer and 1 mL of TPPO 5 ppm in acetonitrile were added to a 1.5 mL microcentrifuge tube. All prepared tubes were then incubated at room temperature in the shaking incubator for the duration of the binding time studied and then centrifuged at 6,000 rpm for 5 min. The supernatant was then separated into another microcentrifuge tube, and the absorbance of TPPO at λmax = 272 nm was determined using a UV-Visible Spectrophotometer. The concentration of TPPO in the supernatant was then calculated from the calibration curve of TPPO (y = 0.0063x+0.0008, R2 = 0.999). The %Bound and imprinting factor (α) of the polymer were calculated following equations (2) and (3), which indicated the efficiency of rebinding and selectivity of polymer,26 respectively.
where Q0 is initial concentration of TPPO before binding with polymer (ppm), Q is the average concentration of TPPO in supernatant after binding with polymer (ppm)
where %BoundMIP is %Bound of MIP, %BoundNIP is %Bound of corresponding NIP.
2.3 Reusability of MIP
The reusability of MIP was determined using the selected polymer that demonstrated the highest performance in the binding study. TPPO solution (5 ppm) was added to selected MIP in the ratio of MIP:TPPO as 5 mg: 1 mL. Firstly, the binding study was conducted according to the method described previously, using 50 mg of MIP in 10 mL of TPPO solution for the first cycle. After UV-Visible spectrophotometer analysis of the supernatant, the left MIP was washed with 100 mL of methanol: acetic acid solution (8:2 v/v) for 2 h (5 times) to remove TPPO from MIP and washed with water, acetonitrile and acetone, respectively, before being dried in a hot air oven. The prepared MIP was then weighted as 40 mg for the second cycle of binding studies and combined with 8 mL of TPPO solution (as previously determined by the MIP:TPPO ratio) to avoid MIP loss during template removal and washing step, which recovered less than 50 mg. The reusability was continued to the fifth cycle by using 30, 20, and 10 mg of MIP in a solution of 6, 4, and 2 mL of TPPOs, respectively, and the study was done in triplicate.
3 Results and Discussions
3.1 Synthesis of MIPs-TPPO
Three different types of functional monomer systems were used for synthesis: 4-vinylpyridine (4VP), styrene (Sty), and 4-vinylpyridine-co-styrene (4VP-co-Sty), with two types of cross-linkers: ethylene glycol dimethacrylate (EGDMA) and divinylbenzene (DVB) (Figure 1). All MIPs and their corresponding NIPs were synthesized via precipitation polymerization using TPPO as a template molecule. Each polymer was obtained as a white powder, and the %yield was expressed in Table 1. SEM with a diameter less than 1 μm revealed semi-spherical beads morphology in synthesized polymers (Figure 2).
According to the results shown in Table 1, the %yield of the synthesized polymer was between 62.05 and 80.65 when EGDMA was used as cross-linkers (entry 1–3) and between 15.41 and 43.76, which less than when using DVB as a cross-linkers (entry 4–6). Using EGDMA as a cross-linker appeared to yield a higher yield than DVB; additionally, none of the polymers (MIPs and NIP) could be obtained when 4VP was combined with DVB (entry 4). This could be explained by the effect of the structure of the functional monomer and the cross-linker. Since EGDMA contains two branch chains containing two vinyl groups (−CH=CH2) in the molecule, this structure may be the more favorable structure for the polymerization more than DVB, which is a more rigid structure.27,28 Moreover, it was discovered that some prepared NIPs had a higher yield than MIPs. This could be explained by the synthetic process, in which NIPs were prepared solely through the polymerization step, whereas MIPs were synthesized via self-assembly step prior to polymerization, with the binding interaction between monomer and template having a significant effect on the completion of the polymerization reaction. Due to the very low yield of polymers synthesized using DVB as a cross-linker, the binding study will be conducted using three different types of MIPs prepared using EGDMA as a cross-linker.
3.2 Binding study of MIPs
The efficiency of MIPs was investigated through a binding study in which TPPO was rebound to MIPs and their corresponding NIPs. The %Bound and imprinting factor (α) indicated the binding affinity and selectivity of MIPs, respectively, as previously described. Firstly, three different types of synthesized polymer according to entries 1–3 in Table 1 were investigated using 5 mg polymers bound with 1 mL of TPPO solution (5 ppm) for 24 h at room temperature. The results of the binding study are depicted in Figure 3. The low binding efficiency was observed with low %Bound as 12.12% and 26.35% for MIPs synthesized using 4VP and 4VP-co-Sty, respectively. Moreover, non-selective binding was observed in both previous MIPs, which could be shown by the low value of imprinting factor (α nearly or less than 1) since NIPs were synthesized by the absence of TPPO and had no cavity inside the polymer. However, MIPs synthesized by Sty showed a very good binding efficiency to template molecules with %Bound = 84.81 and imprinting factor value of 1.88. This would be caused by the strong binding interaction between the cavities inside MIPs with functionalized by π- π interaction between phenyl ring of TPPO and Sty, as shown in Figure 4. This MIP and their corresponding NIP were therefore selected for further study.
3.3 Effect of binding time
MIP-TPPO synthesized by Sty with EGDMA was selected to study the effect of binding time. The binding experiments were also done by previous procedure with varied incubation times as 30 min, 1 h, 2 h, 4 h, 8 h, 12 h, and 24 h at room temperature. Results in Figure 5 showed the uncertainly %Bound of NIPs when increasing time which could be explained by non-specific binding.29 For MIPs, high %Bound was observed from both of polymers from only 30 min (65.11%) of binding time then increased when increasing of time before slightly decreased and stabled. This binding behavior is attributed to the rebinding of the template at the cavities created from the self-assembly process and binding equilibrium.30 Nevertheless, the highest %Bound (86.67%) and the highest imprinting factor value (2.25) were observed with a binding time of 1 h. Therefore, this protocol was selected to study the reusability of MIPs.
3.4 Reusability of MIPs
The reusability of selected MIP-TPPO was studied in five cycles for binding with 5 ppm of TPPO solution for 1 h, and results showed in Table 2. The ratio between MIP:TPPO solution was 5 mg:1 mL, and all MIPs were scaled up to 50 mg in the first cycle to prevent the loss of MIPs during the study in five cycles. It could be seen that the %Bound of MIP was slightly decreased when continuously reused from the first cycle to the fifth cycle, which was from 86.12% to 81.28%. However, this MIP-TPPO could be reused at least five times with less than 5% of %Bound decreasing.
4 Conclusions
Molecularly imprinted polymers selective for triphenylphosphine oxide (MIPs-TPPO) were first synthesized via precipitation polymerization using various types of functional monomer and cross-linkers. The binding efficiency of synthesized MIPs was determined under various conditions, including polymer type, binding time, and reusability. Styrene (Sty) was chosen as the functional monomer and divinylbenzene (DVB) as the cross-linkers for the synthesis of MIP-TPPO, which demonstrated the highest performance of MIP with high binding affinity and high selectivity at only 1 h of binding time. Additionally, when compared to their initial use, the studied MIPs could be reused at least five times with moderate affinity.
References
Charette A B, Janes M K and Boezio A A 2001 Mitsunobu reaction using triphenylphosphine linked to non-cross-linked polystyrene J. Org. Chem. 66 2178
Westman J 2001 An efficient combination of microwave dielectric heating and the use of solid-supported triphenylphosphine for Wittig reactions Org. Lett. 23 3745
Haack K L, Ahn Y M and George G I 2005 A convenient method to remove ruthenium byproducts from olefin metathesis reactions using polymer-bound triphenylphosphine oxide (TPPO) Mol. Divers. 9 301
Batesky D C, Goldfogel M J and Weix D J 2017 Removal of triphenylphosphine oxide by precipitation with zinc chloride in polar solvents J. Org. Chem. 82 9331
Edwards N A, Fisher G, Harris G H and Kellichan N 2014 A general method for the separation of triphenylphosphine oxide and reaction products using high performance countercurrent chromatography J. Chromatogr. A 1323 49
Byrne P A, Rajendran K V, Muldoon J and Gilheany D G 2012 A convenient and mild chromatography-free method for the purification of the products of Wittig and Appel reactions Org. Biomol. Chem. 10 3531
Cao Y, Sheng T, Yang Z, Huang D and Sheng L 2021 Synthesis of molecular-imprinting polymer coated magnetic nanocomposites for selective capture and fast removal of environmental tricyclic analogs Chem. Eng. Sci. 426 128768
Hu T, Chen R, Wang Q, He C and Liu S 2021 Recent advances and applications of molecularly imprintedpolymers in solid-phase extraction for real sample analysis J. Sep. Sci. 44 274
Kupai J, Razali M, Buyuktiryaki S, Kecili R and Szekely G 2017 Long-term stability and reusability of molecularly imprinted polymers Polym. Chem. 8 666
Haupt K 2003 Molecularly imprinted polymers: the next generation Anal. Chem. 17 376A
Sun Y 2014 Molecularly imprinted polymer for 2, 4-dichlorophenoxyacetic acid prepared by a sol-gel method J. Chem. Sci. 126 1005
Tadi K K and Motghare R V 2013 Computational and experimental studies on oxalic acid imprinted polymer J. Chem. Sci. 125 413
BelBruno J J 2019 Molecularly imprinted polymers Chem. Rev. 119 94
Zarejousheghani M, Lorenz W, Vanninen P, Alizadeh T, Cämmerer M and Borsdorf H 2019 Molecularly imprinted polymer materials as selective recognition sorbents for explosives: a review Polymers 11 1
Lanza F and Sellegren B 2001 The application of molecular imprinting technology to solid phase extraction Chromatographia 53 599
Huang Y P, Zheng C and Liu Z S 2011 Molecularly imprinted polymers for the separation of organic compounds in capillary electrochromatography Curr. Org. Chem. 15 1863
Guo T Y, Xia Y Q, Wang J, Song M D and Zhang B H 2005 Chitosan beads as molecularly imprinted polymer matrix for selective separation of proteins Biomaterials 28 5757
Yanez-Sedeno P, Campuzano S and Pingarron J M 2017 Electrochemical sensors based on magnetic molecularly imprinted polymers: a review Anal. Chim. Acta 960 1
Tiwari M P and Prasad A 2015 Molecularly imprinted polymer based enantioselective sensing devices: a review Anal. Chim. Acta 853 1
Allender C J, Richardson C, Woodhouse B, Heard C M and Brain K R 2000 Pharmaceutical applications for molecularly imprinted polymers Int. J. Pharm. 195 39
Olwill A, Hughes H, O’Riordain M and McLoughlin P 2004 The use of molecularly imprinted sol–gels in pharmaceutical separations Biosens. Bioelectron. 20 1045
Yu Y, Ye L, Haupt K and Mosbach K 2002 Formation of a class of enzyme inhibitors (drugs), including a chiral compound, by using imprinted polymers or biomolecules as molecular-scale reaction vessels Angew. Chem. Int. 41 4459
Arabi M, Chaedi M, Ostovan A and Wang S 2016 Synthesis of lab-in-a-pipette-tip extraction using hydrophilic nano-sized dummy molecularly imprinted polymer for purification and analysis of prednisolone J. Colloid Interface Sci. 480 232
Bae S Y, Southard G L and Murray G M 1999 Molecularly imprinted ion exchange resin for purification, preconcentration and determination of UO22+ by spectrophotometry and plasma spectrometry Anal. Chim. Acta 397 173
Karuehanon W, Wongthep T and Wanggorn C 2018 Synthesis of molecularly imprinted polymers for extraction of quercetin in mulberry leaves Prawarun Agr. J. 15 295
Pattarawarapan M, Komkham S and Karuehanon W 2008 Synthesis of nicotinamide-imprinted polymers and their binding performances in organic and aqueous media e-Polymers 91 1
Lloyd L L 1991 Rigid macroporous copolymers as stationary phases in high-performance liquid chromatography J. Chromatogr. A 544 201
de Rezende S M, de Castro Reis M, Reid M G, Silva Jr PL, Coutinho F M, da Silva San Gil R A and Lachter E R 2008 Transesterification of vegetable oils promoted by poly(styrene-divinylbenzene) and poly(divinylbenzene) Appl. Catal. A 349 198
Kantarovich K, Belbont A S, Haupt K, Ilana B and Gheber L A 2009 Detection of template binding to molecularly imprinted polymers by Raman microspectroscopy Appl. Phys. Lett. 94 194103
Ansell R J 2015 Characterization of the binding properties of molecularly imprinted polymers Adv. Biochem. Engin./Biotechnol. 150 51
Acknowledgements
The authors acknowledge the Institute of Research and Development, Lamapang Rajabhat University for a scholarship. They are also grateful to the Center of Excellence for Innovation of Chemistry (PERCH-CIC) and Faculty of Science, Lampang Rajabhat University and Assoc. Prof. Mookda Pattarawarapan, Department of Chemistry, Faculty of Science, Chiang Mai University for providing some chemicals and facilities.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
KARUEHANON, W., FUNFUENHA, W. & PHUTTAWONG, N. Selective triphenylphosphine oxide imprinted polymer for solid scavenger application in organic synthesis. J Chem Sci 134, 21 (2022). https://doi.org/10.1007/s12039-021-02021-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-021-02021-1