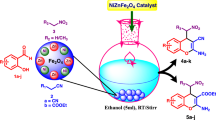
Abstract
This paper describes a very mild, quick and simple protocol for the synthesis of phenols using \(\hbox {CuFe}_{2}\hbox {O}_{4}\) magnetic nanoparticles as a catalyst. The nanosized catalyst has an average diameter of 17.63 nm. The magnetic nanoparticles were characterized by SEM, EDX, VSM, XRD and TEM analysis. The synthesis of phenols from phenylboronic acids using \(\hbox {H}_{2}\hbox {O}_{2}\) as an oxidant proceeded very well with excellent yields. Heterogeneous catalyst, easy recyclability, mild reaction conditions, short reaction time added as an advantage for the present protocol.
Graphical Abstract
A very mild, quick and efficient protocol has been designed for the preparation of phenols from phenyl boronic acids using \(\hbox {CuFe}_{2}\hbox {O}_{4}\) Magnetic Nanoparticles (MNPs) as a catalyst. Heterogeneous catalyst, easy recyclability added as an advantage for the protocol.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phenolic compounds are produced as secondary metabolites by most plants. These compounds play an important role in the growth and reproduction of plants, and provide protection against pathogens and predators,[1] besides contributing towards the colour and sensory characteristics of fruits and vegetables.[2] Phenols exhibit a wide range of physiological properties, such as anti-allergenic, anti-atherogenic, anti-inflammatory, anti-microbial, anti-thrombotic, cardioprotective and vasodilatory effects.[3,4,5,6,7] Phenolic compounds also have a wide range of applications both in the industrial world and in nature. Antioxidant activity[8] is a beneficial effect derived from phenolic compounds.
The production of phenols has begun since the 1860s. Moreover, towards the end of the \(19^{\mathrm{th}}\) century, phenols have been used in the synthesis of dyes, explosives i.e., picric acid, etc. Formaldehyde resins are the basis of the oldest plastics and are still used in electrical equipment to make low-cost thermosetting plastics such as melamine and bakelite.
Looking at the immense value of phenols, scientists have tried to synthesize it through various reaction pathways. The laboratory scale synthesis of phenols uses nucleophilic substitution of aryl halides activated by electron-withdrawing substituents catalysed by copper, palladium,[9] etc. These conditions suffer from several drawbacks like harsh reaction condition, poor functional group compatibility, less substrate scope, etc. Among them, the synthesis of phenols from arylboronic acids to phenols has received significant attention in organic synthesis, because arylboronic acids are diverse, less toxic, and highly stable under air. The transformations of aryl boronic acids to phenols via ipso-hydroxylation have been reported by several scientists using different reaction conditions. Several research groups have reported different catalysts for the synthesis of phenols using arylboronic acids which includes iron(III)oxide,[10] (\(\hbox {NH}_{4})_{2} \hbox {S}_{2} \hbox {O}_{8}\),[11] potassium peroxymonosulfate,[12] hydrazine hydrate, [13] \(\hbox {I}_{2}\),[14] Amberlite IR 120 resin,[15] \(\hbox {H}_{3}\hbox {BO}_{3}\)–\(\hbox {H}_{2}\hbox {O}_{2}\),[16] \(\hbox {NaClO}_{2}\).[17] Recently, many scientists have carried out ipso-hydroxylations using CNT-chitosan,[18] ascorbic acid,[19] \(\hbox {Fe}_{2} \hbox {O}_{3} @\hbox {SiO}_{2}\) nanoparticles,[20] \(\hbox {Cu}_{2}\hbox {O}\) nanoparticles,[21] etc. But these conditions suffer one or more disadvantages like longer reaction time and/or high amount of catalyst loading. Nowadays, the use of nanoparticles as a catalyst is growing over other catalyst systems, but because of their nanosize, the isolation and recovery of the nanocatalyst from the reaction mixture has been one of the major drawbacks.
To overcome all these difficulties, the use of magnetic nanoparticles (MNPs) as a catalyst is preferred over choosing other catalysts because of their stability, easy synthesis, better selectivity, excellent surface area to the volume ratio, and easy recyclability.
Various magnetic nanoparticles have been synthesized in the laboratory which include \(\hbox {Fe}_{3} \hbox {O}_{4}\),[22, 23] \(\hbox {Cu/SB-Fe}_{3} \hbox {O}_{4}\),[24] etc.
Herein we have synthesized \(\hbox {CuFe}_{2}\hbox {O}_{4}\,\hbox {MNPs}\) by a very convenient and simple procedure and used it as a catalyst in the production of phenols from aryl boronic acids.
2 Experimental
2.1 Preparation of the catalyst using Coprecipitation Method
0.5 g of \(\hbox {FeCl}_{3}\) and 0.5 g of copper acetate were added in a round bottom flask and 70 mL of deionised water was added to it and stirred at room temperature. 5 mL of 0.1 M NaOH was added to the stirring solution. After 30 min 0.1 M \(\hbox {NaBH}_{4}\) was added to the solution and stirred for about 1 h. The resulting solution was centrifuged at 1200 rpm for 10 min and washed with ethanol. The centrifuged product was dried and the desired product of \(\hbox {CuFe}_{2}\hbox {O}_{4}\,\hbox {MNPs}\) was obtained.
2.2 General procedure for the Ipso-Hydroxylation
In a round bottom flask 1 mmol of phenylboronic acid, 0.03 mmol (3 mol%) of \(\hbox {CuFe}_{2}\hbox {O}_{4}\,\hbox {MNPs}\), \(200\,\upmu \hbox {L}\) of \(\hbox {H}_{2}\hbox {O}_{2}\) (30%) were added and stirred at room temperature. The desired product was obtained after about 2 min which was monitored by TLC. The reaction mixture was diluted with 20 mL of diethyl ether and the combined organic layer was washed with brine and dried over by anhydrous \(\hbox {Na}_{2} \hbox {SO}_{4}\) and evaporated in a rotary evaporator. The products were confirmed by \(^{1}\hbox {H NMR}\) and \(^{13}\hbox {C NMR}\) spectroscopy without any further purification.
3 Results and Discussion
3.1 Characterization of the catalyst
The powder XRD diffraction pattern of the prepared \(\hbox {CuFe}_{2}\hbox {O}_{4}\,\hbox {MNPs}\) is shown in Figure 1. The various diffraction peaks at \(2\Theta = 18.3, 30.3, 35.6, 42.8, 57.1, 62.95\) corresponds to the planes (110), (111), (200), (220), (311), (222) respectively. The sharp diffraction peaks clearly shows the highly crystalline nature of \(\hbox {CuFe}_{2}\hbox {O}_{4}\,\hbox {MNPs}\).
The SEM images showed the structure and morphology of the nanoparticles (Figure 2). The SEM images depicted the structure of nanoparticles to be spherical with slight aggregation. The purity of the nanoparticles was observed from the Energy Dispersive X-ray (EDX) spectrum which shows the presence of copper, iron and oxygen element.
The morphology of the nanoparticles was determined from the TEM images (Figure 3a). The selected area electron diffraction (SAED) pattern of the \(\hbox {CuFe}_{2}\hbox {O}_{4}\hbox {MNPs}\) showed bright fringes, which indicated the crystalline nature of the nanoparticles (Figure 3b). The average diameter of the nanoparticle was found to be 17.636 nm (Figure 3c).
Furthermore, to observe the magnetic behaviour the VSM analysis of the nanoparticles was studied (Figure 4). From the VSM study, the coercivity (Hci) was found to be 53.387 G.
3.2 Application of the Catalyst
The prepared catalyst was used for the synthesis of various substituted phenols from arylboronic acids. We began our experiment with the optimization parameter for which we took phenyl boronic acid (1 mmol) as the model substrate and the reaction was carried out at room temperature (Scheme 1). The product was formed within 2 min with 96% yield.
Initially, we optimized our reaction using different amounts of \(\hbox {H}_{2}\hbox {O}_{2}\). The reaction preceded very smoothly using only \(200\, \upmu \hbox {L}\) of \(\hbox {H}_{2}\hbox {O}_{2}\) within 2 min (Table 1, entry 4). After the identification of the proper amount of \(\hbox {H}_{2}\hbox {O}_{2}\) the reaction was further optimized for different amount of the catalyst (Table 2). Moreover, with the use of \(\hbox {H}_{2}\hbox {O}_{2}\) only as an oxidant, trace amount of yields was obtained after 1 h (Table 2, entry 1). The yield of the product was further increased by using only 3 mol% of the catalyst without the use of any solvent.
Using the optimized conditions provided in the previous section, the scope of the reaction was studied for a number of phenyl boronic acids to phenols (Table 3). It was observed that both electron withdrawing and electron donating groups had little effect on the reactivity conditions. Moreover, hetero arylboronic acids also gave high yields in less time.
We compared the use of \(\hbox {CuFe}_{2}\hbox {O}_{4}\,\hbox {MNPs}\) as a catalyst for the ipso-hydroxylation of aryl boronic acids to phenols with other reported catalyst,[25,26,27,28] which showed the better catalytic efficiency of our catalyst. The easy recyclability, less amount of catalyst loading, less reaction time, easy preparation of the catalyst proved to be the advantages of the present protocol. The comparison efficiency of phenylboronic acid to phenol with other reported catalyst is shown in Table 4.
3.3 Recyclability
Recyclability is one of the main advantages of a heterogeneous catalyst. To test the recyclability, we observed the ipso-hydroxylation of phenyl boronic acid to phenol (Figure 5). The catalyst was easily recycled up to \(5^{\mathrm{th}}\) cycle without any loss in the catalytic cycle. After the completion of the reaction the catalyst was extracted with the help of an external magnet, centrifuged and washed properly with ethanol. It was then dried and reused for a fresh batch of reaction.
The sharp XRD peaks taken after the \(5^{\mathrm{th}}\) cycle (Figure 5b) proved the crystallinity of the nanoparticles. There was no filtration required for the recyclability of the catalyst.
4 Conclusions
Herein we have synthesized phenols using a very mild procedure using a heterogeneous, recyclable \(\hbox {CuFe}_{2}\hbox {O}_{4}\hbox {MNPs}\) catalyst for the first time. The easy formation of the product in less reaction time proved to be a very significant procedure for the synthesis of phenols. The magnetic nature of the catalyst added as an advantage for the protocol as the product formation required less amount of catalyst.
References
Bravo L 1998 Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance Nutr. Rev. 56 317
Alasalvar C, Grigor J M, Zhang D and Quantick P C 2001 Comparison of Volatiles, Phenolics, Sugars, Antioxidant Vitamins, and Sensory Quality of Different Colored Carrot Varieties J. Agric. Food Chem. 49 1410
Ferreira I C F R, Barros L, Soares M E, Bastos M L and Pereira J A 2007 Antioxidant activity and phenolic contents of Olea europaea L. leaves sprayed with different copper formulations Food Chem. 103 188
Manach C, Mazur A and Scalbert A 2005 Polyphenols and prevention of cardiovascular diseases Curr. Opin Lipidol. 16 77
Middleton E, Middleton E, Kandaswami C, Kandaswami C and Theoharides T C 2000 The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer Pharmacol. Pharmacol. Rev. 52 673
Puupponen-Pimiä R, Nohynek L and Meier C 2001 Antimicrobial properties of phenolic compounds from berries J. Appl. Microbiol. 90 494
Samman S, Lyons Wall P M and Cook N C 1998 Flavonoids and coronary heart disease: Dietary perspectives In Flavonoids in Health and Disease C A RiceEvans and L Packer (Eds.) (New York: Marcel Dekker) pp. 469–482
Heim K E, Tagliaferro A R and Bobilya D J 2002 Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships J. Nutr. Biochem. 13 572
Yu C W, Chen G S, Huang C W and Chern J W 2012 Efficient microwave-assisted Pd-catalyzed hydroxylation of aryl chlorides in the presence of carbonate Org. Lett. 14 3688
Sawant S D, Hudwekar A D, Aravinda Kumar K A, Venkateswarlu V, Singh P P and Vishwakarma R A 2014 Ligand- and base-free synthesis of phenols by rapid oxidation of arylboronic acids using iron(III) oxide Tetrahedron Lett. 55 811
Claudia A, Celedón C, García L C and Corral N J L 2014 An Efficient Synthesis of Phenols via Oxidative Hydroxylation of Arylboronic Acids Using (\(\text{NH}_{4})_{2} \text{ S }_{2} \text{ O }_{8}\) J. Chem. Article ID 569572
Webb K S and Levy D 1995 A facile oxidation of boronic acids and boronic esters Tetrahedron Lett. 36 5117
Zhong Y, Yuan L, Huang Z, Gu W, Shao Y and Han W 2014 Unexpected Hydrazine Hydrate-Mediated Aerobic Oxidation of Aryl/Heteroaryl Boronic Acids to Phenols with Ambient Air RSC Adv. 4 33164
Gogoi A and Bora U 2012 An iodine-promoted, mild and efficient method for the synthesis of phenols from arylboronic acids Synlett. 23 1079
Mulakayala N, Ismail Kumar K M, Rapolu R K, Kandagatla B, Rao P, Oruganti S and Pal M 2012 Catalysis by Amberlite IR-120 resin: A rapid and green method for the synthesis of phenols from arylboronic acids under metal, ligand, and base-free conditions Tetrahedron Lett. 53 6004
Gogoi K, Dewan A, Gogoi A, Borah G and Bora U 2014 Boric Acid as Highly Efficient Catalyst for the Synthesis of Phenols from Arylboronic Acids Heteroatom Chem. 25 127
Gogoi P, Bezboruah P, Gogoi J and Boruah R C 2013 Ipso-hydroxylation of arylboronic acids and boronate esters by using sodium chlorite as an oxidant in water Eur. J. Org. Chem. 2013 7291
Kim H S, Joo S R, Shin U S and Kim S H 2018 Recyclable CNT-chitosan nanohybrid film utilized in copper-catalyzed aerobic ipso-hydroxylation of arylboronic acids in aqueous media Tetrahedron Lett. 59 4597
Das S K, Bhattacharjee P and Bora U 2018 Ascorbic Acid as a Highly Efficient Organocatalyst for ipso-Hydroxylation of Arylboronic Acid Chem. Select. 3 2131
Saikia I, Hazarika M, Hussian N, Das M R and Tamuly C 2017 Biogenic synthesis of \(\text{ Fe }_{2} \text{ O }_{3} @ \text{ SiO }_{2}\) nanoparticles for ipso-hydroxylation of boronic acid in water Tetrahedron Lett. 58 4255
Borah R, Saikia E, Bora S J and Chetia B 2016 On-water synthesis of phenols using biogenic \(\text{ Cu }_{2}\text{ O }\) nanoparticles without using \(\text{ H }_{2} \text{ O }_{2}\) RSC Adv. 6 100443
Chacko P and Shivashankar K 2018 Synthesis of aminomethylphenol derivatives via magnetic nano \(\text{ Fe }_{3} \text{ O }_{4}\) catalyzed one pot Petasis borono-Mannich reaction J. Chem. Sci. 130 1
Dadras A, Jamal M R N, Moghaddam M F and Ayati S E 2018 Green and selective oxidation of alcohols by immobilized Pd ontotriazole functionalized \(\text{ Fe }_{3} \text{ O }_{4}\) magnetic nanoparticles J. Chem. Sci. 130 1
Elhamifar D, Mofatehnia P and Faal M 2017 Magnetic nanoparticles supported Schiff-base/copper complex: An efficient nanocatalyst for preparation of biologically active3,4-dihydropyrimidinones J. Colloid Interface Sci. 504 268
Begum T, Gogoi A, Gogoi P K and Bora U 2015 Catalysis by mont K-10 supported silver nanoparticles: A rapid and green protocol for the efficient ipso-hydroxylation of arylboronic acids Tetrahedron Lett. 56 95
Mahanta A, Adhikari P, Bora U and Thakur A J 2015 Biosilica as an efficient heterogeneous catalyst for ipso-hydroxylation of arylboronic acids Tetrahedron Lett. 56 1780
Gogoi A and Bora U 2013 A mild and efficient protocol for the ipso-hydroxylation of arylboronic acids Tetrahedron Lett. 54 1821
Borah R, Saikia E, Bora S J and Chetia B 2017 Banana pulp extract mediated synthesis of \(\text{ Cu }_{2}\text{ O }\) nanoparticles: An efficient heterogeneous catalyst for the ipso-hydroxylation of arylboronic acids Tetrahedron Lett. 58 1211
Acknowledgements
BC gratefully acknowledges DST-SERB (project No.SB/FT/CS-161/2012) UGC-SAP for financial assistance, and SAIF, NEHU Shillong for spectral data, CSIC Dibrugarh University for XRD, SEM, EDX analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chutia, R., Chetia, B. A simple, fast and excellent protocol for the synthesis of phenols using \(\hbox {CuFe}_{2}\hbox {O}_{4}\) magnetic nanoparticles. J Chem Sci 131, 48 (2019). https://doi.org/10.1007/s12039-019-1624-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-019-1624-7