Abstract
A novel library of aminomethylphenol has been developed using magnetic \(\hbox {Fe}_{3}\hbox {O}_{4}\) nanoparticles via Petasis borono-Mannich reaction of salicylaldehydes, secondary amines and phenyl boronic acids. This one-pot protocol features mild reaction conditions, excellent yields in short reaction times, readily available starting materials, good functional group tolerance and reusability of the catalyst for four consecutive cycles without significant loss in its activity.
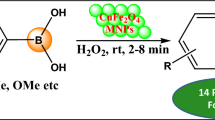
Graphical abstract
A novel library of aminomethylphenol has been developed using magnetic \(\hbox {Fe}_{3}\hbox {O}_{4}\) nanoparticles via Petasis borono-Mannich reaction of salicylaldehydes, secondary amines and phenyl boronic acids. This one-pot protocol features mild reaction conditions, excellent yields in short reaction times, readily available starting materials, good functional group tolerance and reusability of the catalyst for four consecutive cycles without significant loss in its activity.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Aminomethylphenol units are privileged structural motifs which have drawn much attention from the medicinal[1] and material science[2] communities owing to their biological and industrial significance. Enormous compounds belong to this family have entered preclinical and clinical trials over a longer period. Synthetic pharmaceuticals bearing this structural unit have been widely applied to clinical treatment as antibacterial,[3] anti-inflammatory,[4] antimicrobial[5] and antimalarial[6] agents. Some representative pharmacologically important drugs incorporating aminomethylphenol skeleton are WR-194,965, JPC-2997, MK-4815, JPC-3186 and JPC-3210 (Figure 1).[7, 8] Notably, a class of 2-aminomethylphenol displays saluretic profiles and can be used in the treatment of hypertension or edematous disorders.[9] In addition, aminomethylphenol figure presents a key structural motif to prepare human hair dye coupler compounds,[10] heat curable thermosetting surface coating,[11] corrosion inhibiting coating to a metal surface,[12] and as additives for lubricating oils.[13]
Considering the spectacular biological and chemo-physical properties of aminomethylphenol derivatives and their significant role in organic synthesis, the development of versatile, convenient, and effective methods for the design of these scaffolds have been invited considerable attention from both the academic and industrial researchers. The known reactions for the aminomethylphenol motifs in synthetic chemistry are (i) three-component reaction among organoboronic acids, amines and salicylaldehydes,[14] (ii) the reduction of iminomethylphenol derivatives,[15] (iii) the reaction of 2-aminopyridine, benzaldehydes and phenols.[16] Petasis borono-Mannich reaction had reported for pyridine and electron poor aromatic amines.[17] Petasis reaction had reported at room temperature[18] as well as 0 \({^{\circ }}\hbox {C}\).[19] The simplest and the most practical protocol, reported by Petasis borono-Mannich involves the three-component reaction of salicylaldehyde, secondary amine and boronic acid. However, these procedures were found to be sluggish, required a longer reaction time of more than 24 h, failed to proceed full conversion and microwave irradiation or heating was necessary. In the past two decades, several modifications to Petasis borono-Mannich reaction have been reported using catalysts such as \(\hbox {CoFe}_{2}\hbox {O}_{4}\),[20] chitosan[21] and [bmim]\(\hbox {BF}_{4}\).[22] The other interesting works describing this reaction were carried out using protonated trititanate (\(\hbox {H}_{2}\hbox {Ti}_{3}\hbox {O}_{7})\) nanotubes[23] and tetranuclear \(\hbox {Zn}_{2}\hbox {Ln}_{2}\) coordination clusters as catalysts.[24] Despite that, the development of an efficient and simple methodology for the synthesis of aminomethylphenol should take into consideration the reduction in the reaction time, simple reaction conditions, and reusability of the catalyst.
In recent years, magnetic nanoparticles have gained increased attention as a highly useful catalyst for organic synthesis. In particular, environmentally benign heterogeneous magnetic nano \(\hbox {Fe}_{3}\hbox {O}_{4}\) (magnetite) have been achieved much interest owing to its ease of handling, lower cost, non-toxicity, the comfort of recovery with an external magnetic field, oxidative stability and biological compatibility.[25] In the last few years, nano \(\hbox {Fe}_{3}\hbox {O}_{4}\) catalyst has been used for different organic transformation such as Sonogashira–Hagihara reaction,[26] Biginelli reactions,[27] synthesis of imidazoles,[28] Baeyer–Villiger oxidation[29] and as a support for homogeneous catalysts.[30]
Despite these advances, to the best of our knowledge, the utilization of nano \(\hbox {Fe}_{3}\hbox {O}_{4}\) catalyst in the three-component Petasis borono-Mannich reaction has not yet been documented. In continuation of our efforts to develop new synthetic methods for the important organic compounds,[31] in this paper, we disclose the synthesis of aminomethylphenol library via one-pot three-component reaction of salicylaldehydes, secondary amines, and phenylboronic acids in the presence of catalytic amount of magnetic \(\hbox {Fe}_{3}\hbox {O}_{4}\) nanoparticles.
2 Experimental
2.1 General information
Commercially available organic and inorganic compounds purchased from Sigma-Aldrich and Clearsynth Labs Limited (Hyderabad) were used without further purification. Solvents were dried and stored over microwave-activated 4 Å molecular sieves. Melting points were determined on an electric melting point apparatus. Infrared spectra were taken with KBr pellets on an Agilent Cary 630 FT-IR spectrophotometer (only the structurally most important peaks are given). \({}^{1}\hbox {H}\) NMR (400 MHz) spectra were recorded on a Bruker WH-200 spectrometer and \({}^{13}\hbox {C}\) NMR (100 MHz) on Agilent VNRMS spectrometer using \(\hbox {CDCl}_{3}\) as solvent and TMS as an internal standard. Chemical shifts were reported in parts per million (ppm) and coupling constant (J) in hertz (Hz). Data are reported as follows: chemical shift, multiplicity (\(\hbox {s} = \hbox {singlet}\), \(\hbox {d} = \hbox {doublet}\), \(\hbox {t} = \hbox {triplet}\), \(\hbox {q} = \hbox {quartet}\), \(\hbox {m} = \hbox {multiplet}\)). Mass spectra were recorded on an Agilent LC-MS. High-resolution mass spectra (HMRS) were recorded using ion electrospray. Thin layer chromatography was performed on silica gel 60 F254 plates. Elemental analysis was performed on an Elemental Vario Micro Cube rapid analyzer.
2.2 Typical experimental procedure for the synthesis of \((\mathbf{4a})\)
To a stirred solution of salicylaldehyde (0.5 g, 4.09 mmol) in 1,4-dioxane (5 mL) was added nano \(\hbox {Fe}_{3}\hbox {O}_{4}\) (0.0189 g, 2 mol%) and the reaction mixture was stirred at room temperature for 5 min. 2-(Piperidin-4-yl)-1H-benzo[d]imidazole (0.82 g, 4.09 mmol) was added to this reaction mixture, stirred for another 10 min at the same temperature followed by the addition of 4-bromophenylboronic acid (0.82 g, 4.09 mmol) and stirring was continued until the completion of the reaction as indicated by TLC. The \(\hbox {Fe}_{3}\hbox {O}_{4}\) nanoparticles were recovered by absorbing on to the magnetic stirring bar. The reaction mixture was extracted with ethyl acetate (\(3\times 50\) mL). The extract was washed with water, and finally with brine. The organic solution was dried with anhydrous \(\hbox {Na}_{2}\hbox {SO}_{4}\) and concentrated by rotary evaporator. Finally, the residue was purified by recrystallization from ethanol.
2.2.1 2-((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(4-bromophenyl) methyl)phenol \((\mathbf{4a})\)
Yield: 90% (1.697 g); Yellow solid; M.p.: 210–\(212\,{^{\circ }}\hbox {C}\); IR (ATR, \(\hbox {cm}^{-1})\): 3374 (NH), 3500 (OH); \({}^{1}\hbox {H}\) NMR (\(\hbox {CDCl}_{3}\), 400 MHz) \(\delta \): 1.57 (m, 4H), 2.34 (t, 4H, \(J = 6.4\,\hbox {Hz}\)), 2.74 (m, 1H), 4.75 (s, 1H, NH), 5.04 (s, 1H), 5.41 (s, 1H, OH), 6.96 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.08 (t, 1H, \(J = 6.4\,\hbox {Hz}\)), 7.39 (t, 1H, \(J = 6.8\,\hbox {Hz}\)), 7.49 (d, 2H, \(J = 9.6\,\hbox {Hz}\)), 7.73 (t, 2H, \(J = 7.2\,\hbox {Hz}\)), 7.88 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 8.02 (d, 2H, \(J = 9.6\,\hbox {Hz}\)), 8.17 (d, 2H, \(J = 9.2\,\hbox {Hz}\)) ppm; \({}^{13}\hbox {C}\) NMR (\(\hbox {CDCl}_{3}\), 100 MHz) \(\delta \): 30.0 (2C), 35.4, 50.9 (2C), 76.0, 115.1 (2C), 116.4, 119.3, 120.5, 121.8, 123.1 (2C), 127.5, 130.0 (2C), 131.2, 132.4 (2C), 138.8 (2C), 141.5, 141.9, 157.9 ppm; LCMS: m/z Calcd. for \(\hbox {C}_{25}\hbox {H}_{25}\hbox {BrN}_{3}\hbox {O}\) 462.1, found 462.9 \([\hbox {M}+\hbox {H}]^{+}\); Elem. anal. Calcd. (%) for \(\hbox {C}_{25}\hbox {H}_{24}\hbox {BrN}_{3}\hbox {O}\): C, 64.94; H, 5.23; N, 9.09; found (%): C, 64.90; H, 5.18; N, 9.00.
2.2.2 2-((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(4-chlorophenyl) methyl)phenol \((\mathbf{4b})\)
Yield: 89% (1.521 g); Yellow solid; M.p.: 200–\(202\,{^{\circ }}\hbox {C}\); IR (ATR, \(\hbox {cm}^{-1})\): 3380 (NH), 3524 (OH); \({}^{1}\hbox {H}\) NMR (\(\hbox {CDCl}_{3}\), 400 MHz) \(\delta \): 1.57 (m, 4H, \(J = 9.2\,\hbox {Hz}\)), 2.18 (t, 4H, \(J = 6.4\,\hbox {Hz}\)), 2.74 (m, 1H, \(J = 9.6\,\hbox {Hz}\)), 4.61 (s, 1H, NH), 4.94 (s, 1H), 5.32 (s, 1H, OH), 6.97 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.04 (t, 1H, \(J = 6.4\,\hbox {Hz}\)), 7.39 (t, 1H, \(J = 6.8\,\hbox {Hz}\)), 7.50 (d, 2H, \(J = 9.6\,\hbox {Hz}\)), 7.73 (t, 2H, \(J = 7.2\,\hbox {Hz}\)), 7.83 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.97 (d, 2H, \(J = 8.8\,\hbox {Hz}\)), 8.13 (d, 2H, \(J = 9.2\,\hbox {Hz}\)) ppm; \({}^{13}\hbox {C}\) NMR (\(\hbox {CDCl}_{3}\), 100 MHz) \(\delta \): 29.9 (2C), 35.5, 51.0 (2C), 76.2, 115.2 (2C), 116.3, 119.3, 121.6, 123.2 (2C), 127.6, 129.3 (2C), 130.0 (2C), 131.3, 131.8, 138.9 (2C), 140.8, 141.5, 157.9 ppm; LCMS: m/z Calcd. for \(\hbox {C}_{25}\hbox {H}_{25}\hbox {ClN}_{3}\hbox {O}\) 418.9, found 418.9 \([\hbox {M}+\hbox {H}]^{+}\); Elem. anal. Calcd. (%) for \(\hbox {C}_{25}\hbox {H}_{24}\hbox {ClN}_{3}\hbox {O}\): C, 71.85; H, 5.79; N, 10.05; found (%): C, 71.79; H, 5.71; N, 10.01.
2.2.3 2-((4-(1H-benzo[d]imidazol-2-yl) piperidin-1-yl)(4-chlorophenyl)methyl)-4-bromophenol \((\mathbf{4c})\)
Yield: 85% (1.727 g); White solid; M.p.: 201–\(203\,{^{\circ }}\hbox {C}\); IR (ATR, \(\hbox {cm}^{-1})\): 3365 (NH), 3526 (OH); \({}^{1}\hbox {H}\) NMR (\(\hbox {CDCl}_{3}\), 400 MHz) \(\delta \): 1.40 (m, 4H, \(J = 9.6\,\hbox {Hz}\)), 2.16 (t, 4H, \(J = 7.6\,\hbox {Hz}\)), 2.73 (m, 1H, \(J = 9.6\,\hbox {Hz}\)), 4.73 (s, 1H, NH), 5.09 (s, 1H), 5.34 (s, 1H, OH), 6.86 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 6.95 (d, 2H, \(J = 8.8\,\hbox {Hz}\)), 7.09 (s, 1H), 7.39 (t, 2H, \(J = 6.8\,\hbox {Hz}\)), 7.50 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.71 (d, 2H, \(J = 9.2\,\hbox {Hz}\)), 8.00 (d, 2H, \(J = 9.6\,\hbox {Hz}\)) ppm; \({}^{13}\hbox {C}\) NMR (\(\hbox {CDCl}_{3}\), 100 MHz) \(\delta \): 30.1 (2C), 35.6, 51.4 (2C), 75.5, 115.2 (2C), 116.2, 119.2, 121.7, 123.0 (2C), 123.3, 129.3 (2C), 129.6 (2C), 131.8, 134.4, 138.8 (2C), 140.8, 141.4, 156.8 ppm; LCMS: m/z Calcd. for \(\hbox {C}_{25}\hbox {H}_{24}\hbox {BrClN}_{3}\hbox {O}\) 497.0, found 497.4 \([\hbox {M}+\hbox {H}]^{+}\); Elem. anal. Calcd. (%) for \(\hbox {C}_{25}\hbox {H}_{23}\hbox {BrClN}_{3}\hbox {O}\): C, 60.44; H, 4.67; N, 8.46; found (%): C, 60.38; H, 4.60; N, 8.39.
2.2.4 2-((4-(1H-benzo[d]imidazol-2-yl) piperidin-1-yl)(phenyl)methyl)-4-bromophenol \((\mathbf{4d})\)
Yield: 86% (1.626 g); White solid; M.p.: 199–\(201\,{^{\circ }}\hbox {C}\); IR (ATR, \(\hbox {cm}^{-1})\): 3361 (NH), 3530 (OH); \({}^{1}\hbox {H}\) NMR (\(\hbox {CDCl}_{3}\), 400 MHz) \(\delta \): 1.56 (m, 4H, \(J = 9.6\,\hbox {Hz}\)), 2.36 (t, 4H, \(J = 7.2\,\hbox {Hz}\)), 2.75 (m, 1H, \(J = 7.6\,\hbox {Hz}\)), 4.87 (s, 1H, NH), 5.04 (s, 1H), 5.32 (s, 1H, OH), 6.69 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.09 (s, 1H), 7.36 (t, 2H, \(J = 6.8\,\hbox {Hz}\)), 7.42 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.67 (t, 1H, \(J = 6.4\,\hbox {Hz}\)), 7.88 (t, 2H, \(J = 6.8\,\hbox {Hz}\)), 8.02 (d, 2H, \(J = 9.6\,\hbox {Hz}\)), 8.17 (d, 2H, \(J = 9.2\,\hbox {Hz}\)) ppm; \({}^{13}\hbox {C}\) NMR (\(\hbox {CDCl}_{3}\), 100 MHz) \(\delta \): 30.0 (2C), 35.5, 51.2 (2C), 75.5, 115.2 (2C), 116.4, 119.2, 121.7, 123.1 (2C), 123.3, 126.5, 128.3 (2C), 129.4 (2C), 134.3, 138.8 (2C), 141.5, 142.7, 156.8 ppm; LCMS: m/z Calcd. for \(\hbox {C}_{25}\hbox {H}_{25}\hbox {BrN}_{3}\hbox {O}\) 463.3, found 463.3 \([\hbox {M}+\hbox {H}]^{+}\); Elem. anal. Calcd. (%) for \(\hbox {C}_{25}\hbox {H}_{24}\hbox {BrN}_{3}\hbox {O}\): C, 64.94; H, 5.23; N, 9.09; found (%): C, 64.88; H, 5.19; N, 9.01.
2.2.5 2-((4-(1H-benzo[d]imidazol-2-yl) piperidin-1-yl)(phenyl)methyl)-4-nitrophenol \((\mathbf{4e})\)
Yield: 80% (1.401 g); White solid; M.p.: 200–\(202\,{^{\circ }}\hbox {C}\); IR (ATR, \(\hbox {cm}^{-1})\): 3369 (NH), 3528 (OH); \({}^{1}\hbox {H}\) NMR (\(\hbox {CDCl}_{3}\), 400 MHz) \(\delta \): 1.56 (m, 4H, \(J = 9.6\,\hbox {Hz}\)), 2.37 (t, 4H, \(J = 6.8\,\hbox {Hz}\)), 2.76 (m, 1H, \(J = 7.2\,\hbox {Hz}\)), 4.87 (s, 1H, NH), 5.12 (s, 1H), 5.37 (s, 1H, OH), 7.07 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 7.36 (t, 2H, \(J = 7.2\,\hbox {Hz}\)), 7.43 (t, 1H, \(J = 6.8\,\hbox {Hz}\)), 7.68 (t, 2H, \(J = 6.4\,\hbox {Hz}\)), 7.87 (d, 2H, \(J = 9.6\,\hbox {Hz}\)), 7.95 (d, 2H, \(J = 9.6\,\hbox {Hz}\)), 8.07 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 8.16 (s, 1H) ppm; \({}^{13}\hbox {C}\) NMR (\(\hbox {CDCl}_{3}\), 100 MHz) \(\delta \): 29.7 (2C), 35.4, 51.8 (2C), 75.0, 115.1 (2C), 116.3, 120.5, 123.2 (2C), 126.1 (2C), 126.7, 128.4 (2C), 129.2 (2C), 138.9 (2C), 141.0, 141.5, 142.8, 164.2 ppm; LCMS: m/z Calcd. for \(\hbox {C}_{25}\hbox {H}_{25}\hbox {N}_{4}\hbox {O}_{3}\) 429.9, found 429.4 \([\hbox {M}+\hbox {H}]^{+}\); Elem. anal. Calcd. (%) for \(\hbox {C}_{25}\hbox {H}_{24}\hbox {N}_{4}\hbox {O}_{3}\): C, 70.08; H, 5.65; N, 13.08; found (%): C, 70.00; H, 5.59; N, 13.00.
2.2.6 2-((4-(1H-benzo[d]imidazol-2-yl) piperidin-1-yl)(phenyl)methyl)-4-methoxyphenol \((\mathbf{4f})\)
Yield: 94% (1.589 g); White solid; M.p.: 205–\(207\,{^{\circ }}\hbox {C}\); IR (ATR, \(\hbox {cm}^{-1})\): 3374 (NH), 3527 (OH); \({}^{1}\hbox {H}\) NMR (\(\hbox {CDCl}_{3}\), 400 MHz) \(\delta \): 1.57 (m, 4H, \(J = 9.2\,\hbox {Hz}\)), 2.33 (t, 4H, \(J = 6.8\,\hbox {Hz}\)), 2.75 (m, 1H, \(J = 7.2\,\hbox {Hz}\)), 3.81 (s, 3H, \(\hbox {OCH}_{3})\), 4.90 (s, 1H, NH), 5.06 (s, 1H), 5.36 (s, 1H, OH), 6.63 (d, 1H, \(J = 8.8\,\hbox {Hz}\)), 6.74 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.03 (s, 1H), 7.36 (t, 2H, \(J = 6.8\,\hbox {Hz}\)), 7.43 (t, 1H, \(J = 6.4\,\hbox {Hz}\)), 7.67 (t, 2H, \(J = 6.4\,\hbox {Hz}\)), 7.87 (d, 2H, \(J = 9.6\,\hbox {Hz}\)), 8.02 (d, 2H, \(J = 9.6\,\hbox {Hz}\)) ppm; \({}^{13}\hbox {C}\) NMR (\(\hbox {CDCl}_{3}\), 100 MHz) \(\delta \): 29.9 (2C), 35.4, 51.6 (2C), 55.8, 76.1, 113.1, 113.8, 115.2 (2C), 117.5, 120.5, 123.2 (2C), 126.0, 128.2 (2C), 129.3 (2C), 138.8 (2C), 141.5, 142.6, 150.2, 153.9 ppm; LCMS: m/z Calcd. for \(\hbox {C}_{26}\hbox {H}_{28}\hbox {N}_{3}\hbox {O}_{2}\) 414.2, found 414.4 \([\hbox {M}+\hbox {H}]^{+}\); Elem. anal. Calcd. (%) for \(\hbox {C}_{26}\hbox {H}_{27}\hbox {N}_{3}\hbox {O}_{2}\): C, 75.52; H, 6.58; N, 10.16; found (%): C, 75.48; H, 6.49; N, 10.09.

2.2.7 -((4-(1H-benzo[d]imidazol-2-yl) piperidin-1-yl)(phenyl)methyl)-4-methylphenol \((\mathbf{4g})\)
Yield: 91% (1.479 g); White solid; M.p.: 204–\(206\,{^{\circ }}\hbox {C}\); IR (ATR, \(\hbox {cm}^{-1})\): 3371 (NH), 3525 (OH); \({}^{1}\hbox {H}\) NMR (\(\hbox {CDCl}_{3}\), 400 MHz) \(\delta \): 1.56 (m, 4H, \(J = 9.6\,\hbox {Hz}\)), 2.20 (s, 3H, \(\hbox {CH}_{3})\), 2.39 (t, 4H, \(J = 6.4\,\hbox {Hz}\)), 2.75 (m, 1H, \(J = 9.6\,\hbox {Hz}\)), 4.77 (s, 1H, NH), 5.07 (s, 1H), 5.32 (s, 1H, OH), 6.78 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 6.88 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 7.00 (s, 1H), 7.39 (t, 2H, \(J = 6.8\,\hbox {Hz}\)), 7.50 (t, 1H, \(J = 6.4\,\hbox {Hz}\)), 7.73 (t, 2H, \(J = 7.2\,\hbox {Hz}\)), 7.84 (d, 2H, \(J = 9.2\,\hbox {Hz}\)), 7.97 (d, 2H, \(J = 8.8\,\hbox {Hz}\)) ppm; \({}^{13}\hbox {C}\) NMR (\(\hbox {CDCl}_{3}\), 100 MHz) \(\delta \): 21.6, 30.1 (2C), 35.6, 51.4 (2C), 76.1, 115.2 (2C), 116.1, 119.3, 123.0 (2C), 126.0, 127.8, 128.2 (2C), 129.3 (2C), 131.5 (2C), 138.8 (2C), 141.5, 142.6, 154.8 ppm; LCMS: m/z Calcd. for \(\hbox {C}_{26}\hbox {H}_{26}\hbox {N}_{3}\hbox {O}\) 396.2, found 396.4 \([\hbox {M}-\hbox {H}]^{-}\); Elem. anal. Calcd. (%) for \(\hbox {C}_{26}\hbox {H}_{27}\hbox {N}_{3}\hbox {O}\): C, 78.56; H, 6.85; N, 10.57; found (%): C, 78.48; H, 6.78; N, 10.49.
2.2.8 2-((5-Bromo-1H-indol-1-yl)(phenyl) methyl)phenol \((\mathbf{4h})\)
Yield: 88% (1.361 g); White solid; M.p.: 189–\(191\,{^{\circ }}\hbox {C}\); IR (ATR, \(\hbox {cm}^{-1})\): 3521 (OH). \({}^{1}\hbox {H}\) NMR (\(\hbox {CDCl}_{3}\), 400 MHz) \(\delta \): 5.33 (s, 1H, OH), 6.24 (s, 1H), 6.42 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 6.69 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.07 (t, 1H, \(J = 6.4\,\hbox {Hz}\)), 7.34 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.43 (t, 1H, \(J = 6.8\,\hbox {Hz}\)), 7.52 (d, 2H, \(J = 8.8\,\hbox {Hz}\)), 7.67 (t, 1H, \(J = 6.4\,\hbox {Hz}\)), 7.88 (t, 2H, \(J = 6.8\,\hbox {Hz}\)), 7.97 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 8.08 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 8.16 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 8.22 (s, 1H) ppm; \({}^{13}\hbox {C}\) NMR (\(\hbox {CDCl}_{3}\), 100 MHz) \(\delta \): 73.7, 100.9, 110.0, 113.3, 116.4, 121.0, 121.7, 124.7, 126.5, 127.5, 127.9, 128.1 (2C), 128.9, 129.4 (2C), 129.8, 130.7, 135.3, 137.8, 155.4 ppm; LCMS: m/z Calcd. for \(\hbox {C}_{21}\hbox {H}_{15}\)BrNO 377.2, found 377.4 \([\hbox {M}-\hbox {H}]^{-}\); Elem. anal. Calcd. (%) for \(\hbox {C}_{21}\hbox {H}_{16}\)BrNO: C, 66.68; H, 4.26; N, 3.70; found (%): C, 66.59; H, 4.19; N, 3.65.
2.2.9 2-((5-Bromo-1H-indol-1-yl)(4-chlorophenyl) methyl)phenol \((\mathbf{4i})\)
Yield: 86% (1.451 g); White solid; M.p.: 189–\(191\,{^{\circ }}\hbox {C}\); IR (ATR, \(\hbox {cm}^{-1})\): 3524 (OH); \({}^{1}\hbox {H}\) NMR (\(\hbox {CDCl}_{3}\), 400 MHz) \(\delta \): 5.35 (s, 1H, OH), 6.10 (s, 1H), 6.23 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 6.69 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 6.85 (t, 1H, \(J = 6.8\,\hbox {Hz}\)), 7.06 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.30 (t, 1H, \(J = 6.4\,\hbox {Hz}\)), 7.42 (d, 2H, \(J = 9.6\,\hbox {Hz}\)), 7.47 (d, 2H, \(J = 9.2\,\hbox {Hz}\)), 7.66 (d, 1H, \(J = 8.8\,\hbox {Hz}\)), 7.87 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 8.02 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 8.17 (s, 1H) ppm; \({}^{13}\hbox {C}\) NMR (\(\hbox {CDCl}_{3}\), 100 MHz) \(\delta \): 73.8, 100.9, 110.2, 113.2, 116.3, 121.0, 121.7, 124.8, 127.4, 127.9, 128.4 (2C), 128.8, 129.5 (2C), 129.8, 130.7, 132.0, 135.4, 135.9, 154.5 ppm; LCMS: m/z Calcd. for \(\hbox {C}_{21}\hbox {H}_{16}\)BrClNO 413.7, found 413.4 \([\hbox {M}+\hbox {H}]^{+}\); Elem. anal. Calcd. (%) for \(\hbox {C}_{21}\hbox {H}_{15}\hbox {BrClNO}\): C, 61.11; H, 3.66; N, 3.39; found (%): C, 61.04; H, 3.59; N, 3.31.
2.2.10 2-((5-Bromo-1H-indol-1-yl)(4-chlorophenyl) methyl)-4-methoxyphenol \((\mathbf{4j})\)
Yield: 93% (1.683 g); White solid; M.p.: 179–\(181\,{^{\circ }}\hbox {C}\); IR (ATR, \(\hbox {cm}^{-1})\): 3531 (OH); \({}^{1}\hbox {H}\) NMR (\(\hbox {CDCl}_{3}\), 400 MHz) \(\delta \): 3.80, (s, 3H, -\(\hbox {OCH}_{3})\), 5.37 (s, 1H, OH), 6.29 (s, 1H), 6.36 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 6.63 (d, 1H, \(J = 8.8\,\hbox {Hz}\)), 6.74 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.00 (s, 1H), 7.13 (d, 2H, \(J = 9.6\,\hbox {Hz}\)), 7.35 (d, 2H, \(J = 8.8\,\hbox {Hz}\)), 7.44 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.66 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 7.87 (d, 1H, \(J = 8.8\,\hbox {Hz}\)), 8.12 (s, 1H) ppm; \({}^{13}\hbox {C}\) NMR (\(\hbox {CDCl}_{3}\), 100 MHz) \(\delta \): 55.7, 74.0, 100.9, 110.2, 113.2 (2C), 113.7, 117.5, 121.0, 124.7, 128.3 (2C), 128.9 (2C), 129.4 (2C), 130.7, 131.8, 135.4 (2C), 147.5, 153.5 ppm; LCMS: m/z Calcd. for \(\hbox {C}_{22}\hbox {H}_{18}\hbox {BrClNO}_{2 }\)443.0, found 443.2 \([\hbox {M}+\hbox {H}]^{+}\); Elem. anal. Calcd. (%) for \(\hbox {C}_{22}\hbox {H}_{17}\hbox {BrClNO}_{2}\): C, 59.68; H, 3.87; N, 3.16; found (%): C, 59.61; H, 3.79; N, 3.09.
2.2.11 2-((5-Bromo-1H-indol-1-yl)(4-chlorophenyl) methyl)-4-nitrophenol \((\mathbf{4k})\)
Yield: 82% (1.535 g); White solid; M.p.: 170–\(172\,{^{\circ }}\hbox {C}\); IR (ATR, \(\hbox {cm}^{-1})\): 3528 (OH); \({}^{1}\hbox {H}\) NMR (\(\hbox {CDCl}_{3}\), 400 MHz) \(\delta \): 5.39 (s, 1H, OH), 6.20 (s, 1H), 6.30 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 7.00 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.12 (d, 2H, \(J = 8.8\,\hbox {Hz}\)), 7.29 (d, 2H, \(J = 9.2\,\hbox {Hz}\)), 7.40 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.64 (d, 1H, \(J = 8.8\,\hbox {Hz}\)), 7.75 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 7.87 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 8.03 (s, 1H), 8.18 (s, 1H) ppm; \({}^{13}\hbox {C}\) NMR (\(\hbox {CDCl}_{3}\), 100 MHz) \(\delta \): 72.8, 100.8, 110.1, 113.2, 116.1, 121.0, 124.8, 126.0, 126.5, 128.5 (2C), 128.8 (2C), 129.4 (2C), 130.7, 131.9, 135.5 (2C), 141.0, 161.3 ppm; LCMS: m/z Calcd. for \(\hbox {C}_{21}\hbox {H}_{15}\hbox {BrClN}_{2}\hbox {O}_{3 }\)457.0, found 457.2 \([\hbox {M}+\hbox {H}]^{+}\); Elem. anal. Calcd. (%) for \(\hbox {C}_{21}\hbox {H}_{14}\hbox {BrClN}_{2}\hbox {O}_{3}\): C, 55.11; H, 3.08; N, 6.12; found (%): C, 55.06; H, 3.00; N, 6.03.
2.2.12 2-((5-Bromo-1H-indol-1-yl)(phenyl) methyl)-4-methylphenol \((\mathbf{4l})\)
Yield: 90% (1.444 g); White solid; M.p.: 175–\(177\,{^{\circ }}\hbox {C}\); IR (ATR, \(\hbox {cm}^{-1})\): 3526 (OH); \({}^{1}\hbox {H}\) NMR (\(\hbox {CDCl}_{3}\), 400 MHz) \(\delta \): 2.22 (s, 3H, \(\hbox {CH}_{3})\), 5.33 (s, 1H, OH), 6.10 (s, 1H), 6.27 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 6.56 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 6.78 (d, 1H, \(J = 8.8\,\hbox {Hz}\)), 7.03 (s, 1H), 7.12 (d, 2H, \(J = 8.8\,\hbox {Hz}\)), 7.30 (t, 1H, \(J = 6.4\,\hbox {Hz}\)), 7.41 (t, 2H, \(J = 7.2\,\hbox {Hz}\)), 7.64 (d, 1H, \(J = 8.8\,\hbox {Hz}\)), 7.76 (d, 1H, \(J = 8.8\,\hbox {Hz}\)), 7.87 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 8.02 (s, 1H) ppm; \({}^{13}\hbox {C}\) NMR (\(\hbox {CDCl}_{3}\), 100 MHz) \(\delta \): 21.5, 74.0, 100.9, 110.1, 113.2, 116.3, 121.0, 124.7, 126.2, 127.9 (2C), 128.3 (2C), 128.9, 129.5 (2C), 130.8, 131.5 (2C), 135.6, 137.4, 152.4 ppm; LCMS: m/z Calcd. for \(\hbox {C}_{22}\hbox {H}_{19}\)BrNO 393.3, found 393.4 \([\hbox {M}+\hbox {H}]^{+}\); Elem. anal. Calcd (%) for \(\hbox {C}_{22}\hbox {H}_{18}\)BrNO: C, 67.36; H, 4.62; N, 3.57; found (%): C, 67.29; H, 4.58; N, 3.49.
2.2.13 4-Bromo-2-((5-bromo-1H-indol-1-yl)(phenyl) methyl)phenol \((\mathbf{4m})\)
Yield: 87% (1.626 g); White solid; M.p.: 173–\(175\,{^{\circ }}\hbox {C}\); IR (ATR, \(\hbox {cm}^{-1})\): 3523 (OH); \({}^{1}\hbox {H}\) NMR (\(\hbox {CDCl}_{3}\), 400 MHz) \(\delta \): 5.35 (s, 1H, OH), 6.14 (s, 1H), 6.31 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 6.61 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 6.80 (s, 1H), 7.00 (d, 2H, \(J = 8.8\,\hbox {Hz}\)), 7.13 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 7.31 (t, 1H, \(J = 6.8\,\hbox {Hz}\)), 7.41 (t, 2H, \(J = 7.6\,\hbox {Hz}\)), 7.48 (d, 1H, \(J = 10\,\hbox {Hz}\)), 7.76 (d, 1H, \(J = 8.8\,\hbox {Hz}\)), 7.87 (d, 1H, \(J = 8.8\,\hbox {Hz}\)), 8.02 (s, 1H) ppm; \({}^{13}\hbox {C}\) NMR (\(\hbox {CDCl}_{3}\), 100 MHz) \(\delta \): 73.0, 100.9, 110.2, 113.1, 116.1, 119.2, 121.0, 123.2, 124.6, 126.1, 128.2 (2C), 128.9, 129.3 (2C), 130.1, 130.6, 134.4, 135.7, 137.6, 154.3 ppm; HRMS: m/z Calcd. for \(\hbox {C}_{21}\hbox {H}_{15}\hbox {Br}_{2}\hbox {NONa}\) 480.1600, found 480.1170 \([\hbox {M}+\hbox {Na}]^{+}\); Elem. anal. Calcd. (%) for \(\hbox {C}_{21}\hbox {H}_{15}\hbox {Br}_{2}\)NO: C, 55.17; H, 3.31; N, 3.06; found (%): C, 55.11; H, 3.25; N, 2.99.
2.2.14 4-Bromo-2-((5-bromo-1H-indol-1-yl)(4-chlorophenyl)methyl)phenol \((\mathbf{4n})\)
Yield: 84% (1.688 g); White solid; M.p.: 163–\(165\,{^{\circ }}\hbox {C}\); IR (ATR, \(\hbox {cm}^{-1})\): 3527 (OH); \({}^{1}\hbox {H}\) NMR (\(\hbox {CDCl}_{3}\), 400 MHz) \(\delta \): 5.35 (s, 1H, OH), 6.19 (s, 1H), 6.32 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 6.89 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 7.03 (s, 1H), 7.12 (d, 2H, \(J = 8.8\,\hbox {Hz}\)), 7.29 (d, 1H, \(J = 9.2\,\hbox {Hz}\)), 7.39 (d, 2H, \(J = 9.6\,\hbox {Hz}\)), 7.47 (d, 1H, \(J = 8.8\,\hbox {Hz}\)), 7.76 (d, 1H, \(J = 8.8\,\hbox {Hz}\)), 7.87 (d, 1H, \(J = 9.6\,\hbox {Hz}\)), 8.17 (s, 1H) ppm; \({}^{13}\hbox {C}\) NMR (\(\hbox {CDCl}_{3}\), 100 MHz) \(\delta \): 73.2, 100.9, 110.1, 113.2, 116.0, 119.2, 121.0, 123.2, 124.7, 128.3 (2C), 128.9, 129.5 (2C), 130.1, 130.7, 131.8, 134.4, 135.5 (2C), 154.4 ppm; HRMS: m/z Calcd. for \(\hbox {C}_{21}\hbox {H}_{14}\hbox {Br}_{2}\)ClNONa 514.6000, found 514.0392 \([\hbox {M}+\hbox {Na}]^{+}\); Elem. anal. Calcd. (%) for \(\hbox {C}_{21}\hbox {H}_{14}\hbox {Br}_{2}\)ClNO: C, 51.31; H, 2.87; N, 2.85; found (%): C, 51.25; H, 2.80; N, 2.79.
3 Results and Discussion
We initiated our investigation with the reaction of salicylaldehyde (1a), 4-bromophenylboronic acid (2a) and 2-(piperidin-4-yl)-1H-benzo[d]imidazole (3a) to optimize various reaction conditions in DMF solvent at room temperature. Product formation did not happen when the reaction was performed in the absence of a catalyst. When the reaction was performed in the presence of bromodimethylsulfonium bromide (BDMS), and iodine, under the same reaction conditions, 28% and 29% of the desired adduct (4a) was obtained respectively. However, in all catalysts evaluated, the reaction was slow and stalled at low conversions. Eventually, we focussed on metal catalysts and its screen revealed a 5 mol% nano \(\hbox {Fe}_{3}\hbox {O}_{4}\) provided superior to all catalysts with the benefit of an improved isolated yield (47%) of (4a) (Table 1, entry 9).
The next parameter explored was solvents and the result obtained in dry 1,4-dioxane was significantly better than those conducted in dry DMF, \(\hbox {CH}_{3}\hbox {CN}\), DMSO and toluene. Subsequently, the investigation of the effect of catalyst loading found that the best yield was obtained when 2 mol% nano \(\hbox {Fe}_{3}\hbox {O}_{4}\) was used (Table 1, entry 16) in the present reaction system. On increasing the load of catalyst, the yield of (4a) decreases. This is due to dissociation of the product.
Further optimization of various reactants showed that optimum reaction condition was set at a molar ratio of 1a/2a/3a = 1:1:1. When a mixture of salicylaldehyde (1a), 4-bromophenylboronic acid (2a) and 2-(piperidin-4-yl)-1H-benzo[d]imidazole (3a) in 1,4-dioxane was stirred in the presence of 2 mol% of nano \(\hbox {Fe}_{3}\hbox {O}_{4}\) at room temperature for 2 h, the product 2-((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(4-bromophenyl)methyl)phenol (4a) was obtained in excellent yield (90%).
Under the established reaction conditions, the scope of the nano \(\hbox {Fe}_{3}\hbox {O}_{4}\) mediated Petasis borono-Mannich reaction was explored, with the results summarized in Table 2. Various boronic acids bearing halogen substituents, such as bromo and chloro were well tolerated leading to the expected products (4a–c), (4i–k) and (4n) in excellent yields. The study was further extended to a variety of salicylaldehydes. Salicylaldehydes with electron donating substituents were well-tolerated under the standard reaction conditions, generating the corresponding products (4f and 4g) in 94% and 91% yields respectively. On the other hand, electron withdrawing groups such as bromo was compatible and gave the corresponding product (4c) and (4d) in 85 and 86% yields, respectively. Moreover, when a strong electron withdrawing nitro group was used, the desired product (4e) was obtained in 80% yield. The electron donating salicylaldehydes exhibited relatively higher reactivities than electron withdrawing salicylaldehydes.
In the light of a successful process for the synthesis of 2-((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(phenyl)methyl)phenol, we sought to further extend the scope of this practical approach by replacing 2-(piperidin-4-yl)-1H-benzo[d]imidazole with 5-bromo-1H-indole under the optimal reaction conditions. Following the above protocol, gratifyingly, the reaction worked equally well and gave the corresponding products (4h–n) in excellent yields.
One of the added advantages of this catalyst is that it can readily be separated from the reaction mixture by simply applying an external magnetic field and then reused without any significant loss of catalytic activity. The recovery and reuse of the nano \(\hbox {Fe}_{3}\hbox {O}_{4}\) catalyst were studied for salicylaldehyde (1a), 4-bromophenylboronic acid (2a) and 2-(piperidin-4-yl)-1H-benzo[d]imidazole (3a) in 1,4-dioxane under the established optimal reaction conditions at room temperature. The reaction time was maintained constant in each cycle (2 h), and the results are collected in Table 3. The catalyst was recovered after each cycle by magnetic separation, washed with 1,4-dioxane, dried, weighed and reused in the next cycle. The results showed that the catalyst can be reused four successive cycles without a noticeable drop in its activity.
On the basis of the present results, a plausible mechanism for this magnetite catalyzed Petasis borono-Mannich reaction is illustrated in Scheme 1. Salicylaldehyde is activated by the \(\hbox {Fe}_{3}\hbox {O}_{4}\) catalyst because of its Lewis acid property.[32] The nucleophilic addition of the secondary amine to activated salicylaldehyde produces carbinolamine intermediate, followed by its dehydration to produce iminium ion intermediate. This iminium intermediate would coordinate to the organoboronic acid. The carbon–carbon bond formation would occur by migration of the boronic acid substituent to the electropositive carbon. The final product would be obtained by the liberation of \(\hbox {H}_{3}\hbox {BO}_{3}\). Further, when 5-bromo-1H-indole is used as amine, resonance donating effect is facilitated by bromo group in the aromatic ring. Since bromo group is present in position 5 of the indole ring, it reduces the chance of electron withdrawing effect. So nitrogen in the indole ring facilitates nucleophilic addition.[33]
4 Conclusion
In summary, we have accomplished a novel and convenient one-pot protocol for the synthesis of aminomethylphenol libraries via three component Petasis borono-Mannich reaction. This versatile, environmentally benign and straightforward procedure features a broad substrate scope with inexpensive, non-hygroscopic and non-toxic \(\hbox {Fe}_{3}\hbox {O}_{4}\) magnetic nano catalyst, which is easily recoverable and reusable for four cycles.
References
Kragler A, Hofner G and Wanner K T 2008 Synthesis and Biological Evaluation of Aminomethylphenol Derivatives as Inhibitors of the Murine GABA Transporters mGAT1–mGAT4 Eur. J. Med. Chem. 43 2404
Zettlemoyer A C and Nace D M 1950 Use of Amine Additives to Prevent Drying Loss on Aging Ind. Eng. Chem. 42 491
Halve A K, Dubey P K, Kankoriya A and Tiwari K 2009 4-Phenyldiazenyl 2-(Phenylimino methyl) Phenols; Synthesis and In-Vitro Biological Evaluation as Potential Antibacterial Agents J. Enzyme Inhib. Med. Chem. 24 176
a) Zhang Y S, Cao G K, Peng S X, Dni D Z and Jin L 1986 Studies on Nonsteroidal Anti-inflammatory Drugs: Synthesis and Biological Activity of 4-Hydroxy-3-Aminomethyl-Diphenyl and 4(2)-Cyclohexyl-2(4)-Aminomethyl-Phenol Derivatives Acta Pharm. Sin. 21 345; (b) Itoh H, Konno M, Tokuhiro T, Iguchi S and Hayashi M 1981 2-Acyl-6-Aminomethylphenol Derivatives U.S. Patent 4245099 A; (c) Xia Z N, Cao G K and Peng S X 1988 Synthesis and Quantum Chemical Calculation of 4-Heteroaryl-Mono/Di-Aminomethylphenol Derivatives Acta Pharm Sin. 23 574
Ade S B, Kolhatkar D G and Deshpande M N 2012 Synthesis, Characterization and Biological Studies of 2-[(2-Hydroxy-5-Nitro Benzylidene)-Amino]-4-Methyl-Phenol with Ti(IV) and Zr(IV) Complexes Int. J. Pharma Bio Sci. 3 6
Birrell G W, Chavchich M, Ager A L, Shieh H M, Heffernan G D, Zhao W, Krasucki P E, Saionz K W, Terpinski J, Schiehser G A, Jacobus L R, Shanks G D, Jacobus D P and Edstein M D 2015 JPC-2997, a New Aminomethylphenol with High In Vitro and In Vivo Antimalarial Activities against Blood Stages of Plasmodium Antimicrob. Agents Chemother. 59 170
McCallum F, Harris I, Breda K V, De S L, Stanisic D I, Good M F, Jacobus D P and Edstein M D 2016 Evaluation of the 2-Aminomethylphenol JPC-2997 in Aotus Monkeys Infected with Plasmodium falciparum Antimicrob. Agents Chemother. 60 1948
Chavchich M, Birrell G W, Ager A L, MacKenzie D O, Heffernan G D, Schiehser G A, Jacobus L R, Shanks G D, Jacobus D P and Edstein M D 2016 Lead Selection of a New Aminomethylphenol, JPC-3210, for Malaria Treatment and Prevention Antimicrob. Agents Chemother. 60 3115
Englert H C, Lang H J, Hropot M and Kaiser J 1988 2-Aminomethylphenol Derivatives, a Process for Their Preparation, Their Use, and Pharmaceutical Formulations Based on These Compounds CA 1238908 A
(a) Pan Y G, Chan A and Hochman L 1993 Hair Dye Coupler Compounds U. S. Patent 5183941 A; (b) Umbricht G Dr, Rosato F J and Braun H J Dr 2006 3-Amino-2-Aminomethylphenol Derivatives and Colorants Comprising These Compounds WO 2006086374 A2
Evans J M and Woo J T K 1983 Aminomethyl-Phenol Cross-Linking Compounds U.S. Patent 4369290 A
Lawson J R 1992 Composition and Method CA 2066307 A1
(a) Babaev E R 2006 Aminomethyl Derivatives of 2,4-Di-\(\alpha \)-Methylbenzylphenol as Multifunctional Additives for Lubricating Oils Pet. Chem. 46 206; (b) Parlman R M and Burns L D 1982 Bis(Disubstituted Aminomethyl)Phenols as Ashless Hydrocarbon Additives U. S. Patent 4322304 A
Petasis N A and Boral S 2001 One-Step Three-Component Reaction Among Organoboronic Acids, Amines Tetrahedron Lett. 42 539
Palaska E, Erdogan H, Safak C, Sarac S and Yulug N 1993 Turk J. Med. Sci. 18 209
Shushizadeh M R and Azizyan S 2014 Solvent-Free Preparation of Novel 2-[Phenyl (Pyridine-2-Ylamino) Methyl] Phenols as Pseudo-Betti Processor for Natural Products Jundishapur J. Nat. Pharm. Prod. 9 e17808
(a) Morin M S T, Lu Y, Black D A and Arndtsen B A 2012 Copper-Catalyzed Petasis-Type Reaction: A General Route to \(\alpha \)-Substituted Amides From Imines, Acid Chlorides, and Organoboron Reagents J. Org. Chem. 77 2013; (b) Wang J, Li P, Shenm Q and Song G 2014 Concise Synthesis of Aromatic Tertiary Amines via a Double Petasis–Borono Mannich Reaction Of Aromatic Amines, Formaldehyde, and Organoboronic Acids Tetrahedron Lett. 55 3888
Nanda K K and Trotter B W 2005 Diastereoselective Petasis Mannich Reactions Accelerated by Hexafluoroisopropanol: A Pyrrolidine-Derived Arylglycine Synthesis Tetrahedron Lett. 46 2025
Han W Y, Zuo J, Zhang X M and Yuan W C 2013 Enantioselective Petasis Reaction Among Salicylaldehydes, Amines, and Organoboronic Acids Catalyzed by BINOL Tetrahedron 69 537
Kulkarni A M, Pandit K S, Chavan P V, Desai U V and Wadgaonkar P P 2015 Cobalt Ferrite Nanoparticles: A Magnetically Separable and Reusable Catalyst for Petasis-Borono–Mannich Reaction RSC Adv. 5 70586
Reddy S R S, Reddy B R P and Reddy P V G 2015 Chitosan: Highly Efficient, Green, and Reusable Biopolymer Catalyst for the Synthesis of Alkylaminophenols via Petasis Borono Mannich-Reaction Tetrahedron Lett. 56 4984
Yadav J S, Reddy B V S and Lakshmi P N 2007 Ionic Liquid Accelerated Petasis Reaction: A Green Protocol for the Synthesis of Alkylaminophenols J. Mol. Catal. A: Chem. 274 101
Reddy B R P, Reddy P V G, Kumar D P, Reddy B N and Shankar M V 2016 Rapid Synthesis of Alkylaminophenols via the Petasis Borono–Mannich Reaction Using Protonated Trititanate Nanotubes as Robust Solid–Acid Catalysts RSC Adv. 6 14682
Kumar P, Griffiths K, Lymperopoulou S and Kostakis G E 2016 Tetranuclear \(\text{ Zn }_{2}\text{ Ln }_{2}\) Coordination Clusters as Catalysts in the Petasis Borono-Mannich Multicomponent Reaction RSC Adv. 6 79180
(a) Zeng T, Chen W W, Cirtiu C M, Moores A, Song G H and Li C J 2010 \(\text{ Fe }_{3}\text{ O }_{4}\) Nanoparticles: A Robust and Magnetically Recoverable Catalyst for Three-Component Coupling of Aldehyde, Alkyne and Amine Green Chem. 12 570; (b) Muller R H, Maa Ben S, Weyhers H, Specht F and Lucks J S 1996 Cytotoxicity of Magnetite-Loaded Polylactide, Polylactide/Glycolide Particles and Solid Lipid Nanoparticles Int. J. Pharm. 138 85; (c) Zhang Z H, Lu H Y, Yang S H and Gao J W 2010 Synthesis of 2,3-Dihydroquinazolin-4(1\(H)\)-Ones by Three-Component Coupling of Isatoic Anhydride, Amines, and Aldehydes Catalyzed by Magnetic \(\text{ Fe }_{3}\text{ O }_{4}\) Nanoparticles in Water J. Comb. Chem. 12 643; (d) Sreedhar B, Kumar A S and Reddy P S 2010 Magnetically Separable \(\text{ Fe }_{3}\text{ O }_{4}\) Nanoparticles: An Efficient Catalyst for the Synthesis of Propargylamines Tetrahedron Lett. 51 1891; (e) Kassaee M Z, Motamedi E, Movassagh B and Poursadeghi S 2013 Iron-Catalyzed Formation of C–Se and C–Te Bonds Through Cross Coupling of Aryl Halides with Se(0) and Te(0)/Nano-\(\text{ Fe }_{3}\text{ O }_{4}\)@GO Synthesis 45 2337
Firouzabadi H, Iranpoor N, Gholinejad M and Hoseini J 2011 Magnetite (\(\text{ Fe }_{3}\text{ O }_{4})\) Nanoparticles-Catalyzed Sonogashira-Hagihara Reactions in Ethylene Glycol under Ligand-Free Conditions Adv. Synth. Catal. 353 125
Esfahani M N, Hoseini S J and Mohammadi F 2011 \(\text{ Fe }_{3}\text{ O }_{4}\) Nanoparticles as an Efficient and Magnetically Recoverable Catalyst for the Synthesis of 3,4-Dihydropyrimidin-2(1\(H)\)-Ones Under Solvent-Free Conditions Chin. J. Catal. 32 1484
Safari J and Zarnegar Z 2012 Magnetic \(\text{ Fe }_{3}\text{ O }_{4}\) Nanoparticles as a Highly Efficient Catalyst for the Synthesis of Imidazoles Under Ultrasound Irradiation Iran. J. Catal. 2 121
Saikia P K, Sarmah P P, Borah B J, Saikia L, Saikia K and Dutta D K 2016 Stabilized \(\text{ Fe }_{3}\text{ O }_{4}\) Magnetic Nanoparticles Into Nanopores of Modified Montmorillonite Clay: A Highly Efficient Catalyst for the Baeyer–Villiger Oxidation Under Solvent Free Conditions Green Chem. 18 2843
Govan J and Gunko Y K 2014 Recent Advances in the Application of Magnetic Nanoparticles as a Support for Homogeneous Catalysts Nanomaterials 4 222
(a) Chacko P and Shivashankar K 2017 Nano Structured Spinel \(\text{ Co }_{3}\text{ O }_{4}\)-Catalyzed Four Component Reaction: A Novel Synthesis of Ugi Adducts from Aryl Alcohols as a Key Reagent Chin. Chem. Lett. 28 1619; (b) Chacko P and Shivashankar K 2018 \(\text{ I }_{2}\)-Catalyzed One-Pot Synthesis of Benzofuro/Thieno[2,3-\(b\)]Pyrrole Motifs Tetrahedron 74 1520; (c) Chacko P and Shivashankar K 2018 Montmorillonite K10-Catalyzed Synthesis of N-Fused Imino-1,2,4-Thiadiazolo Isoquinoline Derivatives Synth. Commun. 48 1363
Shekouhy M, Moaddeli A and Nezhad A K 2016 Magnetic \(\text{ Fe }_{3}\text{ O }_{4}\)–\(\text{ BF }_{3}\): Highly Efficient Lewis Acid Catalyst for the Synthesis of \(\alpha \)-Aminonitriles Res. Chem. Intermed. 42 3805
Follmann M, Gaul F, Schafer T, Kopec S and Hamley P 2005 Petasis Boronic Mannich Reactions of Electron-Poor Aromatic Amines under Microwave Conditions Synlett 1009
Acknowledgements
We are grateful for the financial support from the Department of Science and Technology - Science and Engineering Research Board (DST-SERB), India, under Fast Track Young Scientist Scheme (No. SB/FT/CS-028/2013 dated: 09.06.2014, 24.09.2015 and 12.09.2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chacko, P., Shivashankar, K. Synthesis of aminomethylphenol derivatives via magnetic nano \(\hbox {Fe}_{3}\hbox {O}_{4}\) catalyzed one pot Petasis borono-Mannich reaction. J Chem Sci 130, 154 (2018). https://doi.org/10.1007/s12039-018-1560-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1560-y