Abstract
Direct dehydrogenative alkenylation of N-arylpyrazoles by acrylates has proven to be efficient under Ru(II) carboxylate complexes SPS-BPY and SPS-DB-BPY catalyst along with \(\hbox {Cu(OAc)}_{2}{\cdot }\hbox {H}_{2}\hbox {O}\) as an oxidant. The reaction was found to be atom-economical and was characterized by a number of substituted N-arylpyrazoles with excellent chemoselectivity. The reaction allowed a direct alkenylation to be performed under the eco-friendly solvent system. The diverse functional group tolerance of this protocol opened up a new insight to the use of ruthenium(II) bipyridine complexes as a productive method for the oxidative heterocoupling of N-arylpyrazole.
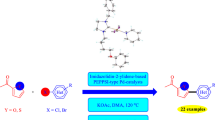
Graphical Abstract
Synopsis Ruthenium(II)-Bipyridine catalyst was used for directive C–C alkenylation of arylpyrazoles and aryloxazolines under additive- and co-catalyst-free mild conditions. The comprehensive substrate scope and high chemoselectivity make the protocol versatile. The method prevents the formation of homo-coupled byproducts thereby challenging the traditional alkenylation reactions and opens up a new genre in ruthenium catalysis.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The traditional Heck coupling reaction for the construction of new C–C bonds can be bartered with transition metal-catalyzed alkenylation of arenes with alkynes which is a more efficient atom-economical reaction. [1] Early reports on catalytic C–C bond formation via ortho-metalated complexes have laid the foundation. [2] The transition metal-catalyzed ortho-alkenylation of substituted aromatics with alkenes via C–H bond activation is one of the powerful methods to synthesize substituted alkenes in a highly regio- and stereoselective manner. [3] In 1968, Fujiwara’s group reported a palladium mediated alkenylation of electron-rich aromatics with alkenes via an electrophilic metallation pathway. [4]\({}^{\mathrm{a,b}}\) In 1993, Murai’s group reported a ruthenium-catalyzed highly regioselective ortho-alkylation of electron-deficient aromatic ketones with alkenes via a chelation-assisted oxidative addition pathway. [4]\({}^{\mathrm{c}}\) Recently, a less expensive and easily affordable ruthenium catalyst has gained much attention for this reaction. [5] Use of \([\hbox {RuH}_{2}(\hbox {CO})(\hbox {PPh}_{3})_{3}]\) as catalyst in the C–H-activated alkenylation of arenes with alkynes was first pioneered by Kakiuchi and Trost, [6] following which several reports on different transition metals like ruthenium, [7] rhodium, [8] palladium, [9] iridium, [10] rhenium, [11] nickel [12] and cobalt [13] have been successfully applied in C–H bond activation. One of the most powerful strategies for the dissociation and functionalization of C–H bonds is the chelation-assisted C–H activation approach that allows several directing groups like pyridines, [14] pyrazoles, [15] nitroso, [16] sulfoximine, [17] \(\hbox {P}{=}\hbox {O}\) bonds [18] and unsaturated C–C bonds [19] to assist the selective C–H bond functionalization.
Alkenylated products have profound applications in light of their existence in many natural products, agrochemicals, pharmaceuticals, functional materials and also some synthones for production of chemicals. [20] Devising synthetic protocols toward these scaffolds has remained the focus of general interest. Traditionally, multistep reactions like Wittig and Peterson olefination processes have been performed to access these scaffolds. Hydroarylation of alkenes [21] and Mizoroki–Heck coupling [22] have earlier been reported as a pronounced method for the synthesis of alkenylated products. However, the pre-functionalization of starting materials limits the scope of these reactions towards this pivotal framework. Normally, in the C–H bond functionalization reaction via deprotonation pathway, the oxidation step such as a metal with lower oxidation state into the higher oxidation state [Pd(0) to Pd(II), Rh(I) to Rh(III), and Ru(0) to Ru(II)] is required to regenerate the active catalyst. Generally, a stoichiometric amount of inorganic or organic oxidant such as AgOAc, \(\hbox {Ag}_{2}\hbox {O}\), \(\hbox {Cu(OAc)}_{2}\), \(\hbox {Fe(OAc)}_{2}\), \(\hbox {PhI(OAc)}_{2}\), benzoquinone, and \(\hbox {K}_{2}\hbox {S}_{2}\hbox {O}_{8}\) is required to regenerate the active catalyst. In most of the reported metal-catalyzed alkenylation reaction, a stoichiometric amount of \(\hbox {Cu(OAc)}_{2}\) has been used as an oxidant. [23] Recent reports also emphasized on the use of a catalytic amount of \(\hbox {Cu(OAc)}_{2}\) along with air or oxygen for C–C alkenylation reactions. [24, 25] Meanwhile, reports of some palladium-catalyzed alkenylation reactions require the use of organic acid along with molecular oxygen [26] with high reaction temperature. The use of high reaction temperature for the alkenylation of substituted aromatics with alkenes, may lead to the dimerization of alkenes as a side product in most cases. [27] Not only palladium but also ruthenium catalyzed alkenylation reactions, required higher reaction temperature. Therefore, it is high time that a convenient protocol for the alkenylation reaction is developed that avoids the use of drastic conditions thereby, suppressing the formation of homocoupled products.
In this work, we have tried to employ our earlier developed catalyst SPS-BPY and SPS-DB-BPY for the alkenylation of aryl pyrazoles. These two catalysts showed pronounced reactivity in hydroxylation reactions [28] and were expected to show similar results in the case of alkenylation. Eminent catalyst Ru(p-cymene)\((\hbox {MesCO}_{2})_{2}\) was earlier seen to be efficient for coupling reactions. However, substituted Ru(p-cymene)\((\hbox {MesCO}_{2})_{2 }\)(SPS-BPY and SPS-DB-BPY) complexes were also studied in the same aspect and emerged as a promising catalyst for alkenylation.
2 Experimental
2.1 General Information
Thin-layer chromatography plates were visualized by exposure to UV light/iodine. \({}^{1}\hbox {H NMR}\) spectra were obtained on 300 and 400 MHz spectrometers whereas \({}^{13}\hbox {C NMR}\) spectra were obtained on 75 and 125 MHz spectrometers with tetramethylsilane and chloroform-d, respectively, as the internal standard and solvent. Chemical shifts (\(\delta \)) are reported in ppm relative to the residual solvent signal (\(\delta =7.26\) for \({}^{1}\hbox {H NMR}\) and \(\delta =77.0\) for \({}^{13}\hbox {C NMR}\)). Data for \({}^{1}\hbox {H NMR}\) are reported as follows: chemical shift (multiplicity, coupling constant, number of hydrogens). Multiplicity is abbreviated as follows: s (singlet), d (doublet), dd (double doublet), bs (broad singlet), bd (broad doublet), t (triplet), q (quartet), m (multiplet). Mass spectra were recorded on the Shimadzu model LCMS-2010EV system that was equipped with electrospray ionization (ESI) probe.
2.2 Materials
The following chemicals were obtained from Sigma-Aldrich, Alfa Aesar and used as received: Iodo benzene (98% pure, Sigma Aldrich, India) and its derivatives, 1H-pyrazole (98% pure, Sigma Aldrich, India), styrene (99% pure, Alfa Aesar, India) and its derivatives, ethyl acrylate, butylacrylate (\({>}99\%\) pure, Sigma Aldrich, India), \(\hbox {Cu(OAc)}_{2}{\cdot }\hbox {H}_{2}\hbox {O}\) (98% pure, Alfa Aesar, India), CuI (99.5% pure, Sigma Aldrich, India), \(\hbox {Cs}_{2}\hbox {CO}_{3}\) (99% pure, Sigma Aldrich, India), \(\hbox {Fe(acac)}_{3}\) (97% pure, Sigma Aldrich, India). The solvents used for the reactions were AR grade FINAR and solvents used for column chromatography were LR grade. Silica used for column chromatography was either 60–120 or 100–200 as per requirement.
2.3 General procedure for Ruthenium catalyzed alkenylation of aryl pyrazoles and oxazoline
To set up the reaction, 100 mg (2 mol%) \(\hbox {Cu(OAc)}_{2}{\cdot }\hbox {H}_{2}\hbox {O}\), 72 mg (0.5 mmol, 1 equiv.) of 1-phenylpyrazole and alkene (1.25 mmol, 2.5 equiv.) were taken in a cleaned and dried R.B. Then, 5 mol% of \(\hbox {Ru}(\hbox {MesCO}_{2})(\hbox {L})\) (p-cymene) [L-2,2\(^\prime \)-bypyridine or 4,4\(^\prime \)-dibromobipyridine] was added to the R.B. under argon. Thereafter, the reaction was kept under reflux conditions at \(100{-}120~^{\circ }\hbox {C}\) under a sealed condition. After cooling the reaction mixture at room temperature, 10 mL of water and 15 mL EtOAc were added; separated organic layer was washed with 10 mL of \(\hbox {NaHCO}_{3}\) solution several times and dried over anhydrous \(\hbox {Na}_{2}\hbox {SO}_{4}\). The final crude was obtained by evaporating the solvent under reduced pressure. Finally, the products were purified by column chromatography using EtOAc: Hexane mixture.
2.4 Synthesis and characterisation of the phenylpyrazoles derivatives
2.4.1 (E)-Butyl 3-(2-(1H-pyrazol-1-yl) phenyl) acrylate 1a[15]
The general procedure was followed using 1-phenylpyrazole (\(66~\upmu \hbox {L}\), 0.5 mmol) and butyl acrylate (0.049 mL, 0.55 mmol) in ethanol. Purification by column chromatography (Hexane/EtOAc: 90/10) yielded 1a. \({}^{1}\hbox {H NMR}\) (300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 7.77 (d, 1H), 7.71 (d, 1H), 7.63 (d, 1H), 7.59 (d, \(J=16.0\) Hz, 1H), 7.52–7.41 (m, 3H), 6.49 (t, 1H), 6.37 (d, \(J=16.0\) Hz, 1H); 4.16 (t, 2H), 1.67–1.61 (m, 2H), 1.42–1.36 (m, 2H), 0.94 (t, 3H); \(\hbox {ESI-MS: [M+H]}^{+}\) 271.
2.4.2 (E)-Butyl 3-(5-methoxy-2-(1H-pyrazol-1-yl) phenyl) acrylate 1b[15]
The general procedure was followed using 4-methoxy phenylpyrazole (87 mg, 0.5 mmol) and butyl acrylate (0.049 mL, 0.55 mmol) in ethanol. Purification by column chromatography (Hexane/EtOAc: 90/10) yielded 1b.\({}^{1}\hbox {H NMR}\)(300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 7.79 (d, 1H), 7.56 (d, 1H); 7.37 (d, 1H), 7.17 (d, 1H), 7.04 (d, \(J=16.0\) Hz, 1H), 7.00 (dd, 1H), 6.52 (t, 1H), 6.27 (d, \(J=16.0\) Hz, 1H), 4.12 (t, 2H), 3.91 (s,3H), 1.63–1.58 (m, 2H), 1.39–1.35 (m, 2H), 0.88 (t, 3H); \(\hbox {ESI-MS [M+H]}^{+ }301\).
2.4.3 (E)-butyl 3-(5-fluoro-2-(1H-pyrazol-1-yl)phenyl) acrylate 1c[15]
The general procedure was followed using 4-fluoro phenylpyrazole (87 mg, 0.5 mmol) and butyl acrylate (0.049 mL, 0.55 mmol) in ethanol. Purification by column chromatography (Hexane/EtOAc: 90/10) yielded 1c.\({}^{1}\hbox {H NMR}\) (300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 7.76 (d,1H), 7.79 (d,1H), 7.48 (d, \(J=16.0\) Hz, 1H), 7.45–7.38 (m, 2H), 7.20–7.15 (m, 1H), 6.49 (t, 1H), 6.36 (d, \(J=16.0\) Hz, 1H), 4.16 (t, 2H), 1.67–1.61 (m, 2H), 1.44–1.35 (m, 2H), 0.94 (t, 3H); \(\hbox {ESI-MS (M+H)}^{+}\) 289.
2.4.4 (E)-Butyl 3-(2-fluoro-6-(1H-pyrazol-1-yl) phenyl) acrylate 1d[15]
The general procedure was followed using 3-fluoro pheylpyrazoles (87 mg, 0.5 mmol) and butyl acrylate (0.049 mL, 0.55 mmol) in ethanol. Purification by column chromatography (Hexane/EtOAc: 90/10) yielded 1d. \({}^{1}\hbox {H NMR}\) (300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 7.79 (d, 1H), 7.70 (m, 1H), 7.64 (d, 1H), 7.60 (d, \(J=16.021\) Hz, 1H), 7.25 (dd, 1H), 7.17–7.13 (m, 1H), 6.51 (t, 1H), 6.34 (d, \(J=16.021\) Hz, 1H), 4.17 (t, 2H), 1.68–1.61 (m, 2H), 1.43–1.37 (m, 2H), 0.95 (t, 3H); \(\hbox {ESI-MS (M+H)}^{+}\) 289.
2.4.5 1-{2,6-Bis[E-2-(Butoxycarbonyl)ethenyl]p-fluorophenyl}-1H-pyrazole 1e[15]
The general procedure was followed using 4-fluoro phenylpyrazoles (87 mg, 0.5 mmol) and butyl acrylate (0.147 mL, 1.5 mmol) in ethanol under sealed tube conditions for more than 20 h. Purification by column chromatography (Hexane/EtOAc: 90/10) yielded 1e. \({}^{1}\hbox {H NMR}\) (300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 7.82 (d, 1H); 7.49 (d, 1H), 7.02 (d, \(J=16.017\) Hz, 2H), 6.55 (t, 1H), 6.30 (d, \(J=16.017\) Hz, 2H); 4.13 (t, 4H), 1.64–1.58 (m, 4H), 1.39–1.33 (m, 4H), 0.93 (t, 6H); \(\hbox {ESI-MS [M+H]}^{+}\) 415.
2.4.6 1-{2,6-Bis[E-2-(Butoxycarbonyl)ethenyl]m-fluorophenyl}-1H-pyrazole 1f[15]
The general procedure was followed using 3-fluoro phenylpyrazoles (87 mg, 0.5 mmol) and butyl acrylate (0.147 mL, 1.5 mmol) in ethanol under sealed tube conditions for more than 20 h. Purification by column chromatography (Hexane/EtOAc: 90/10) yielded 1f. \({}^{1}\hbox {H NMR}\) (300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 7.84 (d, 1H), 7.70 (dd, 1H), 7.51 (d, 1H), 7.33–7.27 (m, 2H), 6.98 (dd, 2H), 6.56 (t, 1H), 6.31 (dd, 2H), 4.31 (t, 4H), 1.64–1.57 (m, 4H), 1.40–1.33 (m, 4H), 0.93 (t, 6H); \(\hbox {ESI-MS [M+H]}^{+}\) 415.
2.4.7 1-{2,6-Bis[(E)-2-(Butoxycarbonyl)ethenyl]p-methoxyphenyl}-1H-pyrazole 1g[15]
The general procedure was followed using 4-methoxy phenylpyrazoles (87 mg, 0.5 mmol) and butyl acrylate (0.147 mL, 1.5 mmol) in ethanol under sealed tube conditions for more than 20 h. Purification by column chromatography (petroleum ether/\(\hbox {Et}_{2}\hbox {O}: 90/10\)) yielded 1g. \({}^{1}\hbox {H NMR}\) (300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 7.8 (d 1H), 7.47 (d, 1H), 7.22 (s, 2H), 7.04 (d, \(J=16.02\) Hz, 1H), 6.52 (t, 1H), 6.27 (d, \(J=16.02\) Hz, 2H), 4.12 (t, 4H), 3.92 (s, 3H), 1.65–1.59 (m, 4H), 1.40–1.34 (m, 4H), 0.93 (t, 6H). \(\hbox {ESI-MS [M+H]}^{+}\) 427.
2.4.8 (2E)-Butyl 3-(2-(4,5-dihydrooxazol-2-yl)phenyl)acrylate 1h[15]
The general procedure was followed using phenyloxazoline (\(66~\upmu \hbox {L}\), 0.5 mmol) and butylacrylate (0.07 mL, 0.55 mmol) in ethanol for 4 h. Purification by column chromatography (Hexane/EtOAc: 70/30) yielded 1h. \({}^{1}\hbox {H NMR}\) (300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 8.53 (d, \(J=16.0\) Hz, 1H), 7.86 (dd,1H), 7.65 (dd, 1H), 7.51–7.41 (m, 2H), 6.36 (d, \( J=16.0\) Hz, 1H), 4.45 (t, 2H), 4.21 (t, 2H), 4.07 (t, 2H), 1.73–1.65 (m, 2H), 1.49–1.43 (m, 2H), 0.97 (t, 3H); \(\hbox {ESI-MS: (M+H)}^{+}=274\).
2.4.9 (2E)-butyl 3-(2-(4,5-dihydro-4,4-dimethyloxazol-2-yl)phenyl)acrylate 1i[15]
The general procedure was followed using 4,4\(^{\prime }\)-dimethylphenyloxazoline (\(66~\upmu \hbox {L}\), 0.5 mmol) and butylacrylate (0.07 mL, 0.55 mmol) in ethanol for 6 h. Purification by column chromatography (Hexane/EtOAc: 70/30) yielded 1i. \({}^{1}\hbox {H NMR}\) (300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 8.48 (d, \(J=16.0\) Hz, 1H), 7.81 (dd, 1H), 7.65 (dd, 1H), 7.48–7.38 (m, 2H), 6.37 (d, \(J=16\) Hz, 1H), 4.21 (t, 2H), 4.13 (s, 2H), 1.72–1.65 (m,2H), 1.50–1.45 (m, 2H), 1.25 (s, 6H), 0.97 (t, 3H); \(\hbox {ESI-MS: (M+H)}^{+}=302\).
2.4.10 1-{2,6-Bis[(E)-2-(Butoxycarbonyl)ethenyl]p-acetylphenyl}-1H-pyrazole 1j[15]
The general procedure was followed using 1-(4-(1H-pyrazol-1-yl)phenyl)ethanone (93 mg, 0.5 mmol) and butyl acrylate (0.147 mL, 1.5 mmol) in ethanol under sealed tube conditions for more than 20 h. Purification by column chromatography (Hexane/EtOAc: 70/30) yielded 1j. \({}^{1}\hbox {H NMR}\) (300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 8.28 (s, 2H), 7.86 (d, 1H), 7.53 (d, 1H), 7.15 (d, \(J=16\) Hz, 2H), 6.58 (t, 1H), 6.42 (d, \(J=16\) Hz, 2H), 4.15 (t, 4H), 2.71 (s, 3H), 1.65–1.42 (m, 4H), 1.41–1.37 (m, 4H), 0.94 (t, 6H); \(\hbox {ESI-MS: (M+H)}^{+}=439\).
2.4.11 1-{2,6-Bis[(E)-2-(Butoxycarbonyl)ethenyl] benzamidophenyl}-1H-pyrazole 1k[15]
The general procedure was followed using 4-(1H-pyrazol-1-yl)benzonitrile (43 mg, 0.25 mmol) and butyl acrylate (0.147 mL, 1.5 mmol) in ethanol under sealed tube conditions for more than 20 h. Purification by column chromatography (Hexane/EtOAc: 70/30) yielded 1k. \({}^{1}\hbox {H NMR}\) (300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 8.16 (s, 2H), 7.85 (d, 1H), 7.52 (d,1H), 7.11 (d, \(J=16\) Hz, 2H), 6.58 (t, 1H), 6.41 (d, \(J=16\) Hz, 2H), 4.14 (t, 4H), 1.66–1.58 (m, 4H), 1.42–1.34 (m, 4H), 0.93 (t, 6H); \(\hbox {ESI-MS: (M+H)}^{+}=440\).
2.4.12 (E)-ethyl 3-(2-(1H-pyrazol-1-yl)phenyl)acrylate 1l
The general procedure was followed using 1-phenylpyrazole (\(66~\upmu \hbox {L}\), 0.5 mmol) and ethyl acrylate (0.52 mmol) in ethanol. Purification by column chromatography (Hexane/EtOAc: 97/3) yielded 1l. \({}^{1}\hbox {H NMR}\) (300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 7.76 (d, 1H), 7.69 (d, 1H), 7.62 (d, 1H), 7.59 (d, \(J=16.0\) Hz, 1H), 7.53–7.40 (m, 3H), 6.51 (t, 1H), 6.35 (d, \(J=16.0\) Hz, 1H); 4.16 (t, 2H), 0.97 (t, 3H); \(\hbox {ESI-MS: [M+H]}^{+}\) 243.
2.4.13 (E)-ethyl 3-(5-methoxy-2-(1H-pyrazol-1-yl)phenyl)acrylate 1m
The general procedure was followed using 1-phenylpyrazole (\(66 ~\upmu \hbox {L}\), 0.5 mmol) and ethyl acrylate (0.52 mmol) in ethanol. Purification by column chromatography (Hexane/EtOAc: 97/3) yielded 1 m. \({}^{1}\hbox {H NMR}\) (300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 7.71 (d, 1H), 7.55 (d, 1H), 7.36 (dd, 1H), 7.17 (d, 1H), 7.06 (d, \(J=16.0\) Hz, 1H), 6.97–7.04 (m, 1H), 6.51 (t, 1H), 6.36 (d, \(J=16.0\) Hz, 1H); 4.13 (t, 2H), 3.91 (s, 3H), 1.02 (t, 3H); \(\hbox {ESI-MS: [M+H]}^{+}\) 273.
2.4.14 (E)-ethyl 3-(5-fluoro-2-(1H-pyrazol-1-yl)phenyl)acrylate 1n
The general procedure was followed using 1-phenylpyrazole (\(66~\upmu \hbox {L}\), 0.5 mmol) and ethyl acrylate (0.52 mmol) in ethanol. Purification by column chromatography (Hexane/EtOAc: 95/5) yielded 1n. \({}^{1}\hbox {H NMR}\) (300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 7.70 (d, 1H), 7.63 (d, 1H), 7.50 (d, \(J=16.0\) Hz, 1H), 7.36–7.46 (m, 2H), 7.15–7.20 (m, 1H), 6.49 (t, 1H), 6.36 (d, \(J=16.0\) Hz, 1H); 4.16 (t, 2H), 1.10 (t, 3H); \(\hbox {ESI-MS: [M+H]}^{+}\) 261.
3 Results and Discussion
Earlier, phenylpyrazole was chosen to react with acrylates for C–C coupling reactions under solvent-free conditions using \(\hbox {Ru}(\hbox {MesCO}_{2})_{2}\)(p-cymene) catalyst by our group. [15] We were further interested to study the catalytic behaviour of bipyridine ligands on of ruthenium(II) catalyst in C–C alkenylation. Therefore, our as-prepared catalyst SPS-BPY and SPS-DB-BPY that were earlier reported for hydroxylation reactions [27] were further examined for \(\hbox {sp}^{2}\hbox {--}\hbox {sp}^{2}\) alkenylation as depicted in Scheme 1 . The inceptive screening was done using simple phenyl pyrazole and butyl acrylate, SPS-BPY and \(\hbox {Cu(OAc)}_{2}\) as a well-known oxidant for coupling reactions. After some initial success with different catalysts, oxidant and solvent screening was performed towards obtaining improved results. Polar aprotic solvents like DMF, acetonitrile, acetone, etc., did not produce good yields though a relatively moderate yield was obtained under 1,2-DCE medium.
On the other hand, the yield dramatically increased (by 19%) when the reaction was performed under EtOH medium, as detailed in Table 1. The addition of alcoholic solvent facilitated direct coordination and the subsequent H-bond interactions thereby enhancing the solubility and catalytic activity. [29] The respective percentage yields for selective products are tabulated in Table 3. Apart from increasing yields, minimum homocoupling product was obtained under SPS-DB-BPY catalyst in the presence of an alcoholic solvent, e.g., EtOH. Screening with alcoholic solvents of longer chain length, a sharp rise in the steric crowding decreases the yields of the reaction.
The yields of the reaction in comparison to our earlier report [15] were found to diminish which may be attributed to the strong \(\sigma \)-donating power of the bipyridine ligands used in the catalyst. Several oxidant screenings were performed and the best results were found to be with \(\hbox {Cu(OAc)}_{2}\) (2 mol%), as shown in Table 2.
The reaction of phenylpyrazoles and substituted phenylpyrazoles with butyl acrylates and 2 mol% of \(\hbox {Cu(OAc)}_{2}{\cdot } \hbox {H}_{2}\hbox {O}\) exhibited a wealth of functional group tolerance leading to 60–80% yield of E-monoalkenylated derivatives (1a–1d,1h, 1i) and E-dialkenylated derivatives (1e–1g,1j, 1k). The lowest yield was found with methoxy phenylpyrazole compared to its parent compound proving that electron donating groups decelerated the reaction. The presence of electron withdrawing groups at para position increased the yield of the reaction whereas same groups in meta position slows down the reaction. On adding butyl acrylate about 1.5–2 equiv. under sealed tube conditions for more than 20 h and 20 mol% of catalyst, a significant amount of dialkenylated pyrazoles (1e–1g,1j, 1k) were obtained. Initially, with 10 mol% of catalyst and 1.25 mmol of the corresponding alkene partner, only 8% of the dialkenylated 1e product was obtained. Therefore, to enhance the yields of the dialkenylated products excess of catalyst, about double the equivalent of alkene and longer reaction time were employed. On implication of the above mole percentages, a clear hike of 24% was observed for the dialkenylated product 1e.
The reaction is not restricted to the use of butyl acrylates but is also applicable with other acrylates. We have conducted the above alkenylation with ethyl acrylates (Figure 1) exhibiting satisfactory yields of 1l, 1m and 1n. The results are incorporated in Table 3. Apart from ester-substituted alkenes, several other electron-deficient, as well as electron-rich alkenes, are expected to show similar results which are under investigation. The yields of the above derivatives with both catalysts SPS-BPY and SPS-DB-BPY are depicted in Table 3. The highest yield of about 72% was found with para-fluoro derivative, a mild electron withdrawing substituent. Under the optimized conditions, the formation of bis-alkenylated products of oxazolines was difficult. The oxidative addition during the di-alkenylation step is retarded due to the presence of the weak directing group.
Since no additional phosphine ligand was used, the directing atom is unable to coordinate well with the catalyst thereby preventing the oxidative addition. [30] Therefore, the existing reaction conditions lead to exclusive monoalkenylated 1h–1i. In the case of strong electron withdrawing group substituted phenyl-pyrazole derivatives 1j–1k, we have found exclusively bi-coupling products. The electron withdrawing groups highly facilitate the oxidative addition process and probably leads to the formation of bis-alkenylated products. On using 0.5 mmol of the acrylate, 12% of monoalkenylated product and 43% of bis-alkenylated product (1j) were formed. Since with 1:1 ratio of the coupling partners substantial amount of bis-alkenylated product was formed, we enhanced the equivalents of the acrylate and found di-coupled products in 68% yield of 1j. When the reactions were carried out with derivatives that are prone to oxidation in the presence of the oxidant \(\hbox {Cu(OAc)}_{2}\) (e.g., 1j, 1k), it was found that these substrates exclusively show the alkenylated product without affecting the functional group. Therefore, this method can prove to be efficient in terms of functional group tolerance.
4 Conclusions
We have reported the ruthenium-catalyzed C–H alkenylation of phenylpyrazoles and phenyl oxazolines. It is presumed that C–C bond formation enables effective intermolecular alkenylation which proceeded with excellent positional selectivity. The optimized catalyst SPS-BPY and SPS-DB-BPY were found to be highly chemoselective along with \(\hbox {Cu(OAc)}_{2}{\cdot }\hbox {H}_{2}\hbox {O}\) as an oxidant. The use of alcoholic solvents to get better yields make the reaction environment-friendly and reduces the difficulty in work-up procedures, thereby resulting in a cleaner reaction. A range of functional group tolerance was achieved with these catalysts and it opens up an alternative to the conventional routes for alkenylation. Mechanistic details of these catalysts are still under sporadic investigation.
References
Heck R F 1967 Alkyl- and Acylcobalt Carbonyls Containing Olefinic Unsaturation. II. Cyclization of 5-Hexenoylcobalt Tetracarbonyl and Nonterminal Olefin Complexes J. Am. Chem. Soc. 89 5518
Lewis L N and Smith J F 1986 Catalytic carbon-carbon bond formation via ortho-metalated complexes J. Am. Chem. Soc. 108 2728
Arockiam P B, Bruneau C and Dixneuf P H 2012 Ruthenium(II)-Catalyzed C–H Bond Activation and Functionalization Chem. Rev. 112 5879
(a) Fujiwara Y Moritani I Matsuda M and Teranishi S 1968 Cationic Pd(II)-Catalyzed Fujiwara-Moritani Reactions at Room Temperature in Water Tetrahedron Lett. 9 3863; (b) Fujiwara Y, Noritani I, Danno, S Asano R and Teranishi S 1969 Aromatic substitution of olefins. VI. Arylation of olefins with palladium(II) acetate J. Am. Chem. Soc. 91 7166; (c) Kakiuchi F and Murai S 2002 Catalytic C-H/Olefin Coupling Acc. Chem. Res. 35 826
Suzuki C, Morimoto K, Hirano K, Satoh T and Miura M 2014 Ruthenium- and Rhodium-Catalyzed Dehydrogenative ortho-Alkenylation of Benzylamines via Free Amino Group Directed C-H Bond Cleavage Adv. Synth. Catal. 356 1521
Murai S, Kakiuchi F, Sekine S, Tanaka Y, Kamatani A, Sonoda M and Chatani N 1993 Efficient catalytic addition of aromatic carbon-hydrogen bonds to olefins Nature 366 529
Cheng K, Yao B, Zhao J and Zhang Y 2008 \(\text{ RuCl }_{3}\)-Catalyzed Alkenylation of Aromatic C-H Bonds with Terminal Alkynes Org. Lett. 10 5309
Patureau F W, Besset T, Kuhl N and Glorius F 2011 Diverse Strategies toward Indenol and Fulvene Derivatives: Rh-Catalyzed C-H Activation of Aryl Ketones Followed by Coupling with Internal Alkynes J. Am. Chem. Soc. 133 2154
Tsukada N, Mitsuboshi T, Setoguchi H and Inoue Y 2003 Stereoselective cis-Addition of Aromatic C-H Bonds to Alkynes Catalyzed by Dinuclear Palladium Complexes J. Am. Chem. Soc. 125 12102
Satoh T, Nishinaka Y, Miura M and Nomura M 1999 Iridium-Catalyzed Regioselective Reaction of 1-Naphthols with Alkynes at the peri-Position Chem. Lett. 28 615
Kuninobu Y, Kawata A and Takai K 2005 Rhenium-Catalyzed Formation of Indene Frameworks via C-H Bond Activation: [3+2] Annulation of Aromatic Aldimines and Acetylenes J. Am. Chem. Soc. 127 13498
Nakao Y, Kanyiva K S, Oda S and Hiyama T 2006 Hydroheteroarylation of Alkynes under Mild Nickel Catalysis J. Am. Chem. Soc. 128 8146
Gao K, Lee P S, Fujita T and Yoshikai N 2010 Cobalt-Catalyzed Hydroarylation of Alkynes through Chelation-Assisted C-H Bond Activation J. Am. Chem. Soc. 132 12249
Cho S H, Hwang S J and Chang S 2008 Palladium-Catalyzed C-H Functionalization of Pyridine N-Oxides: Highly Selective Alkenylation and Direct Arylation with Unactivated Arenes J. Am. Chem. Soc. 29 9254
Shome S and Singh S P 2015 Solvent-Free Ru(II)-Catalyzed C-H Activation: Synthesis of Alkenyl Arylpyrazole Derivatives Eur. J. Org. 6025
Liu B, Fan Y, Gao Y, Sun C, Xu C and Zhu J 2013 Rhodium(III)-Catalyzed N-Nitroso-Directed C–H Olefination of Arenes. High-Yield, Versatile Coupling under Mild Conditions J. Am. Chem. Soc. 135 468
Dong W, Wang L, Parthasarathy K, Pan F and Bolm C 2013 Rhodium-Catalyzed Oxidative Annulation of Sulfoximines and Alkynes as an Approach to 1,2-Benzothiazines Angew. Chem. Int. Ed. 52 11573
Chary B C, Kim S, Park Y, Kim J and Lee P H 2013 Palladium-Catalyzed C–H Arylation Using Phosphoramidate as a Directing Group at Room Temperature Org. Lett. 15 2692
Gandeepan P and Cheng C H 2012 Allylic Carbon–Carbon Double Bond Directed Pd-Catalyzed Oxidative ortho-Olefination of Arenes J. Am. Chem. Soc. 134 5738
(a) Kumar V and Shaw A K 2008 First total synthesis of (+)-varitriol J. Org. Chem. 73 7526; (b) Ioset J R, Marston A, Gupta M P and Hostettmann K 2001 Five New Prenylated Stilbenes from the Root Bark of Lonchocarpus chiricanus J. Nat. Prod. 64 710
Zhou C and Larock R C 2005 Regio- and Stereoselective Route to Tetrasubstituted Olefins by the Palladium-Catalyzed Three-Component Coupling of Aryl Iodides, Internal Alkynes, and Arylboronic Acids J. Org. Chem. 70 3765
Guis M, Marra C and Farina A 2002 A new efficient resveratrol synthesis Tetrahedron Lett. 43 597
(a) Patureau F W and Glorius F 2010 Rh catalyzed olefination and vinylation of unactivated acetanilides J. Am. Chem. Soc. 132 9982; (b) Brasse M, Cámpora J, Ellman J A and Bergman R G 2013 Mechanistic study of the oxidative coupling of styrene with 2-phenylpyridine derivatives catalyzed by cationic Rhodium (III) via C–H activation J. Am. Chem. Soc. 135 6427
(a) Padala K and Jeganmohan M 2011 Ruthenium-Catalyzed Ortho-Alkenylation of Aromatic Ketones with Alkenes by C–H Bond Activation Org. Lett. 13 6144; (b) Padala K and Jeganmohan M 2012 Highly Regio- and Stereoselective Ruthenium(II)-Catalyzed Direct ortho-Alkenylation of Aromatic and Heteroaromatic Aldehydes with Activated Alkenes under Open Atmosphere Org. Lett. 14 1134
Manikandan R and Jeganmohan M 2017 Recent advances in the ruthenium(II)-catalyzed chelation-assisted C–H olefination of substituted aromatics, alkenes and heteroaromatics with alkenes via the deprotonation pathway Chem. Commun. 53 8931
Wang D H, Engle K M, Shi B F and Yu J Q 2010 Ligand-enabled reactivity and selectivity in a synthetically versatile aryl C–H olefination Science 327 315
Hu X H, Zhang J, Yang X F, Xu Y H and Loh T P 2015 Stereo- and Chemoselective Cross-Coupling between Two Electron-Deficient Acrylates: An Efficient Route to (Z,E)-Muconate Derivatives J. Am. Chem. Soc. 137 3169
Shome S and Singh S P 2017 Design and synthesis of ruthenium bipyridine catalyst: An approach towards low-cost hydroxylation of arenes and heteroarenes Tetrahedron Lett. 58 3743
Dyson P J and Jessop P G 2016 Solvent effects in catalysis: rational improvements of catalysts via manipulation of solvent interactions Catal. Sci. Technol. 6 3302
Li B, Devaraj K, Darcel C and Dixneuf P H 2012 Catalytic C–H bond arylation of aryl imines and oxazolines in water with ruthenium(II)-acetate catalyst Tetrahedron 68 5179
Acknowledgements
SPS and SS thank DST Fast Track Young Scientist Project (CS-83/2012) for funding. SS also thanks AcSIR for PhD registration.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue on Modern Trends in Inorganic Chemistry
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shome, S., Singh, S.P. Ruthenium-bipyridine complex catalyzed C–H alkenylation of arylpyrazole derivatives. J Chem Sci 130, 87 (2018). https://doi.org/10.1007/s12039-018-1478-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1478-4