Abstract
5rolGLP-HV is a promising dual-function peptide for the treatment of diabetes and thrombosis simultaneously. For investigating the therapeutic mechanism of 5rolGLP-HV for type 2 diabetes mellitus (T2DM), STZ-induced diabetic mice were established and treated with 5rolGLP-HV. The results showed that daily water and food intake, blood glucose, serum and pancreatic insulin levels significantly decreased after 5rolGLP-HV treatment with various oral concentrations, and 16 mg/kg was the optimal dose for controlling diabetes. 5rolGLP-HV treatment decreased the MDA levels and the T-SOD activity in serum and pancreatic of diabetic mice (but not up to significant difference), and significantly increased the expression of signal pathways related genes of rolGLP-1, also the density of insulin expression and the numbers of apoptosis cells in islets of diabetic mice were significantly decreased in comparison to the negative diabetic mice. These effects above may be clarified the hypoglycemic mechanisms of 5rolGLP-HV, and 5rolGLP-HV may be as a potential drug for diabetes in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Diabetes mellitus (DM) is a serious systematic metabolic disease characterized by variable degrees of insulin resistance and β-cell dysfunction, insulin deficiency, and persistent hyperglycemia in diabetes is a major factor in developing the serious pathological complications that accompany this disease, such as cardiovascular disease (Thomas et al. 2009; Honardoost et al. 2014; Turner et al. 2016). Type 2 diabetes mellitus (T2DM) has increased dramatically and it accounts for 90–95% of all diabetes (Moller 2001). The pathophysiology of T2DM is a complex process and β-cell function is decreased by about 75% when fasting hyperglycaemia is present in patients with T2DM (Kim and Caprio 2011; Coskun and Bolkent 2014). Because of the rapidly prevalence and strongly associated with enormous social and economic burden of T2DM, assessment of β-cell function in individuals at risk of developing diabetes has been of interest and substantial efforts to understand the pathophysiology and develop new preventive and therapeutic approaches for T2DM has been made in recent years (Kahn et al. 2006; Garcia-Jimenez et al. 2016a; Kwak and Park 2016; Turner et al. 2016).
However, treatment of hyperglycemia in T2DM is usually with oral antidiabetic drug monotherapy, initially. Following the disease progresses, many complications such as cardiovascular diseases would appeared. In order to reduce cardiovascular morbidity and mortality, multitargeted treatment, including antihypertensive, lipid-lowering and antiplatelet drugs, is employing in T2DM patients. Thus, this patient group is at an increased risk of harmful drug–drug interactions (DDIs) (Tornio et al. 2012). 5rolGLP-HV is a fusion peptide that combined functions of rolGLP-1 and rHV. Treatment of 5rolGLP-HV significantly decreased the levels of blood glucose in diabetes mice and delayed the formation of thrombus in thrombosis mice (Ni et al. 2016c). In addition, oral administration of 5rolGLP-HV significantly elevated HOMA-IR but decreased HOMA-IS and HOMA-β, which implied that the islet function and glucose tolerance were impaired in saline solution-treated diabetic mice (Ni et al. 2016a, d). The oral dose of 5rolGLP-HV was selected following the pharmacokinetics of rolGLP-1 (Ma et al. 2014). The toxicity study of 5rolGLP-HV was thoroughly investigated and clearly demonstrated the safety of 5rolGLP-HV (Ni et al. 2016b). Although 5rolGLP-HV is a promising drug in treating diabetes and its complication of thrombus in the future, the molecular mechanisms underlying the hypoglycemic effect of 5rolGLP-HV remain unclear. The aim of this analysis was to evaluate the potential mechanisms of 5rolGLP-HV to treat T2DM by analyzing the signaling pathways of 5rolGLP-HV, decreasing the expression of insulin in islet β cells and restraining apoptosis and oxidation resistance of 5rolGLP-HV in STZ-induced diabetic mice model.
2 Materials and methods
2.1 Animals and treatment
Male C57/BL mice, 3 weeks of age, specific pathogen free (SPF), were used in this experiment. They were purchased from Laboratory Animal Center of the Academy of Military Medical Sciences of China. The animals were acclimated to the housing environment for 7 days and were fastened overnight before being used. Mice were housed in a temperature and humidity controlled room (21 ± 2°C, 50 ± 20%) with a 12-h light/dark cycle. The mice were provided free access to water and food. After a week of adaptive feeding, the mice were randomly divided into normal control group and diabetic group. The diabetic models were developed using a high-fat diet (HFD) for 4 weeks and the normal control groups were given a standard diet (STD). High fat diet consisting of 60% standard diet, 15% lard, 15% sucrose, 5% whole milk powder and 5% yolk.
The experiments were approved and supervised by the Institutional Animal Care and Use Committee of Nankai University. All efforts were made to minimize the number of animals used.
Numerous researchers have reported that STZ was widely used to induce the diabetic animal models (Cemek et al. 2008; Gürpınar et al. 2010; Szkudelski 2001). C57BL/6 J mice were intraperitoneally injected with STZ (Sigma, USA) after 4 weeks of dietary intervention to get the diabetes mice model. The STZ was dissolved in citrate buffer (pH=4.5) at a dose of 50 mg/kg/day for four consecutive days to induce hyperglycemia, while control group was fed standard chow and injected with the equal volume of citrate buffer vehicle alone. One week after injection, the blood was collected from the tail vein to determine the glucose level by using GlucoLeader™ Enhance Glucose Meter and test strips (HMD BioMedical Inc., Hsinchu, Taiwan). Mice whose fasting blood glucose levels were greater than 11.1 mM were considered to be type 2 diabetes mellitus and selected for the study. The diabetes mice were fed on the high-glucose-fat diet for another a week and, then all the normal control mice were divided into 2 groups and diabetic mice were divided into 5 groups, randomly. They were list as below:
-
Group 1 (normal control+saline solution), was treated with saline solution solution in a matched volume;
-
Group 2 (normal control+5rolGLP-HV), receiving 5rolGLP-HV in a matched volume;
-
Group 3 (negative diabetic control+saline solution), was treated with saline solution in a matched volume;
-
Group 4, Group 5, Group 6 and Group 7 (diabetic+5rolGLP-HV) were orally given different dose of 5rolGLP-HV (8, 16, 32, 64 mg/kg), respectively.
All animals were given orally once daily for 3 weeks. Food and water intake, and body weight was measured daily throughout the study. Blood samples were collected from tail vein and fasting blood glucose (FBG) was monitored after 12 h fasting by a blood glucose meter (ONETOUCH UltraEasy, Johnson Medical Devices Co.,Ltd.) before treatment once a week throughout the experimental period.
2.2 Collection of blood and tissue samples
Mice were sacrificed by cervical dislocation at the end of study. Blood samples were collected from the orbital, allowing to clot on ice and subsequently subjected to centrifugation (3500 rpm at 4°C for 10 min). The serum and plasma samples were stored at −80°C for further analysis. Fasting serum insulin was measured with ELISA kits according to the manufacturer’s recommendations. Dissecting the mice and isolating the pancreatic. Small piece of pancreatic (from the tail of the pancreatic to the middle of the body of the pancreatic) was cut and fixed in 10% neutral formalin solution, dehydrated in a graded series of ethanol, and embedded in paraffin. After fixation and embedment, the tissues were sectioned to a thickness of 5 μm for immunohistochemical and apoptosis staining in order to assess the expression of insulin and the numbers of apoptosis cells in islets by optical microscopy. Remaining portion of pancreatic were stored at −80°C for further analysis.
2.3 Lipid peroxidation and T-SOD activity detection
At the end of study period, lipid peroxidation was carried out by measuring the content of malondialdehyde (MDA) in the serum and pancreatic by a MDA assay kit (Jiancheng, China) according to the manufacturer’s recommendations. The activity of antioxidant enzyme which determined by total superoxide dismutase (T-SOD) in serum and pancreatic were determined by using a T-SOD assay kit (Jiancheng, China) according to the manufacturer’s recommendations (Zhou et al. 2015).
2.4 Assessment of serum and pancreatic insulin contents
Pancreatic insulin was extracted by an acid/ethanol method as previously described (Noh et al. 2013). The pancreatic insulin contents were determined using an ELISA kit (KENGEN, China) and normalized by protein content. The serum insulin contents were determined using an ELISA kit according to the manufacturer’s recommendations.
2.5 Analysis of gene expression by reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated from the pancreatic using TRIzol reagent (Takara, Japan) following the protocol provided by the manufacturer. RNA samples were stored at -80°C until analysis. Single stranded complementary DNA (cDNA) was synthesized from 5 µg of total RNA using M-MLV reverse transcriptase (RT) (Takara, Japan) and oligo(dT)15-primers. Real-time PCR was carried out using SYBR Premix Ex TaqTM II (2×, Takara, Japan). Each reaction mixture with a total volume of 20 µl contained 20 ng of cDNA, 10 µl of SYBR Premix Ex TaqTM II (2×), 5 µM of each primer. The thermo-cycle program was set as follows: 95 °C for 30 s followed by 45 cycles of 95°C for 15 s, 60°C for 20 s, and 72 °C for 20s, melting at 60–95°C. The relative gene expression levels were calculated by the 2−∆∆Ct method and all the target genes were corrected by normalization to the expression levels of the housekeeping gene β-actin. Details of the primers for the target gene were listed in table 1.
2.6 Immunohistochemistry and neogenesis examinations of islets
The paraffin embedded sections were de-paraffinated in xylene and rehydrated in series of graded ethanol. The deparaffinized sections were performed to microwave antigen retrieval in Tris-EDTA buffer solution (pH 8.0) for 20 min after boiling of the solution. Cooling the sections to room temperature, and treating them with 3% H2O2 for 10 min to block endogenous peroxidase activity. The sections were incubated with primary antibody of rabbit monoclonal insulin antibody (Abcam, USA) at a dilution of 1:64000 for overnight at 4°C. Subsequently, detected using a one-step immunohistochemistry kit (Sungene, China). Finally the sections were observed with a light microscope. A negative control was performed with the absence of primary antibody. The apoptosis of islets cells were determined by the terminal deoxynucleotidyl transferase (Tdt) mediated dUTP (TUNEL) staining kit (Beyotime, China) according to the manufacturer’s instructions. The negative control sections were prepared by substituting Tdt enzyme with distilled water.
2.7 Statistical analysis
The values were presented as mean ± standard deviation (SD). Student’s t-test was used to determine differences between two groups. Multiple groups were compared by ANOVA with Dunnett’s pair-wise comparisons. The p<0.05 and p<0.01 were considered as statistical significant and dramatically significant, respectively.
3 Results
3.1 Construction of diabetic mice model
After two weeks of STZ-injection, the body weight of diabetic mice were significant increase compared to the control group (18.88±0.28 g vs. 23.08±0.21 g, p<0.05) (figure 1 a), while the average daily food intake and water intake were increased significantly compared to the control mice (3.63±0.53 g/d vs. 2.85±0.32 g/d, p<0.05 and 14.30±1.42 ml/d vs. 4.60±0.25 ml/d, p<0.01) (figure 1 b, c). The blood glucose of diabetic mice also exhibited much higher than the control (24.66±4.57 mmol/L vs. 6.03±0.70 mmol/L, p<0.01) (figure 1d).
3.2 Bioactivity of 5rolGLP-HV in diabetic mice
To obtain the optimal oral dose of 5rolGLP-HV, four dose of 8, 16, 32 and 64 mg/kg body weight were selected for treatment of diabetic mice.
Compared to the normal control, the body weight of diabetic mice was significant decreased during treatments period (p<0.01). The body weight during the treatment within groups exhibited a tendency of increase but had no significant difference compared to the diabetic mice treated with saline solution (table 2).
After 3 weeks of treatment of 5rolGLP-HV, the average daily food intake and water intake were all significantly decreased (p<0.05) within all the groups compared with those before undergoing therapy, while there was no significant change before and after the treatment in normal control groups and negative diabetic control (table 2). After 1-week treatment of 5rolGLP-HV, the descend range of average daily food and water intake presented dose-dependent of different oral doses (table 2). During 2 and 3 week’s treatment, the oral dose at 16, 32 and 64 mg/kg body weight had similar treatment effects, and better than the dose of 8 mg/kg. However, the optimum dose of 5rolGLP-HV was 16 mg/kg.
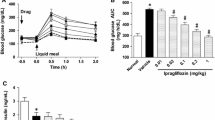
The fasting blood glucoses (FBG) of mice was examined once a week. Similar to the changes of daily food and water intake, the average FBG of negative diabetic mice all significantly increased during treatment period. After 1-week treatment, the decrease range of FBG also presented dose-dependent of different oral doses (table 2). Although the level of FBG among 16, 32 and 64 mg/kg all significantly decreased compared to negative diabetic group, and their hypoglycemic effects were better than 8 mg/kg, the treatment effects were even poorer at the higher doses after 3-week treatment. There was no significant change during the treatment of normal control groups and negative diabetic control (table 2). The results showed that 5rolGLP-HV oral dosage of 16 mg/kg was optimal for maintaining glucose level in diabetic mice.
After 3-week treatment caused the serum insulin level of negative diabetic mice a significant increase in comparison to the normal control (11.49±1.63 mU/L vs. 2.47±0.33 mU/L, p<0.01). 5rolGLP-HV treatment decreased the insulin level significantly within all the groups compared to the negative diabetic mice, and the best oral dose of improving hyperinsulinemia was 16 mg/kg (table 3). Similar to the insulin change in serum, the insulin level in the pancreatic of negative diabetic mice increased significantly compared to the normal control (0.20±0.017 mU/g prot vs. 0.033±0.0055 mU/g prot, p<0.01). Treatment of 5rolGLP-HV significant decreased the insulin level were all within all the groups compared with negative diabetic mice (p<0.05), and their therapeutic effects almost the same (table 3).
3.3 Effects of 5rolGLP-HV on MDA content and SOD activity in serum and pancreatic
The lipid peroxidation was determined by assessing the content of malondialdehyde (MDA) and the activity of antioxidant enzyme was determined by detecting the total superoxide dismutase (T-SOD) in serum and pancreatic of mice. As shown in figure 2, the concentration of MDA in serum and pancreatic in diabetic mice which treated with saline solution were significant increase compared with normal control group (15.37±1.80 nmol/ml vs.8.35±1.20 nmol/ml and 5.99±0.57 nmol/mg prot vs. 1.91±0.68 nmol/mg prot, p<0.05). The MDA content in serum and pancreatic of diabetic mice all decreased after treatment with 5rolGLP-HV, but there were no statistical differences. Similar to the changes of MDA, the activity of T-SOD showed a significant decrease in the diabetic untreated group compared to those of the normal control group (155.7333±12.43 U/ml vs. 105.50±8.97 U/ml and 227.05±15.45 U/mg prot vs. 115.41±22.77 U/mg prot, p<0.05). Oral administration of 5rolGLP-HV to diabetic mice decreased the SOD activity compared to the diabetic mice which treating with saline solution. The content of MDA and the activity of T-SOD in normal mice which oral administration of 5rolGLP-HV were in the normal range all the time.
3.4 Effects of 5rolGLP-HV on signaling pathway related genes expression in pancreatic
The present study measuring the effects of 5rolGLP-HV on the expression of several genes involved in the signal pathways of rolGLP-1 to play its physiological effect in pancreatic. As shown in figure 3, diabetes led to a down-regulation of several rolGLP-1 signal pathway related genes: GLP-1R, PI3K, Akt, MEK and ERK compared with normal control. There was a decreased tendency of PKA in the diabetic mice, although this did not reach statistical significance. After treated by 5rolGLP-HV at an oral dose of 16 mg/kg for 3 weeks, the mRNA levels of GLP-1R, Akt, MEK and ERK were significantly increased versus diabetic mice which treated with saline solution (figure 3), and PKA, PI3K was higher (although not significantly) in 5rolGLP-HV-treated group than the diabetic mice treated with saline solution. These results showed that 5rolGLP-HV play its hypoglycemic action by various signal pathways in pancreatic.
3.5 Effects of 5rolGLP-HV on insulin secretion and apoptosis of islets cells
Immunohistochemistry was used to analysis of pancreatic islets at the end of the study to address primary mechanisms of improving insulin response in 5rolGLP-HV-treatment mice. Pancreatic islets were examined by immunostaining for insulin. Interesting, the immunoreactivity for insulin antibody revealed marked enlargement inside islet-β cells of STZ-induced diabetic mice compared with normal mice. The mean insulin areas in islet-β cells significantly increased in comparison to the normal control group. After treatment of 5rolGLP-HV, the expression of insulin in islet-β cells was reduction and the mean insulin areas in islet-β cells were decreased significantly compared to the diabetic mice which treating with saline solution (figure 4). Consistent with previous research, the diabetic mice had not reached insulin-deficient state and the insulin sensitivity was decreased in the early stage of T2DM. The secretion of insulin in an excessive state to lower the high blood. 5rolGLP-HV treatment increased the insulin sensitivity and the blood glucose was controlled well in a relatively lower level of insulin.
Next, we investigated the apoptosis of islets cells in diabetic mice after treatment with 5rolGLP-HV. Apoptotic cells stained with the terminal dideoxynucleotidyltransferase mediated dUTP nick and end labeling (TUNEL) method well visualized in the pancreata of diabetic mice treated with 5rolGLP-HV. Compared to the normal control group, the brown deposits in islets cells of diabetic mice which induced by STZ were significant increased, indicating that the numbers of apoptosis cells were increased. Treatment with 5rolGLP-HV decreased the areas of brown deposits in diabetic mice (figure 5). The results proved that the apoptosis that induced by diabetes was improve after treatment of 5rolGLP-HV.
4 Discussion
5rolGLP-HV is a dual-function peptide that connect the latent function of GLP-1 and hirudin in treating T2DM and preventing thrombosis (Ni et al. 2016a, d). The present study showed that administration of 5rolGLP-HV improved the “three polys and one little” symptom and decreased the blood glucose and insulin levels in diabetic mice induced by STZ-injection (tables 2 and 3). To the best of our knowledge, this is the first study to provide direct evidence to show the mechanism of action about 5rolGLP-HV playing its biological function in insulin-deficient diabetic mice.
Oxidative stress is the important factor that induce of the occurrence and development of T2DM (Fardoun 2007), and there are now plenty evidence showing that oxidative stress plays a significant role in β-cell deterioration (Pinney and Simmons 2010; Sakai et al. 2003; Sakuraba et al. 2002). Oxidative stress also is a key component in the progressive of diabetic complications (Morsy et al. 2015). Lipid peroxidation degree is considered as one of the most common indicator that used to reflect the oxidative stress level in diabetes. A large number of free radicals were produced which induced by hyperglycemia, and the ability of scavenging free radicals was significantly lower in diabetes. In this study, the lipid peroxidation which determined by assessing the concentration of malondialdehyde (MDA) and the activity of antioxidant enzyme which determined by total superoxide dismutase (T-SOD) in serum and pancreatic tissue were decreased, indicating that the degree of lipid peroxidation and antioxidant defense system dysfunction were improved (figures 2 and 3).
GLP-1 is incapable of enhancing insulin secretion to prevent hypoglycemia when blood glucose is lowered to physiological level (Röder et al. 2016).The actions of GLP-1 are regulated by the activation of a GLP-1 receptor (GLP-1R) (Dailey and Moran 2013). It stimulates the production of intracellular cAMP in PKA-dependent and PKA-independent mechanisms in β cell (Leech et al. 2011). Vitro studies have shown that GLP-1 induces the trans-phosphorylation of EGFR, subsequent the PI3K/Akt pathway was activated in INS cells (Buteau et al. 2001, 2002; Tuduri et al. 2016). In homeostasis, GLP-1 binding to the GLP-1 receptor (GLP-1R) could up-regulate primarily PI3K/Akt by increasing cAMP, and increase the production and cellular uptake of insulin to lower blood glucose (Marathe et al. 2013). the Raf/MEK/ERK (MAPK) signaling pathways also could be activated by the binding of GLP-1 and GLP-1R, leading to the changes of β cells in anti-apoptotic and proliferative (Garcia-Jimenez et al. 2016b; Taniguchi et al. 2006). Most of these effects manifest only at elevated plasma glucose levels, thus accounting for the glucose dependence of GLP-1-potentiated insulin secretion (Ashcroft and Rorsman 2012). In this study, real-time PCR showed that 5rolGLP-HV promoted the genes expression of GLP-1 signaling pathway in the pancreatic of diabetic mice in this study, and the mRNA of GLP-1R, Akt, MEK and ERK were increased 100%, 62%, 55% and 195% compared to the negative diabetic mice, respectively (p<0.05) (figure 3).
Due to the argument of β-cell mass and the expression alterations of key enzymes of β-cell glucose metabolism in insulin-resistant states, the compensatory hypersecretion of insulin will be appeared (Pick et al. 1998). In animal models, as an insulin secretagogue and a β-cell mitogen, GLP-1 had the capable of increasing β-cell proliferation and reducing β-cell apoptosis (Drucker 2006). GLP-1 binding to the GLP-1R, and affecting β-cell function, neogenesis, proliferation, and/or reduce β-cell apoptosis (Kwon et al. 2009). However, this is an essential component of the compensatory mechanisms that maintaining normal glucose tolerance because the Zucker fatty rats with a diabetes-prone sub-line fail to adequately increase their β-cell mass in insulin-resistant states. This is not due to a failure of β-cell proliferation, but rather an enhanced rate of β-cell death due to apoptosis (Bell and Polonsky 2001). Apoptosis is an important factor in inducing the progressive loss of β-cell in T2DM (Rhodes 2005). It has been reported that the β-cell apoptosis was existed in autopsied pancreatic samples of T2DM patients (Butler et al. 2003). Although previous studies have proved treatment with 5rolGLP-HV significantly reversed elevated fasting insulin levels, leading to significant decrease in HOMA-IR but elevation in HOMA-IS, and restored the pancreatic injury induced in the diabetic mice (Ni et al. 2016a), the specific mechanism of 5rolGLP-HV is unclear. The present study showed that oral administration of 5rolGLP-HV decreased the expression of insulin in islet-β cells and the mean insulin areas in islet-β cells were decreased significantly compared to the diabetic mice which treating with saline solution (figure 4). Treatment with 5rolGLP-HV also decreased the areas of brown deposits in diabetic mice, indicating that the apoptosis that induced by diabetes was improve (figure 5).
In summary, the hypoglycemic mechanism of 5rolGLP-HV in typec2 diabetes mice was studied preliminarily for the first time. However, as a dual-function peptide for the treatment of diabetes and thrombosis, the antithrombus study about 5rolGLP-HV relative less than hypoglycemic effect, and the thrombolytic effect of 5rolGLP-HV will remain to be clarified in the future.
Abbreviations
- FBG:
-
fasting blood-glucose
- HV:
-
hirudin
- MDA:
-
malondialdehyde
- rolGLP-1:
-
recombinant oral long-acting GLP-1
- STZ:
-
streptozotocin
- T2DM:
-
type 2 diabetes mellitus
- T-SOD:
-
total superoxide dismutase
References
Ashcroft F and Rorsman P 2012 Diabetes Mellitus and the β Cell: The Last Ten Years. Cell 148 1160–1171
Bell GI and Polonsky KS 2001 Diabetes mellitus and genetically programmed defects in beta-cell function. Nature 414 788–791
Buteau J, Foisy S, and Prentki M 2002 Glucagon-like peptide-1 induces pancreatic beta-cell proliferation via a c-Src-dependent transactivation of the epidermal growth factor receptor. Diabetes 51 A375–A376
Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, and Prentki M 2001 Protein kinase Czeta activation mediates glucagon-like peptide-1-induced pancreatic beta-cell proliferation. Diabetes 50 2237–2243
Butler AE, Janson J, Bonnerweir S, Ritzel R, Rizza RA, and Butler PC 2003 Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52 102–110
Cemek M, Kağa S, Şimşek N, Büyükokuroğlu ME, and Konuk M 2008 Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. J. Nat. Med. 62 284–293
Coskun ZM and Bolkent S 2014 Biochemical and immunohistochemical changes in delta-9-tetrahydrocannabinol-treated type 2 diabetic rats. Acta Histochem. 116 112–116
Dailey MJ and Moran TH 2013 Glucagon-like peptide 1 and appetite. Trends Endocrin. Met. 24 85–91
Drucker DJ 2006 The biology of incretin hormones. Cell Metab. 3 153–165
Fardoun RZ 2007 The use of vitamin E in type 2 diabetes mellitus. Clin. Exp. Hypertension 29 135–148
Gürpınar T, Ekerbiçer N, Uysal N, Barut T, Tarakçı F, and Tuğlu MI 2010 The histologic evaluation of atorvastatin and melatonin treatment on oxidative stress and apoptosis of diabetic rat pancreas. Kafkas Üniversitesi Veteriner Fakültesi Dergisi 16 547–552
Garcia-Jimenez C, Gutierrez-Salmeron M, Chocarro-Calvo A, Garcia-Martinez JM, Castano A, and De la Vieja A 2016a From obesity to diabetes and cancer: epidemiological links and role of therapies. Bri. J. Cancer 114 716–722
Garcia-Jimenez C, Gutierrez-Salmeron M, Chocarro-Calvo A, Garcia-Martinez JM, Castano A, and De la Vieja A 2016b From obesity to diabetes and cancer: epidemiological links and role of therapies. Bri. J. Cancer 114 716–722
Honardoost M, Sarookhani MR, Arefian E, and Soleimani M 2014 Insulin Resistance Associated Genes and miRNAs. Appl.Biochem. Biotechnol. 174 63
Kahn SE, Hull RL, and Utzschneider KM 2006 Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444 840
Kim G and Caprio S 2011 Diabetes and insulin resistance in pediatric obesity. Pediat. Clin. North Am. 58 1355–1361
Kwak SH and Park KS 2016 Recent progress in genetic and epigenetic research on type 2 diabetes. Exp. Mol. Med. 48 e220
Kwon DY, Kim YS, Ahn IS, Kim dS, Kang S, Hong SM, and Park S 2009 Exendin-4 potentiates insulinotropic action partly via increasing beta-cell proliferation and neogenesis and decreasing apoptosis in association with the attenuation of endoplasmic reticulum stress in islets of diabetic rats. J. Pharmacol. Sci. 111 361–371
Leech CA, Dzhura I, Chepurny OG, Kang G, Schwede F, Genieser H, and Holz GG 2011 Molecular physiology of glucagon-like peptide-1 Insulin secretagogue action in pancreatic β cells. Prog. Biophys. Mol. Biol. 107 236–247
Ma B, Hu X, Zhao X, Zhang Y, Li C, Ma Z, Abbas SA, Chen W, et al. 2014 Pharmacokinetics, pharmacodynamics, and cytotoxicity of recombinant orally-administrated long-lasting GLP-1 and its therapeutic effect on db/db mice. Exp. Clin. Endocrinol. Diabetes 226 215–221
Marathe CS, Rayner CK, Jones KL, and Horowitz M 2013 Glucagon-like peptides 1 and 2 in health and disease: A review. Peptides 44 75–86
Moller DE 2001 New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414 821–827
Morsy MA, Heeba GH, and Mahmoud ME 2015 Ameliorative effect of eprosartan on high-fat diet/streptozotocin-induced early diabetic nephropathy in rats. Eur. J. Pharmacol. 750 90–97
Ni Z, Ma X, Wang B, Wang H, Duan H, Li X, Jiang P, Tu P, et al. 2016a Pharmacological effects and pharmacokinetic properties of a dual-function peptide 5rolGLP-HV. Appl. Biochem. Biotechnol. 181 1–12
Ni Z, Wang B, Ma X, Duan H, Jiang P, Li X, Wei Q, Ji X, et al. 2016b Toxicology assessment of a dual-function peptide 5rolGLP-HV in MICE. Appl. Biochem. Biotechnol. 180 1276–1285
Ni Z, Zhang Y, Wang H, Wei Y, Ma B, Hao J, Tu P, Duan H, et al. 2016c Construction of a fusion peptide 5rolGLP-HV and analysis of its therapeutic effect on type 2 diabetes mellitus and thrombosis in mice. Appl. Biochem. Biotechnol. 179 59–74
Ni Z, Zhang Y, Wang H, Wei Y, Ma B, Hao J, Tu P, Duan H, et al. 2016d Construction of a fusion peptide 5rolGLP-HV and analysis of its therapeutic effect on type 2 diabetes mellitus and thrombosis in mice. Appl. Biochem. Biotechnol. 179 1–16
Noh JR, Hwang JH, Kim YH, Kim KS, Gang GT, Kim SW, Kim DK, Shong M, et al. 2013 The orphan nuclear receptor small heterodimer partner negatively regulates pancreatic beta cell survival and hyperglycemia in multiple low-dose streptozotocin-induced type 1 diabetic mice. Int. J. Biochem. Cell Biol. 45 1538–1545
Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonnerweir S, and Polonsky KS 1998 Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes 47 358–364
Pinney SE and Simmons RA 2010 Epigenetic mechanisms in the development of type 2 diabetes. Trends Endocrinol. Metab. 21 223–229
Röder PV, Wu B, Liu Y, and Han W 2016 Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 48 e219
Rhodes CJ 2005 Type 2 diabetes-a matter of beta-cell life and death? Science 307 380–384
Sakai K, Matsumoto K, Nishikawa T, Suefuji M, Nakamaru K, Hirashima Y, Kawashima J, Shirotani T, et al. 2003 Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic beta-cells. Biochem. Biophys. Research Commun. 300 216–222
Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, and Yagihashi S 2002 Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 45 85–96
Szkudelski T 2001 The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 50 537–546
Taniguchi CM, Emanuelli B, and Kahn CR 2006 Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7 85–96
Thomas HE, Mckenzie MD, Angstetra E, Campbell PD, and Kay TW 2009 Beta cell apoptosis in diabetes. Apoptosis 14 1389–1404
Tornio A, Niemi M, Neuvonen PJ, and Backman JT 2012 Drug interactions with oral antidiabetic agents: pharmacokinetic mechanisms and clinical implications. Trends Pharmacol. Sci. 33 312–322
Tuduri E, Lopez M, Dieguez C, Nadal A, and Nogueiras R 2016 Glucagon-Like Peptide 1 Analogs and their Effects on Pancreatic Islets. Trends Endocrin. Met. 27 304–318
Turner N, Zeng XY, Osborne B, Rogers S, and Ye JM 2016 Repurposing Drugs to Target the Diabetes Epidemic. Trends Pharmacol. Sci. 37 379–389
Zhou J, Yan J, Bai Z, Li K, and Huang K 2015 Hypoglycemic activity and potential mechanism of a polysaccharide from the loach in streptozotocin-induced diabetic mice. Carbohydrate Polymers 121 199–206
Acknowledgments
This study is supported by the Key Technologies R&D Program of Tianjin (14ZCZDSY00013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by BJ RAO.
Corresponding editor: BJ Rao
Rights and permissions
About this article
Cite this article
Wang, Y., Li, M. & Ni, Z. Primary study on the hypoglycemic mechanism of 5rolGLP-HV in STZ-induced type 2 diabetes mellitus mice. J Biosci 43, 921–929 (2018). https://doi.org/10.1007/s12038-018-9809-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-018-9809-7