Abstract
Glucagon-like peptide-1 (GLP-1), is currently used to treat type 2 diabetes mellitus and hirudin (HV), plays an important role in controlling thrombosis and cardiovascular diseases. This investigation aimed to develop a fusion peptide 5rolGLP-HV which combined functions of rolGLP-1 and rHV to treat diabetes and thrombosis. In this study, we constructed a fusion gene including five copies of rolGLP-1 and one copy of rHV (5rolGLP-HV). The optimum expression conditions of 5rolGLP-HV in a soluble form were 0.8 mM IPTG induction when OD600 reached 0.6–0.8 and further growing at 25 °C for 9 h. Isolated rolGLP-1 and rHV were acquired by trypsin digestion in vitro, and the concentration of them was determined by HPLC in vivo. Oral administration of 5rolGLP-HV significantly decreased the levels of blood glucose, GHbA1C, TC, and TG in diabetic mice at the time of 3 weeks compared to the saline-treated group (p < 0.05), while the insulin level was reversed significantly (p < 0.05). 5rolGLP-HV treatment significantly shortened the length of thrombus in thrombosis mice compared to the saline-treated group (p < 0.01). These results indicated that 5rolGLP-HV had dual-function in treating diabetes and preventing thrombosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a heterogeneous disorder characterized by a progressive decline in insulin action, followed by the inability of β cells to compensate for insulin resistance and often leads to thrombosis and cardiovascular failure [1–3]. The natural disease progression of T2DM often requires patients to be prescribed multiple antihyperglycemic drugs to control blood glucose level adequately and reduce the risk of long-term complications [4].
Recent studies have demonstrated that incretin hormones including glucagon-like peptide-1 (GLP-1) and GLP-1 analogs have the potential to control glucose and lipid metabolism [5–9]. GLP-1 not only decreases blood sugar levels in diabetes mellitus patients but also stimulates insulin gene expression and inhibits gastric emptying, gastric acid release, glucagon secretion, and food intake [10]. Animal studies showed that GLP-1 increases β-cell mass, maintains β-cell efficiency, and reduces β-cell apoptosis [11]. The biological half-life of natural GLP-1 was only about 2 min due to the second amino-terminal Ala being recognized and cleaved by dipeptidyl peptidase IV (DPP-IV) [12]. Thus, the majority of pharmaceutical approaches have been focused on developing longer-acting analogs of GLP-1 or DPP-IV inhibitor, and they were demonstrated to have insulintropic effects as well as pleiotropic effects on patients and mice models of diabetes [13–16]. Data from in vitro studies showed that GLP-1 and GLP-1 analogs treatment decreased liver triglyceride and improved insulin sensitivity in high-fat diet (HFD) mice [6]. Significant decreases in serum and hepatic triglyceride levels were observed in DPP-IV-deficient mice [17].
T2DM confers an increased risk of vascular disease, with diabetes induced micro- and macrovascular complications being the major causes of morbidity and mortality in patients with T2DM [18, 19]. The overall risk of cardiovascular disease for people with DM increases two- to threefold in men and three- to fivefold in women when compared with people without DM [20]. Thrombosis was one of the major vascular complications suffered from diabetes. Hirudin (HV), as the best thrombin inhibitor thus far, is a 65 amino acid peptide isolated from salivary glands of Hirudomedieinalis [21]. The recombinant hirudin, without sulfur group on the tyrosine residue, exhibited similar activity and anti-thrombin properties as wild protein [22]. In comparison with other anticoagulants, hirudin had many merits, such as it can bind both circulating and clot-bound thrombin, its activity does not require the assistance of intrinsic cofactors, and it cannot be inhibited by natural inhibitors, etc. [23]. These virtues make hirudin a promising candidate for anticoagulant therapy in clinical states involving thrombosis and disseminated intravascular coagulation [24].
This study attempted to develop a recombinant oral 5rolGLP-HV fusion peptide and analyze its function on treating T2DM and thrombosis. This work laid a foundation for the development of dual function oral drugs in treating diabetes and its complication of thrombus in the future.
Materials and Methods
Materials
Ni-NTA agarose was purchased from Bio-Works (Sweden). STZ, fibrinogen, and thrombin were purchased from Sigma (USA). Modified trypsin (TPCK-treated) was purchased from New England BioLabs (Beijing, China). Mouse glycosylated hemoglobin (GHbA1C) ELISA kit, Mouse Triglyceride (TG) ELISA kit, Mouse cholesterol (TC) ELISA kit, and Mouse Insulin (INS) ELISA kit were purchased from Dingguo Biochemistry Technology (Beijing, China). Carrageenan was purchased from Ruji Biotechnology Co. (Shanghai, China). The host strain E. coli DH5α and E. coli BL21 (DE3), vector pET22b(+) (connected the fragments of rolGLP-1) and pMD18-T (connected the fragments of rolGLP-1 and rolGLP-HV) were maintained in our laboratory.
Animals
Male C57BL/6 J mice (3 weeks old) about 12 ± 1 g, male KM mice (3 weeks old) about 21 ± 2 g, and male Sprague Dawley (SD) rats weighing 250–300 g were purchased from Laboratory Animal Center of the Academy of Military Medical Sciences of China (Animal License Number: SCXK-2007-004). The experiments were approved and supervised by the local ethics committee.
Mice and rats were housed individually in an environmentally controlled room (23 ± 2 °C, 50 % relative humidity). Mice were acclimatized for 1 week and housed under a 12-h light/dark cycle and allowed water and food ad libitum [4].
Construction of Recombinant Plasmid pET22b (+)-5rolGLP-HV
The primers used in this study were listed in Table 1. Phosphorylated the primers of rolGLP-1-F, rolGLP-1-R, and 5rolGLP-HV-F to obtain the primers of P-rolGLP-1-F, P-rolGLP-1-R, and P-5rolGLP-HV-F (F = forward primer, R = reverse primer, P = Phosphorylate). This work used the method of blunt-end ligation to connect the fragments of genes and we connected five copies of rolGLP-1 and single copy of rHV. The steps of constructing the gene of 5rolGLP-HV were as follows (Fig. 1a): ①P-rolGLP-1-F and rolGLP-1-R as forward and reverse primers and pMD18-rolGLP-1 as template to amplify P-rolGLP-1. ②rolGLP-1-F and P-rolGLP-1-R as forward and reverse primers and pMD18-rolGLP-1 as template to obtain rolGLP-1-P. P-rolGLP-1 and rolGLP-1-P were ligated by T4 DNA ligase to get 2rolGLP-1. ③5rolGLP-1-F and P-rolGLP-1-R as forward and reverse primers and 2rolGLP-1 as template to obtain 2rolGLP-1-P. ④P-rolGLP-1-F and rHV-R as forward and reverse primers and pMD18-rolGLP-HV as template to obtain P-rolGLP-HV. 2rolGLP-1-P and P-rolGLP-HV were ligated by T4 DNA ligase to get 3rolGLP-HV. ⑤P-rolGLP-1-F and rHV-R as forward and reverse primers and 3rolGLP-HV as template to obtain P-3rolGLP-HV, then connected 2rolGLP-1-P and P-3rolGLP-HV to obtain 5rolGLP-HV. ⑥5rolGLP-HV-His-F and 5rolGLP-HV-His-R as forward and reverse primers and 5rolGLP-HV as template to obtain 5rolGLP-HV-His. The expression vector pET22b (+)-rolGLP-1 and 5rolGLP-HV-His were digested with Kpn I and Hind III, then, they were ligated by T4 DNA ligase (Fig. 1b). Then, the recombinant plasmids were transformed into E. coli DH5α and sequenced after cultivation at 37 °C. Then, the positive one was transformed into E. coli BL21 (DE3) for protein expression.
Optimization of the Soluble Expression and Purification of 5rolGLP-HV
The pET22b(+)-5rolGLP-HV recombinant plasmids were transformed into E. coli BL21 (DE3) cells, and they were incubated into LB liquid medium containing ampicillin (100 μg/mL) and incubated at 37 °C overnight. Subsequently, the mixture was transferred to fresh LB liquid medium (1:100 dilution) containing ampicillin (100 μg/mL) and cultured at 37 °C. In order to induce gene expression, 1 mM IPTG was added when the optical density at 600 nm (OD600) reached 0.6–0.8, and the mixture was incubated at 37 °C and 30 °C for 2, 4, 6, 8 h, 25 °C for 3, 6, 9, 12 h, 20 °C, and 16 °C for 4, 8, 12, 16 h. The confirmed optimal temperature and time of soluble expression were 25 °C for 9 h. Then the cells were induced with 0.4, 0.6, 0.8, and 1.0 mM IPTG when OD600 reached 0.6–0.8 and further growing at 25 °C for 9 h. After 9 h, these cells were pelleted down by centrifugation at 12,000 rpm for 10 min at 4 °C. The cells were suspended in PBS buffer (pH 8.0) and lysed on ice by sonication. The lysate was centrifuged at 12,000 rpm for 30 min at 4 °C. The supernatant and precipitation were collected and analyzed by SDS-PAGE and Western blot [25]. The supernatant containing 5rolGLP-HV was loaded onto a Ni-NTA resin column that had been pre-equilibrated and washed the non-specific bound protein with binding buffer (Tris-HCl 20 mM, pH 8.0, NaCl 500 mM, Triton X-100 1 %) and washing buffer (Tris-HCl 20 mM pH 8.0, NaCl 500 mM, Imidazole 30 mM) for 10 times, respectively. The fractions containing 5rolGLP-HV were collected from the column with elution buffer (Tris-HCl 20 mM pH 8.0, NaCl 500 mM, Imidazole 300 mM) and dialyzed overnight in PBS buffer to remove imidazole. The desalted protein by dialysis was stored at −80 °C after lyophilized for further analysis. The concentration of 5rolGLP-HV was determined by following the method of Bradford [26].
Digestion Assay of 5rolGLP-HV In Vitro
The digestion assay in vitro was conducted in order to test whether 5rolGLP-HV could be cleaved by trypsin into rolGLP-1 and rHV. Briefly, purified 5rolGLP-HV was mixed with the modified trypsin at a ratio of 20:1 (m/m) at 25 °C for 24 h in the reaction buffer. At the same time, the other group includes the same amount of 5rolGLP-HV without the modified trypsin at 25 °C for 24 h in the reaction buffer as the control [27]. The result of digestion was analyzed by SDS-PAGE.
Detected the Concentration of rolGLP-1 and rHV in SD Rats In Vivo
The SD rats (n = 5) were fasted with access to water for 12 h and treated with 5rolGLP-HV at a single dose of 16 mg/kg. The HPLC method was applied to determine the concentration of single copy rolGLP-1 and rHV in rats after oral administration of 5rolGLP-HV. Blood samples were collected from the suborbital vein into heparinized centrifuge tubes at 1, 1.5, 2, 4, 6, 8, 10, and 12 h post oral administration of 5rolGLP-HV. The whole blood samples were centrifuged at 4000 rpm for 15 min to collect plasma. Plasma samples were stored at −20 °C for later HPLC analysis [28].
Hyperglycemia Mice Induction and 5rolGLP-HV Treatment
After 1 week of acclimation, the C57BL/6 J mice were randomly divided into normal control group (n = 7) and high-fat diet group (n = 42), which were fed normal chow and high-fat diet, respectively. The high-fat diet consisted of 15 % lard, 15 % sucrose, 5 % whole milk powder, 5 % yolk, and 60 % normal chow. Water and food were available ad libitum [29, 30].
After 4 weeks, the high-fat diet group was fasted for 17 h and given intraperitoneal injection of 100-mg/kg streptozotocin (STZ) for 1 week while the control group was given vehicle citrate buffer [31]. The tail blood was collected for blood glucose assess. STZ-treated mice with fast plasma glucose > 11.1 mmol/L were considered the diabetic mice and selected for further pharmacological studies.
Diabetic mice were divided into four groups randomly (n = 7 per group): (1) negative control group was treated with saline, (2) positive control group was treated with metformin (300 mg/kg), (3) rolGLP-1 treatment group was treated with rolGLP-1 (11.5 mg/kg), and (4) 5rolGLP-HV treatment group was treated with 5rolGLP-HV (16 mg/kg). The effects of 5rolGLP-HV on diabetic mice were analyzed after 3 weeks of treatment. GHbA1C, TG, TC, and insulin levels were measured by test kits.
Oral Glucose Tolerance Test (OGTT)
Oral glucose tolerance test (OGTT) was performed after 3 weeks of treatment. Mice were fasted for 12–16 h followed by an oral administration of glucose (2 g/kg). Plasma glucose and insulin levels were determined at 0 (baseline), 15, 30, 60, and 120 min after glucose administration [32]. The area under the curve (AUC) was calculated by the trapezoid method follow the formula: AUC(min ⋅ mmol/L) = [1/2 × BG (0 min) + BG (120 min) ] + [BG (90 min) + BG (60 min) − BG (30 min)] × 30 min.
Anticoagulant Activity of 5rolGLP-HV
The method of thrombin direct titration was employed to determine the anticoagulant activity of 5rolGLP-HV, the concrete steps were as follows: firstly, added 200 ul blood fibrinogen solution (0.5 %) into 96-well plate, then supplemented with 100 ul purified 5rolGLP-HV and 5 ul thrombin (100 NIH/ml). Shook the mixture and observed it for 1 min at room temperature. Added 5uL thrombin (NIH 100/ml) per times until the fibrinogen solution condensed within 1 min. The anticoagulant activity of 5rolGLP-HV in 1-mL sample = instillment times × fusion peptide content in 0.1-mL sample × 10 [33].
Thrombus Induction and 5rolGLP-HV Treatment
The KM mice were divided into two groups randomly (n = 6 per group): (1) negative control group was given subcutaneous injection of carrageenan (50 mg/kg) on the back and treated with saline and (2) 5rolGLP-HV treatment group was given subcutaneous injection of carrageenan (50 mg/kg) on the back and treated with 5rolGLP-HV (16 mg/kg). The antithrombotic ability of 5rolGLP-HV was evaluated according to the appeared time and length of thrombosis.
Data Analysis
Results were expressed as mean ± standard error (S.E.M.). The data analysis was performed using SPSS program version 17. Statistical analysis between multiple groups was determined by the one-way analysis of variance (ANOVA) followed by a Bonferroni’s post hoc test. A p value <0.05 was considered statistical significance.
Results
Identification of Recombinant Plasmid pET22b(+)-5rolGLP-HV
The 5rolGLP-HV was successfully inserted into pET22b(+)-rolGLP-1 plasmid identified by Kpn I and Hind III digestion, and the digested products were evaluated by 1.0 % agarose gel electrophoresis. The amplification products of full-length 5rolGLP-HV gene (686 bp) were also verified by agarose gel (Fig. 2).
Identification of the recombinant plasmid pET22b(+)-5rolGLP-HV. Lane M: DNA Marker. Lane 1: recombinant plasmid pET22b(+)-5rolGLP-HV. Lane 2: recombinant plasmid pET22b(+)-5rolGLP-HV was double digested by Kpn I and Hind III. Lane 3: full length of 5rolGLP-HV gene amplified from recombinant plasmid pET22b(+)-5rolGLP-HV
Soluble Expressions and Purification Analysis of 5rolGLP-HV
The vector of pET22b(+)-5rolGLP-HV was transformed into the expression host E. coli BL21 (DE3), and the cells were induced with IPTG to express the target protein. Based on the analysis of soluble expression, the optimal expression conditions were 0.8 mM IPTG induction when OD600 reached 0.6–0.8 and further growing at 25 °C for 9 h (Fig. 3).
Soluble expression conditions optimization of 5rolGLP-HV. a Induced expression in different times of 5rolGLP-HV at 37 °C. Lane M: protein Marker. Lane 1, 3, 5 and 7: ultra-sonication supernatant of induced cells after 2, 4, 6, and 8 h. Lane 2, 4, 6, and 8: ultra-sonication precipitate of induced cells after 2, 4, 6, and 8 h. b Induced expression in different times of 5rolGLP-HV at 25 °C. Lane 1, 3, 5, and 7: ultra-sonication precipitate of induced cells after 3, 6, 9, and 12 h. Lane 2, 4, 6, and 8: ultra-sonication supernatant of induced cells after 3, 6, 9, and 12 h. c Induced expression in different times of 5rolGLP-HV at 16 °C. Lane 1, 3, 5, and 7: ultra-sonication precipitate of induced cells after 4, 8, 12, and 16 h. Lane 2, 4, 6, and 8: ultra-sonication supernatant of induced cells after 4, 8, 12, and 16 h. d Induced expression in different concentrations of IPTG. Lane 1, 3, 5, and 7: ultra-sonication precipitate of induced cells with 0.4, 0.6, 0.8, and 1.0 mM IPTG. Lane 2, 4, 6 and 8: ultra-sonication supernatant of induced cells with 0.4, 0.6, 0.8, and 1.0 mM IPTG. Arrows point to the purpose protein of 5rolGLP-HV
Crude extracts were released by sonication, and the supernatant fractions containing 5rolGLP-HV were loaded into the Ni-NTA affinity chromatography. The purified protein was analyzed by SDS-PAGE and Western blot (Fig. 4). The proportion of 5rolGLP-HV in total protein was about 23.3 %, and the purity of 5rolGLP-HV could > 90 % after purification according to the results of the grayscale scanning (Fig. 4a). The concentration of purified 5rolGLP-HV, which was determined by the Bradford method, was about 0.25 mg/ml.
The expression and purification analysis of 5rolGLP-HV by SDS-PAGE and Western blot. a SDS-PAGE analysis of 5rolGLP-HV. Lane M: protein Marker. Lane 1: non-induced negative control. Lane. 2: pET22b(+)-5rolGLP-HV-BL21 (DE3) induced by IPTG. Lane 3: supernatant of induced cells after ultrasonication. Lane 4: precipitate of induced cells after ultrasonication. Lane 5: 5rolGLP-HV after purification. b Western blotting analysis of 5rolGLP-HV. Arrows point to the purpose protein of 5rolGLP-HV
Digestion Result of 5rolGLP-HV by Trypsin In Vitro
To investigate whether 5rolGLP-HV could be cleaved by trypsin into single copy of rolGLP-1 and rHV in vitro, the digestion test was conducted, and the digestion result was analyzed by SDS-PAGE (Fig. 5). Although there was a part of 5rolGLP-HV not been cut, most of it was cleaved into independent rolGLP-1 and rHV, which located at about 3 and 7 kDa, respectively (Fig. 5b), implying that 5rolGLP-HV might be cleaved by trypsin in vivo and achieved the double function to treat diabetes and thrombosis.
Analysis of 5rolGLP-HV after cleaved by modified trypsin in vitro. a Sequence of nature GLP-1, rolGLP-1 and 5rolGLP-HV. b 5rolGLP-HV was digested by modified trypsin and subjected to SDS-PAGE. Lane M: protein molecular weight marker; Lane 1: un-digested 5rolGLP-HV; Lane 2: trypsin-digested 5rolGLP-HV. Arrows point to the single copy of rolGLP-1 and rHV based on predicted molecular weight
The Concentration of rolGLP-1 and rHV After Oral Administration of 5rolGLP-HV In Vivo
The mean plasma concentration-time profile following a single oral administration of 5rolGLP-HV was shown in Table 2. We could detect the single copy of rolGLP-1 and rHV at different point-in-time in rats after oral administration of 5rolGLP-HV. The concentration of them reached the peak values (10.27 ± 0.67 ng/ml and 4.99 ± 0.47 ng/ml) at about 8 and 6 h, respectively (Table 2). These data indicated that 5rolGLP-HV could be degraded into single copy of rolGLP-1 and rHV and had a relatively longer duration in vivo.
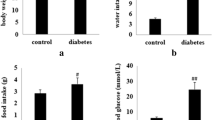
Effects of 5rolGLP-HV on Body Weight, Energy Intake, and Plasma Glucose
Significant increases in water intake, food intake, and plasma glucose level were observed in the STZ-induced diabetic mice, but no significant change in body weight was found compared to the control (Fig. 6). After 3 weeks of treatment, the body weight exhibited similar among the groups (Fig. 6a). Food intake, water intake, and plasma glucose level were significantly decreased compared to the saline-treated group after treating with metformin, rolGLP-1, and 5rolGLP-HV for 3 weeks, and the hypoglycemic effect of 5rolGLP-HV was better than rolGLP-1 (Fig. 6b–d).
Effects of 5rolGLP-HV Treatment on Plasma Glucose Level in OGTT
The OGTT data clearly showed that the plasma glucose of all the mice reached the peak value after 30 min following glucose loading and had a decreasing trend within the next 90 min (Fig. 7a). The diabetic mice exhibited significantly higher postprandial glucose level compared to the control (1474.20 ± 119.29 min · mmol/L vs 382.75 ± 67.88 min · mmol/L, p < 0.05), implying insulin resistance and impaired glucose tolerance (Fig. 7b). Oral administration of 5rolGLP-HV significantly decreased the postprandial glucose level as well as AUC values compared to the saline-treated diabetic mice (889 ± 207.34 min · mmol/L vs 1474.20 ± 119.29 min · mmol/L, p < 0.05) (Fig. 7b).
Effect of 5rolGLP-HV on OGTT in mice. a Blood glucose level of the mice in OGTT. b The AUC of OGTT was calculated. In OGTT, blood samples were collected before and at 30, 60, 90, and 120 min following glucose loading (2 g/kg) on day 21. AUC, the area under the curve. * p < 0.05 vs. control group, # p < 0.05 vs. saline-treated diabetic group
Effects of 5rolGLP-HV Treatment on GHbA1C, TC, TG, and Insulin Levels
Without treatment, the GHbA1C, TC, and TG levels in the saline-treated diabetic mice were significantly higher, and the insulin level was significantly lower than the control group, indicating the occurrence of hyperlipidemia. Treatments of metformin, rolGLP-1, and 5rolGLP-HV significantly decreased the GHbA1C, TC, and TG levels in diabetic mice at the time of 3 weeks compared to the saline-treated group (0.80 ± 0.10 mmol/L vs 1.56 ± 0.18 mmol/L, 4.82 ± 0.22 mmol/L vs 5.96 ± 0.31 mmol/L, 1.44 ± 0.17 mmol/L vs 2.0 ± 0.17 mmol/L, P < 0.05, respectively) (Fig. 8a–c), accompanied by significantly elevated the insulin level (10.65 ± 0.71 mU/L vs 5.5 ± 0.23 mU/L, p < 0.05) (Fig. 8d). Taken together, these results verified that 5rolGLP-HV treatment effectively ameliorated hyperglycemia, and the restoration effect of 5rolGLP-HV was much better than rolGLP-1.
Treatment effects of 5rolGLP-HV on GHbA1C, TC, TG, and insulin levels in mice. a GHbA1C, b TC, c TG, and d insulin levels. Plasma samples were collected from the eyeballs of the mice following glucose loading (2 g/kg) on day 21. GHbA1C, glycosylated hemoglobin A1c; TC total cholesterol, TG triglyceride. *p < 0.05 vs. control group, # p < 0.05 vs. saline-treated diabetic group, ¤ p < 0.05 vs. rolGLP-1 or metformin-treated diabetic group
Anticoagulant and Antithrombotic Activity of 5rolGLP-HV
The titration analysis result indicated that the hirudin activity of 5rolGLP-HV was about 105ATU/mL (Fig. 9a). Oral administration of 5rolGLP-HV extended the appeared time of black tail obviously compared to the saline-treated mice (data not shown). 5rolGLP-HV treatment significantly shortened the length of thrombus in thrombosis mice compared to the saline-treated group (2.92 ± 0.74 cm vs. 7.92 ± 1.15 cm, p < 0.01) (Fig. 9b). These results demonstrated that 5rolGLP-HV could release the active rHV and restrain the thrombin activity thus inhibiting the formation of thrombus.
Results of the thrombin titration and the length change of thrombus in mice tail. a the thrombin titration assay. a1, fibrinogen; a2, condensated fibrinogen. b the length of thrombus in mice tail after treating with 5rolGLP-HV and saline. b1, oral administrated of 5rolGLP-HV (16 mg/kg); b2, oral administrated of saline. The arrows pointing towards the end of the blood clot
Discussion
In our previous study, we used site-directed mutagenesis technology to eliminate the DPP-IV recognition site by substituting Ser for Ala at position 8 and trypsin recognition sites by replacing two Lys residues at positions 26 and 34 with Glu and Asp and obtained a recombinant oral long-acting GLP-1 (rolGLP-1) which could be resistant to DPP-IV and trypsin degradation simultaneously [34]. Animal experiments showed that oral administration of rolGLP-1 could reduce blood glucose significantly and ameliorate the oral glucose tolerance [35, 36]. Hirudin forms a highly stable non-covalent complex with thrombin. It can competitively inhibit the binding of fibrinogen to thrombin thereby blocking the clotting process [37]. These characteristics of them offer a good suitability as oral medicine for T2DM and anti-thrombotic therapy.
In this study, we tried to develop a dual-function peptide 5rolGLP-HV that combined the functions of rolGLP-1 and rHV on one agent to treat diabetes and thrombus at the same time. We designed five copies of rolGLP-1 and one copy of HV in order to ensure that it could offer enough hypoglycemic activity and proper anti-thrombus function to avoid producing too high thrombin activity to cause side-effect like hemophilia.
The digestion assay of 5rolGLP-HV in vitro and the concentration detection about single copy of rolGLP-1 and rHV in vivo demonstrated that 5rolGLP-HV could be degraded into single copy of rolGLP-1 and rHV in vivo and played their biological activity (Fig. 5) (Table 2). Oral administration of 5rolGLP-HV for 3 weeks, the GHbA1C, TC, and TG levels were significantly decreased, and the insulin level was elevated significantly in the blood (Fig. 8). The level of oral glucose tolerance was also improved (Fig. 7). In addition, 5rolGLP-HV also had a stronger anticoagulant and antithrombotic functions (Fig. 9). These results suggested that the 5rolGLP-HV may be decomposed into separate rolGLP-1 and HV after oral administration. It can enter the body through the intestinal absorption and play an important role in prevention on hyperglycemia and thrombosis (Figs. 7, 8 and 9). Although the point for point absorption pathway of 5rolGLP-HV by oral administration is unclear, many studies have showed that oral proteins could be absorbed into blood circulation. When the nattokinase was injected into the mice duodenum, it was absorbed into the bloodstream and played a physiological activity [38]. In addition, a type of protease inhibitor called Bowman-Birk inhibitor (BBI) which is composed of 71 amino acids can play a very good anti-cancer effect by oral administration. The isotope tracer experiment on animals showed that more than half of the BBI entered the blood after oral administration of BBI 2–3 h [39]. However, the concrete mechanism of the protein absorption into the blood circulation after oral administration is unclear and still needs further study.
The track through of 5rolGLP-HV in excreted still remains unknown. Most of 5rolGLP-HV may also go through the kidney and excrete through urine, but the detailed pharmacokinetics experiment is needed to validate it. In addition, the distribution of rolGLP and rHV in the tissues after oral administration of 5rolGLP-HV also needs further study. Moreover, the toxicology research about 5rolGLP-HV is necessary in order to ensure its safety in the future.
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- DPP-IV:
-
Dipeptidyl peptidase IV
- rolGLP-1:
-
Recombinant oral long-acting GLP-1
- HV:
-
Hirudin
- STZ:
-
Streptozotocin
- AUC:
-
Area under the curve
- OD:
-
Optical density
- OGTT:
-
Oral glucose tolerance test
- GHbA1C:
-
Glycosylated hemoglobin A1c
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- FBG:
-
Fasting blood-glucose
- ATU:
-
Antithrombin units
References
Ferroni, P., Basili, S., Falco, A., & Davi, G. (2004). Platelet activation in type 2 diabetes mellitus. Journal of Thrombosis and Haemostasis, 2(8), 1282–1291.
Ajjan, R., & Grant, P. J. (2006). Coagulation and atherothrombotic disease. Atherosclerosis, 186(2), 240–259.
Ajjan, R. A., & Ariens, R. A. (2009). Cardiovascular disease and heritability of the prothrombotic state. Blood Reviews, 23(2), 67–78.
Lennox, R., Porter, D.W., Flatt, P.R., Holscher, C., Irwin, N., Gault, V.A. (2014). Comparison of the independent and combined effects of sub-chronic therapy with metformin and a stable GLP-1 receptor agonist on cognitive function, hippocampal synaptic plasticity and metabolic control in high-fat fed mice. Neuropharmacology, 8622–8630.
Dailey, M. J., & Moran, T. H. (2013). Glucagon-like peptide 1 and appetite. Trends in Endocrinology and Metabolism, 24(2), 85–91.
Samson, S. L., Sathyanarayana, P., Jogi, M., Gonzalez, E. V., Gutierrez, A., Krishnamurthy, R., Muthupillai, R., Chan, L., & Bajaj, M. (2011). Exenatide decreases hepatic fibroblast growth factor 21 resistance in non-alcoholic fatty liver disease in a mouse model of obesity and in a randomised controlled trial. Diabetologia, 54(12), 3093–3100.
Mack, C. M., Moore, C. X., Jodka, C. M., Bhavsar, S., Wilson, J. K., Hoyt, J. A., Roan, J. L., Vu, C., Laugero, K. D., Parkes, D. G., et al. (2006). Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. International Journal of Obesity, 30(9), 1332–1340.
Parlevliet, E. T., de Leeuw van Weenen, J. E., Romijn, J. A., & Pijl, H. (2010). GLP-1 treatment reduces endogenous insulin resistance via activation of central GLP-1 receptors in mice fed a high-fat diet. American Journal of Physiology, Endocrinology and Metabolism, 299(2), E318–E324.
Parlevliet, E. T., Wang, Y., Geerling, J. J., Schroder-Van der Elst, J. P., Picha, K., O’Neil, K., Stojanovic-Susulic, V., Ort, T., Havekes, L. M., Romijn, J. A., et al. (2012). GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE*3-Leiden mice. PLoS ONE, 7(11), e49152.
Gao, M., Tian, H., Ma, C., Gao, X., Guo, W., & Yao, W. (2010). Expression, purification, and C-terminal site-specific PEGylation of cysteine-mutated glucagon-like peptide-1. Applied Biochemistry and Biotechnology, 162(1), 155–165.
Tahrani, A. A., Piya, M. K., Kennedy, A., & Barnett, A. H. (2010). Glycaemic control in type 2 diabetes: targets and new therapies. Pharmacology and Therapeutics, 125(2), 328–361.
Green, B. D., Liu, H. K., McCluskey, J. T., Duffy, N. A., O’Harte, F. P., McClenaghan, N. H., & Flatt, P. R. (2005). Function of a long-term, GLP-1-treated, insulin-secreting cell line is improved by preventing DPP IV-mediated degradation of GLP-1. Diabetes, Obesity & Metabolism, 7(5), 563–569.
Reimann, F., & Gribble, F. M. (2002). Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes, 51(9), 2757–2763.
Drucker, D. J., & Nauck, M. A. (2006). The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet, 368(9548), 1696–1705.
Holst, J. J. (2007). The physiology of glucagon-like peptide 1. Physiological Reviews, 87(4), 1409–1439.
Baggio, L. L., & Drucker, D. J. (2007). Biology of incretins: GLP-1 and GIP. Gastroenterology, 132(6), 2131–2157.
Ben-Shlomo, S., Zvibel, I., Shnell, M., Shlomai, A., Chepurko, E., Halpern, Z., Barzilai, N., Oren, R., & Fishman, S. (2011). Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. Journal of Hepatology, 54(6), 1214–1223.
Madonna, R., & De Caterina, R. (2011). Cellular and molecular mechanisms of vascular injury in diabetes—part I: pathways of vascular disease in diabetes. Vascular Pharmacology, 54(3-6), 68–74.
Honardoost, M., Sarookhani, M. R., Arefian, E., & Soleimani, M. (2014). Insulin resistance associated genes and miRNAs. Applied Biochemistry and Biotechnology, 174(1), 63–80.
Standl, E., Muller, M., & Schnell, O. (2009). The impact of glucose-lowering therapy on cardiovascular outcomes. Best Practice & Research Clinical Endocrinology & Metabolism, 23(3), 401–411.
Markwardt, F. (1985). Pharmacology of hirudin: one hundred years after the first report of the anticoagulant agent in medicinal leeches. Biomedica Biochimica Acta, 44(7-8), 1007–1013.
Donella-Deana, A., Varro, A., Dockray, G. J., & Pinna, L. A. (1991). Substitution of phosphotyrosine for sulphotyrosine in biologically active peptides. Enzymatic phosphorylation of a progastrin peptide confers immunoreactivity reminiscent of the sulphated derivative. Biochimica et Biophysica Acta, 1095(1), 75–77.
Lombardi, A., De Simone, G., Galdiero, S., Staiano, N., Nastri, F., & Pavone, V. (1999). From natural to synthetic multisite thrombin inhibitors. Biopolymers, 51(1), 19–39.
Nowak, G., & Markwardt, F. (1991). Hirudin in disseminated intravascular coagulation. Haemostasis, 21(Suppl), 1142–1148.
Jiang, W., Li, W., Hong, Y., Wang, S., Fang, B. (2015). Cloning, expression, mutagenesis library construction of glycerol dehydratase, and binding mode simulation of its reactivase with ligands. Applied Biochemistry and Biotechnology.
Zafar, A., Aftab, M.N., Ud Din, Z., Aftab, S., Iqbal, I., Ul Haq, I. (2015). Cloning, purification and characterization of a highly thermostable amylase gene of thermotoga petrophila into Escherichia coli. Applied Biochemistry and Biotechnology.
Zhang, Y. F., Wei, Y. M., Ma, B. C., Qiao, K. Y., Ma, Z. H., Li, C., Ma, C., Ji, Y. L., Dong, Z., Hao, J. F., et al. (2013). Expression of rolGLP-HV in E-coli and its dual-function for the treatment of diabetes and thrombosis. International Journal of Peptide Research and Therapeutics, 19(3), 257–263.
Zeng, X.Y., Dong, S., He, N.N., Jiang, C.J., Dai, Y., Xia, Y.F. (2015). Comparative pharmacokinetics of arctigenin in normal and type 2 diabetic rats after oral and intravenous administration. Fitoterapia, 105119–105126.
Rouse, R., Xu, L., Stewart, S., & Zhang, J. (2014). High fat diet and GLP-1 drugs induce pancreatic injury in mice. Toxicology and Applied Pharmacology, 276(2), 104–114.
Shrivastava, A., Chaturvedi, U., Sonkar, R., Khanna, A. K., Saxena, J. K., & Bhatia, G. (2012). Antioxidant effect of Azadirachta indica on high fat diet induced diabetic Charles Foster rats. Applied Biochemistry and Biotechnology, 167(2), 229–236.
Morsy, M.A., Heeba, G.H., Mahmoud, M.E. (2015). Ameliorative effect of eprosartan on high-fat diet/streptozotocin-induced early diabetic nephropathy in rats. European Journal of Pharmacology, 75090–75097.
Liu, C., Hu, M. Y., Zhang, M., Li, F., Li, J., Zhang, J., Li, Y., Guo, H. F., Xu, P., Liu, L., et al. (2014). Association of GLP-1 secretion with anti-hyperlipidemic effect of ginsenosides in high-fat diet fed rats. Metabolism - Clinical and Experimental, 63(10), 1342–1351.
Shi, B. X., Li, J. C., Yu, A. P., Yuan, B., & Wu, C. S. (2006). Two-step ion-exchange chromatographic purification of recombinant hirudin-II and its C-terminal-truncated derivatives expressed in Pichia pastoris. Process Biochemistry, 41(12), 2446–2451.
Ma, B., Tu, P., Zhao, X., Zhang, Y., Wang, Y., Ma, C., Ji, Y., Li, X., Abbas, S. A., & Li, M. (2013). Expression and purification of optimized rolGLP-1, a novel GLP-1 analog, in Escherichia coli BL21(DE3) and its good glucoregulatory effect on type 2 diabetic mice. Current Pharmaceutical Biotechnology, 14(11), 985–994.
Hou, J., Yan, R., Yang, L., Wu, Z., Wang, C., Ding, D., Li, N., Ma, C., & Li, M. (2007). High-level expression of fusion protein containing 10 tandem repeated GLP-1 analogs in yeast Pichia pastoris and its biological activity in a diabetic rat model. Bioscience, Biotechnology, and Biochemistry, 71(6), 1462–1469.
Hou, J., Yan, R., Ding, D., Yang, L., Wang, C., Wu, Z., Yu, X., Li, W., & Li, M. (2007). Oral administration of a fusion protein containing eight GLP-1 analogues produced in Escherichia coli BL21(DE3) in streptozotocin-induced diabetic rats. Biotechnology Letters, 29(10), 1439–1446.
Cen, X., Ni, J., Tan, T., Liu, X., Li, C., Chen, J., Huang, Y., Zhu, S., & Bi, Q. (2006). Investigation on recombinant hirudin via oral route. Peptides, 27(4), 836–840.
Fujita, M., Ito, Y., Hong, K., & Nishimuro, S. (1995). Characterization of nattokinase-degraded products from human fibrinogen or cross-linked fibrin. Fibrinolysis, 9(3), 157–164.
Billings, P. C., St Clair, W. H., Maki, P. A., & Kennedy, A. R. (1992). Distribution of the Bowman Birk protease inhibitor in mice following oral administration. Cancer Letters, 62(3), 191–197.
Acknowledgments
This study is supported by the Key Technologies R&D Program of Tianjin (14ZCZDSY00013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ni, Z., Zhang, Y., Wang, H. et al. Construction of a Fusion Peptide 5rolGLP-HV and Analysis of its Therapeutic Effect on Type 2 Diabetes Mellitus and Thrombosis in Mice. Appl Biochem Biotechnol 179, 59–74 (2016). https://doi.org/10.1007/s12010-016-1979-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-1979-x