Abstract
Leishmania establishes a successful parasitism by evading both oxidative and non-oxidative killing pathways, and its drug resistance against the currently available therapeutics demands for a safe and cheap drug. Since the parasite synthesizes ergosterol instead of cholesterol, using the same biochemical pathway and enzymes, an inhibitor of HMG-CoA-Reductase, Lovastatin, has been tried for its anti-Leishmanial effect. Lovastatin, being an inhibitor of HMG-CoA-Reductase, inhibits infection by cholesterol depletion, while chromium chloride complexes, at their higher concentrations, are reported to exhibit cytotoxicity. In intracellular amastigotes, cytotoxicity has been checked by assessing various manifestation of cell death, viz. DNA fragmentation, AnnexinV-FITC binding and JC-1 fluorescence ratio. Release of hydrogen peroxide (HPO) and nitric oxide (NO) has been assessed in live cell. Lovastatin and CrCl3.6H2O in combination has appeared to be ineffective on promastigotes but has induced cytotoxic effect on the intracellular amastigotes through up-regulation of cellular signalling mechanisms. CrCl3.6H2O stimulates generation of NO, leading to reduction of the number of intracellular amastigote, while Lovastatin shows HPO-mediated killing of the same, keeping the host cell unaffected. This novel therapeutic approach, involving two known safe compounds in suboptimal doses, may resolve human visceral Leishmaniasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Leishmania donovani, the unicellular protozoan parasite mainly responsible for visceral Leishmaniasis, is found in two forms: the extracellular stage is known as promastigotes, residing in the lining of the gut of infected insect vector, having prominent flagella as its locomotory organ, while the intracellular stage in mammalian host (humans) is known as amastigote without flagella (Sharma and Singh 2009). Various compounds from pentavalent antimonial compound sodium Stibogluconate (Roychoudhury and Ali 2008), Amphotericin B, Imiprimane and Pentamidine are known for their anti-Leishmanial therapeutics (Verma and Dey 2004; Jayanarayan and Dey 2005; Mukherjee et al. 2012). These compounds in their long-run therapeutic applications have shown various clinical problems. Sodium Stibogluconate (SAG) has become outdated because of increasing occurrences of drug resistance and toxic side effects (Croft et al. 2006; Chakravarty and Sundar 2010). The effect of Pentamidine treatment on the SAG-resistant visceral Leishmaniasis has serious and deleterious toxic side effects (Moore and Lockwood 2010). Treatment with Amphotericin B through intravenous infusion results in detrimental infusion reactions, thus removing a cost-effective treatment procedure (Khoo et al. 1994) for the Third World countries. Liposomal Amphotericin B is a new-generation drug reported to have relapsing Leishmaniasis in treated patients with variable efficacy, toxic side effects and painful administration (Wortmann et al. 2010). For these reasons, improved drug therapy against Leishmania sp. infection is still needed.
Cholesterol, the major component of eukaryotic plasma membrane, plays a very important role in the membrane lipid bilayer and the integrity of membrane proteins. Leishmania is such a eukaryote which lacks cholesterol in its membrane, instead they use the same synthesizing machinery to produce a ergostane skeleton, similar to cholesterol (Roberts et al. 2003). Moreover, Leishmanial infection has been shown to be modulated by membrane cholesterol (Aditya Kumar et al. 2016a) and the cell surface siglecs (Roy et al. 2016) of host cells and through SREBP2 activation (Mukherjee et al. 2014), and a possible receptor-mediated mechanism of action of cholesterol has been recently proposed (Aditya Kumar et al. 2016b) involving Fc receptors, complementary receptors (CR1, CR2, etc.) for binding and internalization (Pucadyil and Chattopadhyay 2007; Gimpl 2010). Considering the importance of cholesterol and ergosterol in the life cycle of Leishmania, Imiprimane is a proposed drug which acts as an inhibitor for ergosterol synthesis for promastigotes (Mukherjee et al. 2012). Similarly, statins are another group of inhibitors that inhibit the rate limiting enzyme of cholesterol biosynthesis, i.e. 3-hydroxy-3-methylglutyryl coenzyme A reductase (HMG-CoA Reductase), acting upstream of the pathway, than Imiprimane does.
Upon infection, these parasites first paralyse the intracellular oxidative mode of defence mechanism by inhibiting NAD(P)H oxidase (Lodge et al. 2006) and then blocking iNOS activation through insulin-like growth factor (Reis et al. 2013), inhibiting NO synthesis. Statins, the low-molecular-weight drugs, beside lowering the cellular cholesterol level, exert pleiotropic effects specifically on the immune system (Liao and Laufs 2005). Statins are known to reduce the production of superoxide radicals by decreasing the activity of NAD(P)H oxidase (Delbosc et al. 2002), but they induce the synthesis of hydrogen peroxide (HPO) (Crane 2001) since as they restrict the intrinsic biosynthesis of Co-enzyme Q (Kettawan et al. 2007). Thus, statins may have potential anti-Leishmanial protective role due to their inhibiting the infection process and activating the various cell signalling pathways.
On the other hand, some micronutrients like chromium and zinc are also reported to have anti-parasitic property (Al-Mulla Hummadi et al. 2005). Chromium chloride hexahydrate complexes (CrCl3. 6H2O) at their higher concentrations show cytotoxic and genotoxic effects mediating ROS generation, ultimately leading to apoptotsis-like death (Bagchi et al. 2002; Balamurugan et al. 2002; Levis and Majone 1979). Thus, chromium supplementation may help protecting the cells from the parasites through controlled activation of type I immune response.
In the present study we have evaluated the effect and mode of action of Lovastatin and micronutrient chromium chloride hexahydrate individually as well as in combinations, in low doses, on both of the stages of Leishmania donovani. This combination of Lovastatin and a micronutrient (CrCl3.6H2O) at their suboptimal concentrations may become a novel in vitro therapeutic strategy in the future, bypassing the side effects on human health and resolving human visceral Leishmaniasis.
2 Materials and methods
2.1 Isolation of human peripheral blood monocytes (PBMCs) and in vitro infection with L. donovani
The virulent L. donovani strains, AG83 [MHOM/IN/1983/AG83], were originally obtained from an Indian patient with Kala-azar (Ghosh et al. 1985). Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated and cultured in complete RPMI medium, supplemented with FCS (Gibco 10082). Human monocytic leukemia cell-line THP-1 cells were cultured with RPMI-1640 (Gibco 23400-021) supplemented with FCS (Gibco 10082), passaged at every 72 h before being 95% confluent. L. donovani (promastigotes) of AG83 strain had been cultured with M199 (Gibco 1240) supplemented with FCS, passaged at every 72 h. Amastigotes and promastigotes were incubated with isolated PBMCs as well as THP-1 cells at varying multiplicities of infection (MOI) for 18 h (cell: parasite 1:10). Parasites left outside the cells were removed by light centrifugation and stained with Giemsa to quantitate intracellular parasitic load (Ghosh et al. 2003). The same infection regimen with both promastigotes and amastigotes were followed after opsonization with 5% normal human serum at 37°C before infection (McDowell et al. 2002).
2.2 Flow cytometry
Flow cytometric analysis (BD LSR, BD Biosciences) was done to differentiate the surface-attached parasites from internalized ones by quenching technique (Ma et al. 1987). Parasites labelled with FITC were incubated with THP-1 cells (cell : parasite is of 1:10 ratio) for 18 h at 37°C, in the presence and absence of 0.1 mg/mL Crystal Violet. To analyse the viability of the FITC-labelled promastigotes and amastigotes, MTT assay was done (Ma et al. 1987; Ghosh et al. 2006).
2.3 Preparation of various concentrations of drugs and treatment
Lovastatin sodium salt (Cayman Chemicals) was prepared in 25 mM stock, in serum-free RPMI medium using ethanol (0.0002%) (Merck) and HPLC-grade water. Since the concentration of ethanol was very less (<0.02%), it was considered ineffective on parasites or monocytes, and vehicle controls were avoided. Chromium chloride (Sigma Aldrich 27096) 20 mg/mL stock was prepared by dissolving chromium chloride in ultrapure pyrogen-free distilled water.
2.4 Cytotoxicity assay
The viable numbers of treated and untreated L. donovani promastigotes and THP-1 cells were checked by Trypan Blue dye exclusion assay (Himedia TC193). Cytotoxicity of amastigotes were determined by counting the number of intracellular amastigotes per hundred cells from the Giemsa-stained smear of infected THP-1 cells for each treated groups by observing under bright field microscopy at 100× oil immersion. At least 400 THP-1 cells were examined for each coverslip.
2.5 Nucleosomal DNA fragmentation assay
Intracellular amastigotes treated with optimal dose of chromium chloride (150 µg/mL) and Lovastatin (50 µM) alone and in combination for 36 and 48 h were isolated. Genomic DNA was isolated from both untreated and treated amastigote by phenol chloroform isolation (1:1) method (Beck 2002). Further analysis was done via 0.8% Agarose gel electrophoresis using Gel Documentation System (Biorad).
2.6 Detection of apoptosis in isolated amastigotes treated with Lovastatin (50 µM) and CrCl3 (150 µg/mL) in combination using Annexin V-FITC and PI
Apoptosis in the amastigotes was determined using the Annexin V-FITC Apoptosis Detection Kit (BD Pharminogen) according to the manufacturer’s protocol. Amastigotes from combinatorial treated and untreated THP-1 cells were isolated and double-stained with Annexin V-FITC and propidium iodide (PI) as per the manufacturer’s protocol. They were analysed by flow cytometry (BD-LSR, BD Biosciences). Annexin V-FITC-positive amastigotes were assayed as apoptotic population of amastigotes (Kaur et al. 2013).
2.7 Determination of mitochondrial membrane potential
Mitochondrial membrane potential (∆Ψm) is the difference in electric potential between the interior of the mitochondria and its inter-membranous space generated due to proton movement across the mitochondrial inner membrane. This makes the interior of the mitochondria to be electronegative, accumulating the cationic dyes, such as JC-1. ∆Ψm of infected and uninfected THP-1 cells after treatment for 48 h was determined by using JC-I dye (Sigma), which is widely used as a probe in detection of apoptosis, monitoring the mitochondrial health. JC-1 is a cationic dye which concentrates into mitochondria in response to ∆Ψm crossing the mitochondrial membrane due to its lipophilicity. A greater ∆Ψm accumulates more dye, forming J-aggregates which emit at 590 nm. But mitochondrial damage diminishes the ∆Ψm and JC-1 accumulates in lower concentration emitting at 530 nm. Thus, JC-1 dye exhibits the health of a cellular mitochondria through its accumulation depending on the mitochondrial membrane potential difference (∆Ψm), indicated by changing its fluorescence emission between green (530 nm) and red (590 nm). Mitochondrial membrane depolarization or mitochondrial damage is indicated by decrease in the red (590)/green (530) fluorescence intensity. The uninfected THP-1 (negative control) and infected THP-1 cells were collected after 48 h of treatment and were washed with PBS and stained with JC-1 (10 µM) for 7 min at 37°C in the CO2 incubator following the protocol. Thereafter the cells were washed twice with PBS and fluorescence intensity of red and green in both infected and uninfected THP-1 cells stained with JC-1 were observed using the filters at 585 nm and 530 nm. The ratio of fluorescence at 590 to 530 nm was considered to be the relative ∆Ψm value.
2.8 Detection of nitric oxide (NO) and hydrogen peroxide (HPO)
To determine the mediator of parasitic death, the presence of NO and HPO had been detected with Free Radical Analyzer (WPI TBR1025, USA) machine according to the manufacturers’ protocol (Doeller et al. 2005). (supplementary material, section 1.3)
2.9 Statistical analysis
Cytotoxicity in promastigotes as well as amastigotes and mean fluorescence index of JC-1 of all the treated groups were statically checked with respect to their untreated control by one-way ANOVA and multiple comparison test at the significance level of 0.05. The linear curves of NO and HPO production were analysed by determining Pearson’s correlation coefficient and then were transformed into Fisher’s Z-score from which P-value of each group can be calculated and compared with Po of null hypothesis at the significance level of 0.05.
3 Results
3.1 Combinatorial treatment with Lovastatin and CrCl3 inhibits the growth of intracellular amastigotes but fails to exert any cytotoxic effect on L. donovani promastigotes
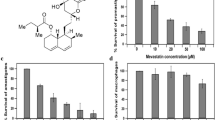
L.donovani AG83 promastigotes (2.5×106 cells/mL) were incubated with CrCl3 (150 µg/mL), Lovastatin (50 µM and 100 µM) alone and in combination for 3 days. The numbers of live promastigotes were counted by Trypan Blue exclusion assay up to day 3; all the treatment protocol failed to induce any growth retardation or killing effect on promastigotes (figure1a), but reduced size, roundish shape and shading of flagella in maximum cases, were observed after treatment. Parasites were also incubated with the lower concentrations of CrCl3.6H2O (10, 50,100 µg/mL) and Lovastatin (10 and 50 µM) along with positive control sodium antimony gluconate (SAG). The treated groups did not show any significant effect in comparison to positive control (supplementary figure 1a). PBMCs from healthy donor and THP-1 cells were grown on coverslips separately were treated with the above-mentioned protocol for 3 days. Percentages of live cells were calculated under microscope by Trypan Blue exclusion method. It was observed that both Lovastatin (50 µM) and CrCl3 (150µg/mL) did not significantly affect the health of THP-1 cells as well as PBMC in comparison with untreated cells (figure 1b). This indicated that proposed therapeutics were safe for normal cell survival. For further experiments monocytic cell-line THP-1 was selected as host for L. donovani infection as they behaved similar to PBMCs after treatment.
In vitro effects of Lovastatin and CrCl3 on promastigotes, live THP-1 and intracellular amastigotes: (a) Promastigotes (2.5×106 cells/mL) were cultured for 3 days in M199 media supplemented with 10% FCS in presence of CrCl3 (150 µg/mL), Lovastatin (50 and 100 μM) alone and in combination. Dose- and time-dependent assessment of live promastigotes percentage was done with proposed treatment protocols. Data are expressed as percentage of live promastigotes and represent mean ± SD of three independent experiments. (b) Effect of treatment over healthy PBMC and THP-1 cells. Healthy PBMCs and THP-1 cells were cultured for 3 days in RPMI 1640 media supplemented with 10% FCS in presence of CrCl3 (150 µg/mL) and Lovastatin (50 µM) alone and in combination. Data are expressed as percentages of live cells and represent mean ± SD of three independent experiments. (c) Cytotoxicity assay of intracellular amastigotes after treatment with different drug combinations: THP-1 cells were allowed to adhere on coverslip in RPMI 1640 media supplemented with 10% FCS before in vitro infection with L. donovani amastigotes followed by further incubation for 2 days at 37°C with CrCl3 (150 µg/mL) and Lovastatin (50 µM) alone and in combination. Anti-Leishmanial activity was determined by calculating the number of amastigotes/100 cells. Experiments were performed thrice and data are represented from three set of these experiments as mean ± SD. *P<0.5 in one-way ANOVA with infected THP-1 cells (Ctrl group) vs CrCl3, Lovastatin and combined treated group (L+C). **P<0.5 in one-way ANOVA with infected THP-1 cells combined treated group (L+C) vs CrCl3 (C) and Lovastatin (Lova)-treated groups.
Healthy THP-1 cells were infected with L. donovani AG83 amastigotes in vitro. Infected cells after subsequent washing were incubated with CrCl3 (150 µg/mL) and Lovastatin (50 µM) alone and in combination for 2 days. Intracellular parasitic burden was measured after Giemsa Staining. After 48 h of treatment Lovastatin (L) reduced burden up to 75%, CrCl3 (C) 49%, while the combinatorial (L+C) treatment reduced up to 96%, in comparison to untreated infected control group (Ctrl) (figure 1c). The infected THP-1 cells were also checked with lower concentrations of CrCl3.6H2O (10, 50, 100 µg/mL) and Lovastatin (10 µM) along with positive control sodium antimony gluconate (SAG) where it has been found that these treatment protocols have graded effect in inducing cytotoxicity with a highest induction at the combination of L50 and C150 (supplementary figure 1b). As Lovastatin 50 µM along with CrCl3 (150 µg/mL) proved efficient enough to reduce parasitic burden, this concentration was selected for further experiments.
3.2 Successful infection of THP-1 cells by the amastigotes form of L. donovani
To study the interaction of L. donovani amastigotes and promastigotes with monocytic cell-line THP-1, a novel quenching technique had been used that differentiated surface-attached parasites from internalized ones (Ma et al. 1987). FITC-labelled metacyclic promastigotes and purified amastigotes were incubated with THP-1 cells (cell : parasite ratio of 1:10, for each form of the parasite). After 18 h of the addition of amastigotes and promastigotes, cells were analysed through flow cytometer in the presence and absence of 0.1 mg/mL Crystal Violet (supplementary materials 1.2). Crystal Violet, at this concentration, quenches all membrane fluorescence of FITC, while intracellular fluorescence remains unaffected, allowing distinction between the parasites attached to the surface with internalized ones. The viability of both the forms of parasite remained unaffected after FITC labeling (data not shown).
To maintain the physiological relevance of in vitro infection with the in vivo system, parasites were opsonized with normal human serum, because in the in vivo system parasites come in contact with serum prior to infection after after entering into the mammalian system through a sand fly. The infection was given with amastigotes and metacyclic promastigotes opsonized with normal human serum and counter-stained by anti-Human IgG-FITC.
When THP-1 cells were incubated with un-opsonized metacyclic L. donovani promastigotes, in the presence of Crystal Violet, a drastic reduction in fluorescence of FITC-positive cells (from 68%–90% to 4%) had been observed. FITC labelling keeps L. donovani promastigotes unaffected, which was proved by MTT assay (data not shown). In contrast to L. donovani promastigotes, both opsonized and un-opsonized L. donovani amastigotes were internalized efficiently by the cells (figure 2). The successful infection of THP-1 cells by amastigotes in comparison to promastigotes, was further confirmed by Giemsa staining of the infected cells by microscopic examination (data not shown).
Successful infection by amastigotes into THP-1 cells: THP-1 cells were incubated with 5% normal human sera-opsonized (30 min at 37°C) and unopsonized FITC-labelled promastigotes and amastigotes (cell: parasite was 1:10) for 18 h at 37°C. Flow cytometric analysis was done both in absence (left) and presence (right) of 0.1mg/mL Crystal Violet to discriminate surface attached parasites from internalized ones by quenching technique. Values in the upper right quadrants are the percentage of fluorescence-positive cells. Results are representation of three similar experiments.
3.3 Oligonucleosomal DNA fragmentation of L. donovani amastigotes indicates apoptosis-like cell death
DNA laddering is a classical sign of apoptosis, which was checked for both promastigotes, amastigotes and the control uninfected THP1 cells. After treatment with Lovastatin (50 µM) and CrCl3 (150 µg/mL) alone and in combination for 48 h, genomic DNA of promastigotes were isolated and analysed. But the treated promastigotes failed to produce any DNA damage (ladder or smear) even after 48 h of treatment (figure 3a). In case of uninfected THP-1 cells the various drug combinations had failed to produce any ladder-like appearance even after 48 h, which clearly indicates that the above-mentioned compounds in suboptimal doses were not genotoxic for normal THP-1 (figure 3b). DNA fragmentation analysis was done with isolated amastigotes from infected THP-1, treated with CrCl3. 6H2O (C), Lovastatin (Lova) and in combination (L+C) with chosen concentrations. It had been observed that the combinatorial treated group (L+C) had shown prominent ladder in only 36 h (figure 3c(i)). At 48 h all the treated groups exhibit the appearance of DNA ladder, among which combinatorial treated group (L+C) has shown much more prominent ladder (figure 3c(ii)). Thus combinatorial treatment has more pronounced apoptotic effect on amastigotes rather than the individually treated groups.
(a) Appearance of genomic DNA of promastigotes after treatment with different drug combination at 48 h: DNA Fragmentation assay of L. donovani promastigote. L1 - 1 kb DNA ladder (L); L2 - untreated (Ctrl); L3 - treated with CrCl3 (CrCl3); L4 - treated with Lovastatin (Lova); L5 - treated with Lovastatin and CrCl3 (L+C) in combination (γ = 0.73). (b) Appearance of genomic DNA of uninfected THP-1 cells after treatment with different drug combinations after 48 h of treatment; L1 - 1kb DNA ladder (L); L2 - untreated (Ctrl); L3 - treated with CrCl3 (CrCl3); L4 - treated with Lovastatin (Lova); L5 - treated with Lovastatin and CrCl3 (L+C) in combination (γ = 0.57). (c) Appearance of genomic DNA of isolated amastigotes after treatment with different drug combinations: at 36 h (i) and 48 h (ii) (γ = 0.61 and 0.36). L1 - 1kb DNA ladder (L); L2 - untreated (Ctrl); L3 - treated with CrCl3 (CrCl3); L4 - treated with Lovastatin (Lova); L5 - treated with Lovastatin and CrCl3 (L+C) in combination. (d) Effect of Lovastatin and CrCl3 on amastigotes: Intracellular amastigotes treated with Lovastatin (50 µM) and CrCl3 (150 µg/mL) for 36 h were isolated and double-stained with Annexin V FITC and PI untreated control (i) treated with Lovastatin (50 µM) and CrCl3 (150 µg/mL) (ii). Given data represents three similar independent experiments, analysed by flow cytometry (BD-LSR, BD Biosciences).
3.4 Combinatorial treatment induce apoptosis-like death in intracellular amastigotes
As the combinatorial treatment (i.e. CrCl3 150 µg/mL and Lovastatin 50 µM) had proved to be much more effective, this protocol was followed to measure apoptotic change of amastigotes. The treated (36 h) amastigotes were checked for apoptotic marker through flow cytometry after Annexin V-FITC/ PI double-staining. Untreated amastigotes (Ctrl) did not show any fluorescence and were displayed at the right lower quadrant. It was evident that untreated amastigotes were very poorly stained by Annexin V-FITC (7.8%) or PI (2.2%): as the Phosphatidylserine were present at the inner part of the plasma membrane, Annexin V- FITC could not bind with Phosphatidylserine, and as the untreated amastigotes have intact cell membrane, PI was not able to enter the cytoplasm (figure 3d(i)). Amastigotes isolated from infected THP-1cells after 36 h of treatment with CrCl3 (150 µg/mL) and Lovastatin (50 µM) in combination have shown changes in cellular morphology. The amastigotes with marks of early apoptosis exhibited a green staining (Annexin V-FITC) in their plasma membranes, confirming that Phosphatidylserine was redistributed to the outer leaflet of the plasma membrane and, as the Annexin V-FITC has a strong affinity for the Phosphatidylserine counterpart, it bound to it exhibiting a green fluorescence (17.1%) (figure 3d(ii)). Thus, a significant increment of (120%) of Annexin-V-positive amastigotes were observed by flow cytometry after treatment in comparison to untreated amastigotes.
3.5 Assaying mitochondrial health of infected and uninfected THP-1 cells by JC-1 dye
The ratio of Mean Fluorescence Index at 590 nm and 530 nm were detected in the treated and untreated infected THP-1 cells after 48 h of treatment. In the case of uninfected THP-1, Lovastatin and CrCl3.6H2O alone or in combination did not exert any significant change in MMP (∆Ψm) with the respective negative controls (uninfected untreated THP-1 cells) (figure 4). In the case of infected and treated THP-1 cells, Lovastatin and CrCl3 (L+C) treated infected THP-1 cells exhibited the lowest 590:529 fluorescence (0.295±0.0171) in comparison with that of the groups Ctrl (2.011±0.049), C (0.938±0.02267) and Lova (0.597±0.0423). A drop in ∆Ψm of 53.3% in CrCl3, 70.3% in Lova and 85.3% in combinatorial (L+C) treated groups was observed with respect to control. This indicated sustained hypo-polarization of the mitochondrial membrane in all the treated groups, but it was more pronounced in the L+C group while exerting least effect on uninfected THP-1 cellular health.
Mitochondrial membrane potential (MMP) of infected and uninfected THP-1 cells in the form of Mean Fluorescence Index. Mean Fluorescence Index in each group was calculated as the fluorescence ratio of red to green (590:530 FI). The MMP was analysed using a Fluorescence Microplate Reader after staining with JC-1 dye. *P<0.5 in one-way ANOVA with infected THP-1 cells (Ctrl group) vs CrCl3, Lovastatin and combined treated group (L+C). **P<0.5 in one-way ANOVA with infected THP-1 cells combined treated group (L+C) vs CrCl3 (C) and Lovastatin (Lova)-treated groups.
3.6 Role of reactive oxygen and non-oxygen species in intracellular amastigote death
The aforesaid results indicate death in intracellular amastigotes with an overall outcome of decreased intracellular parasitic load upon combined treatment with Lovastatin and CrCl3. Since reactive nitrogenous intermediates (RNI) are more effective against the intracellular amastigotes and statins are well known in inducing hydrogen peroxide (HPO) synthesis by depleting the cells from Co-enzyme Q; further investigation of the causative agents of intracellular parasite death was performed by measuring the levels of nitric oxide (NO) and hydrogen peroxide (HPO) by using Free Radical Analyzer (WPI, USA) and radical-specific probes.
3.6.1 Status of nitric oxide released by untreated and treated infected THP-1 cells
The NO secretion from live THP-1 cells upon the aforesaid treatment protocol on both infected and uninfected cells were measured. Uninfected and treated THP-1 cells did not exhibit significant level of NO production in comparison to the uninfected untreated THP-1 cells (supplementary figure 2; table 1), whereas when the infected cells were treated with CrCl3 (C) (figure 5b), and Lovastatin (L) (figure 5c) alone or (L+C) in combination (figure 5d), all the groups except Lova produced significant amount of NO in comparison to the infected untreated THP-1 cells (table 2).
Measurement of nitric oxide (NO) release from THP1 cells infected by L. donovani (up to 2 h) after treatment with Lovastatin (50 µM) and CrCl3 (150 µg/mL); NO released from infected THP-1 cells was assessed by Free Radical Analyzer (WPI, USA) through NO sensor probe after treatment. (a) Untreated control (Ctrl), (b) CrCl3 (150 µg/mL) treated, (c) Lovastatin (50 µM) treated, (d) CrCl3 (150 µg/mL) and Lovastatin (50 µM) treated in combination. Data were analysed by determining Pearson’s correlation coefficient and then were transformed into Fisher’s z-score from which the P-value of each group had been calculated and compared with Po of null hypothesis at the significance level of 0.05.
In case of Lovastatin-treated groups, iNOS activity may be lost through peroxisome proliferator activated receptors (PPARs), while in case of combinatorial treated group (L+C) the NO release is very quick and transient, which may give the intracellular parasites a certain shock, leading to parasitic death. All the infected treated groups except Lovastatin-treated group - produced significant levels of NO compared to that of their respective uninfected THP-1 treated cells (table 3), which proves that NO release in the infected cells is due to drug treatment, i.e. killing the parasites.
The inability to secret NO by the untreated infected cells in comparison with untreated uninfected cells is due to inhibition of NAD(P)H oxidase (Lodge et al. 2006) by these parasites which block iNOS activation through insulin-like growth factor (Reis et al. 2013) for successful parasitism.
3.6.2 Status of hydrogen peroxide (HPO) released by untreated and treated infected THP-1 cells
Measurement of HPO release from uninfected and infected THP-1 cells after the aforesaid treatment regimen showed insignificant level of HPO from differently treated uninfected cells in comparison to uninfected untreated THP-1 cells (supplementary figure 3; table 4).
On comparing with infected untreated control, CrCl3. 6H2O-treated group (figure 6b) had no significant release of HPO, while only Lovastatin-treated infected cells produced HPO quite rapidly up to 2 h (figure 6c). In case of combinatorial treated group (Lovastatin and CrCl3. 6H2O), there was a significant level of HPO release from basal level just after drug treatment which continued up to 2 h (figure 6d; table 5).
Measurement of hydrogen peroxide (HPO) release from THP-1 cells infected by L. donovani up to 2 h after treatment with Lovastatin (50 µM) and CrCl3 (150 µg/mL); HPO released from infected THP-1 cells were analyzed by Free Radical Analyzer (WPI, USA) through HPO sensor probe after treatment. (a) Infected control (Ctrl), (b) CrCl3 (150 µg/mL) treated, (c) Lovastatin (50 µM) treated, (d) CrCl3 (150 µg/mL) and Lovastatin (50µM) treated in combination. Data were analysed by determining Pearson’s correlation coefficient and then were transformed into Fisher’s z-score from which the P-value of each group had been calculated and compared with Po of null hypothesis at the significance level of 0.05.
HPO release in infected untreated THP-1 cells and infected group treated with Lovastatin in comparison to that of their respective uninfected THP-1 cells and uninfected treated group with Lovastatin (table 6) was significant, while the other CrCl3 and L+C treated groups exhibited insignificant HPO release in comparision to their uninfected controls.
This proves that significant HPO release happens after infection, being insufficient to be parasitidal, but in the presence of Lovastatin, HPO surge occurs in infected cells, leading to intracellular parasitic death. In the L+C treated group, the infected cells secreted significant HPO as compared to the untreated infected cells, leading to parasitic death; this secretion becomes insignificant compared to the respective uninfected group, indicating the scavenging role of CrCl3.
Thus, combinatorial treated group was much more effective in producing a quick surge of ROS generation, which may prove to be effective to activate the downstream signalling cascades, responsible for death of intracellular amastigotes.
4 Discussion
In our current study, metacyclic L. donovani promastigotes were the first target (regarding ergosterol depletion) for the combinatorial treatment as ergosterol is an essential component for maintaining trypanosoma membrane, their size and characteristic body shape. The promastigotes were treated with graded concentrations of CrCl3 (from 10 µg/mL up to 150 µg/mL) and Lovastatin (from 10 µM up to 100 µM) and found to be almost unresponsive to both the compounds (figure 1a) with least alteration at their genomic DNA integrity (figure 3a). This indicates that although statin and Imiprimane can block the ergosterol synthesis machinery in the promastigotes detrimentally affecting their shapes and sizes, they fail to alter their integrity at their molecular level, thus recurrence is a possibility.
Since statin is well documented in immune-modulation with a well-fortified prove of CrCl3 of being immune-stimulatory, the effects of Lovastatin and CrCl3 were studied on promastigotes and the intracellular amastigotes i.e. in the presence of THP-1 cell, not on axenic amastigotes. The amastigotes studied were generated through infecting THP1 cells with opsonized amastigotes after confirming both normal human PBMC and THP-1 cells remain unaffected after the treatment of combinatorial protocol, eliminating the cytotoxicity chances of the above-mentioned drugs.
In these intracellular amastigotes, when treated with this drug combination at lower concentrations, the intracellular parasitic burden was evident to be low enough but parasitical effect up to molecular level, i.e. DNA damage, was absent. This led us to select the concentrations of Lovastatin and CrCl3 at 50 µM and 150 µg/mL respectively, which showed clear death signals through almost every possible analysis. CrCl3 reduced 49% and Lovastatin 75%, while combinatorial treatment reduced 96% live intracellular parasite burden in comparison with the untreated control one. The Annexin V FITC-positive amastigotes population increased from 7.8% to 17% (FSC), which clearly indicated that combinatorial treatment induced apoptosis over the treated amastigotes. Nucleosomal DNA fragmentation assay of the treated amastigotes and decrease in mitochondrial membrane potential in amastigotes-infected THP-1 cells evidently proved that Lovastatin (50 µM), CrCl3 (150 µg/mL) and combinatorial treatment (L+C) all were effective in amastigotes killing. But, the combinatorial treatment (L+C) was the most potent one as it produced DNA ladder in treated amastigotes within only 36 h.
In our attempt to find the possible mechanism of the anti-amastigote activity of the proposed protocol, it was observed that Lovastatin alone failed to produce any significant amount of NO (table 2) due to its immune-suppressive action and possible inhibitory action over iNOS through PPARs activation. But CrCl3 alone was successful in inducing NO in infected THP-1 cells. Also, Lovastatin induced HPO synthesis by possibly limiting the production of Co-enzyme Q in the mitochondrial electron transport chain. Thus, the combinatorial treatment was proved to be effective in inducing both NO and HPO within 30 min by giving a oxidative shock to the intracellular amastigotes, which ultimately leads to death. Although our study did not attempt to determine if the mode of action of intracellular parasitic death was due to apoptosis or necrosis, a conclusion can be drawn that the above-said compounds, i.e. CrCl3, 6H2O (150 µg/mL) and Lovastatin sodium salt (50 µM) are most effective on amastigotes only when they are used in combination rather than used separately. CrCl3. 6H2O, when administered, exerts intracellular parasitic death by inducing nitric oxide, while Lovastatin induces parasitic death by HPO as well as inhibits further infection by lowering the cellular cholesterol level. But when both of these agents are administered in combination, they are a double-edged sword. Both NO and HPO play the role of key mediator of the parasitic cell death, keeping the level of generation of these reactive species, NO and HPO, within a safe limit to minimize the cellular damage.
References
Aditya Kumar G, Jafurulla M and Chattopadhyay A 2016a The membrane as the gatekeeper of infection: cholesterol in host–pathogen interaction. Chem. Phys. Lipids 199 179–185
Aditya Kumar G, Roy S, Jafurulla M, Mandal C and Chattopadhyay A 2016b Statin-induced chronic cholesterol depletion inhibits Leishmania donovani infection: relevance of optimum host membrane cholesterol. Biochim. Biophys. Acta 1858 2088–2096
Al-Mulla Hummadi YM, Al-Bashir NM and Najim RA 2005 The mechanism behind the antileishmanial effect of zinc sulphate. II. Effects on the enzymes of the parasites. Ann. Trop. Med. Parasitol. 99 131–139
Bagchi D, Stohs SJ, Downs BW, Bagchi M and Preuss HG 2002 Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology 180 5–22
Balamurugan K, Rajaram R, Ramasami T and Narayanan S 2002 Chromium(III)-induced apoptosis of lymphocytes: death decision by ROS and Src-family tyrosine kinases. Free Radic. Biol. Med. 33 1622–1640
Beck H-P 2002 Extraction and purification of plasmodium parasite DNA. Methods Mol. Med. 72 159–163
Chakravarty J and Sundar S 2010 Drug resistance in leishmaniasis. J. Glob. Infect. Dis. 2 167–176
Crane FL 2001 Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 20 591–598
Croft SL, Sundar S and Fairlamb AH 2006 Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19 111–126
Delbosc S, Morena M, Djouad F, Ledoucen C, Descomps B and Cristol J-P 2002 Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are able to reduce superoxide anion production by NADPH oxidase in THP-1-derived monocytes. J. Cardiovasc. Pharmacol. 40 611–617
Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR, Darley-Usmar VM and Kraus DW 2005 Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal. Biochem. 341 40–51
Ghosh AK, Bhattacharyya FK, Ghosh DK 1985 Leishmania donovani: amastigote inhibition and mode of action of berberine. Exp. Parasitol. 60, 404–413
Ghosh M, Pal C, Ray M, Maitra S, Mandal L and Bandyopadhyay S 2003 Dendritic cell-based immunotherapy combined with antimony-based chemotherapy cures established murine visceral leishmaniasis. J. Immunol. 170 5625–5629
Ghosh M, Mandal L, Maitra S, Rakshit S, Paul K, Bagchi J, Ganguly D, Pal C and Bandyopadhyay S. (2006) Leishmania donovani infection of human myeloid dendritic cells leads to a Th1 response in CD4+ T cells from healthy donors and patients with kala-azar. J. Infect. Dis. 194 294–301
Gimpl G 2010 Cholesterol-protein interaction: methods and cholesterol reporter molecules. Subcell. Biochem. 51 1–45
Jayanarayan KG and Dey CS 2005 Altered tubulin dynamics, localization and post-translational modifications in sodium arsenite resistant Leishmania donovani in response to paclitaxel, trifluralin and a combination of both and induction of apoptosis-like cell death. Parasitology 131 215–230
Kaur J, Dutta S, Chang K-P and Singh N 2013 A member of the Ras oncogene family, RAP1A, mediates antileishmanial activity of monastrol. J. Antimicrob. Chemother. 68 1071–1080
Kettawan A, Takahashi T, Kongkachuichai R, Charoenkiatkul S, Kishi T and Okamoto T 2007 Protective effects of coenzyme Q(10) on decreased oxidative stress resistance induced by simvastatin. J. Clin. Biochem. Nutr. 40 194–202
Khoo’ SH, Bond J and Denning DW 1994 Administering amphotericin B—a practical approach. J. Antimicrob. Chemother. 33 203–213
Levis AG and Majone F 1979 Cytotoxic and clastogenic effects of soluble chromium compounds on mammalian cell cultures. Br. J. Cancer 40 523–533
Liao JK and Laufs U 2005 Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 45 89–118
Lodge R, Diallo TO and Descoteaux A 2006 Leishmania donovani lipophosphoglycan blocks NADPH oxidase assembly at the phagosome membrane. Cell. Microbiol. 8 1922–1931
Ma JA, Chapman GV, Chen SL, Penny R and Breit SN 1987 Flow cytometry with crystal violet to detect intracytoplasmic fluorescence in viable human lymphocytes. Demonstration of antibody entering living cells. J. Immunol. Methods 104 195–200
McDowell MA, Marovich M, Lira R, Braun M and Sacks D 2002 Leishmania priming of human dendritic cells for CD40 ligand-induced interleukin-12p70 secretion is strain and species dependent. Infect. Immun. 70 3994–4001
Moore EM and Lockwood DN 2010 Treatment of visceral leishmaniasis. J. Glob. Infect. Dis. 2 151–158
Mukherjee M, Basu Ball W and Das PK 2014 Leishmania donovani activates SREBP2 to modulate macrophage membrane cholesterol and mitochondrial oxidants for establishment of infection. Int. J. Biochem. Cell Biol. 55 196–208
Mukherjee S, Mukherjee B, Mukhopadhyay R, Naskar K, Sundar S, Dujardin JC, Das AK, Roy S, Clem A, Sundar S, et al. 2012 Imipramine is an orally active drug against both antimony sensitive and resistant Leishmania donovani clinical isolates in experimental infection. PLoS Negl. Trop. Dis. 6 e1987
Pucadyil TJ and Chattopadhyay A 2007 Cholesterol: a potential therapeutic target in Leishmania infection? Trends Parasitol. 23 49–53
Reis LC, Ramos-Sanchez EM and Goto H 2013 The interactions and essential effects of intrinsic insulin-like growth factor-I on Leishmania (Leishmania) major growth within macrophages. Parasite Immunol. 35 239–244
Roberts CW, McLeod R, Rice DW, Ginger M, Chance ML and Goad LJ (2003) Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Mol. Biochem. Parasitol. 126 129–142
Roy K, Mandloi S, Chakrabarti S and Roy S 2016 Cholesterol corrects altered conformation of MHC-II protein in Leishmania donovani infected macrophages: Implication in therapy. Biochimica et Biophysica Acta. 1858 2088–2096
Roychoudhury J and Ali N 2008 Sodium stibogluconate: therapeutic use in the management of leishmaniasis. Indian J. Biochem. Biophys. 45 16–22
Sharma U and Singh S 2009 Immunobiology of leishmaniasis. Indian J. Exp. Biol. 47 412–423
Verma NK and Dey CS 2004 Possible mechanism of miltefosine-mediated death of Leishmania donovani. Antimicrob. Agents Chemother. 48 3010–3015
Wortmann G, Zapor M, Ressner R, Fraser S, Hartzell J, Pierson J, Weintrob A and Magill A 2010 Lipsosomal amphotericin B for treatment of cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 83 1028–1033
Acknowledgements
We acknowledge Dr Santu Bandyopadhyay, Chief Scientist, Indian Institute of Chemical Biology, Jadavpur, Kolkata, for his support in FACS analysis. We also acknowledge Dr Nahid Ali, Ms Roma Sinha and Mr Anirban Manna, Indian Institute of Chemical Biology, Jadavpur, Kolkata, for their support and for providing L. donovani AG83-sensitive strain used in this study. We also acknowledge Department of Biotechnology (DBT) (Grant No. BT/PR15025/GBD/27/284/2010 (Order-I) dated 16.06.2011), India, for their financial support in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Amit Chattopadhyay
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verma, A.K., Laha, B., Pandey, M. et al. Cholesterol-lowering drug, in combination with chromium chloride, induces early apoptotic signals in intracellular L. donovani amastigotes, leading to death. J Biosci 42, 427–438 (2017). https://doi.org/10.1007/s12038-017-9690-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-017-9690-9