Abstract
Type 2 diabetes mellitus (T2D) is a chronic metabolic disorder considered to be the most predominant form of diabetes throughout the world. This study aimed to investigate the possible effects of cinnamaldehyde (CIN) on insulin signaling pathways in STZ-induced T2D rat model. T2D was originated by feeding rats with a high-fat diet (HFD) plus 25% fructose solution plus streptozotocin (STZ) (35 mg/kg, i.p.). CIN effects were investigated on fasting blood glucose, insulin, oral glucose tolerance test (OGTT), insulin tolerance test (ITT), liver biomarkers, lipid profile, oxidative stress biomarkers, serum advanced glycation end products (AGEs) and its receptors (RAGE) in the aorta, and histopathology of the liver and aorta. Additionally, the mRNA expression of hepatic insulin signaling pathway genes, phosphorylated AKT (serine 473) (P-AKT ser473) level, and aortic nitric oxide synthase3 (eNOS) and NADPH oxidase4 (NOX4) were determined. CIN treatment for 30 days significantly decreased OGTT, ITT, fasting blood glucose, insulin, and HOMA-IR and increased HOMA-β index when compared to diabetic rats. CIN also improved lipid profile and decreased serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity, serum AGEs, and aortic RAGE. Additionally, CIN treatment significantly decreased hepatic malondialdehyde (MDA), increased hepatic and aortic glutathione (GSH) and superoxide dismutase (SOD), and decreased steatosis and inflammation observed in liver tissue of rats. Additionally, significant elevation in mRNA expression of insulin receptor substrate1 (IRS1), phosphatidylinositol 3-kinase regulatory subunit1 (PI3K-P85 subunit), and AKT serine/threonine kinase2 (AKT2); increased levels of P-AKT ser473 and aortic eNOS; and decrease in mRNA expression of NOX4 were detected in CIN-treated group when compared to diabetic group. This study suggests antidiabetic and antioxidant effects of CIN probably through upregulation of eNOS and IRS1/PI3K/AKT2 signaling pathway and alleviating AGEs, RAGE, and NOX4 elevation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2D), a chronic metabolic disorder, is the most prevalent form of diabetes throughout the world (Xu et al. 2013). Insulin resistance (IR) and β-cell failure are two major mechanisms which contribute to the pathogenesis of T2D (Kahn 1998). IR is the main etiology of T2D as it is a physiological condition in which cells fail to respond adequately to the normal actions of insulin. The body may continue to produce insulin, but the cells in the body become resistant to its actions and, therefore, are unable to use it effectively, leading to hyperglycemia (Paneni et al. 2014).
The classical pathway of insulin in glucose metabolism is the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signal pathway; it has a role in glucose uptake by the liver, skeletal muscles, and adipose tissues (Cusi et al. 2000; Cantley 2002). This pathway proceeds through the insulin receptor substrates IRS1 and IRS2 by activation of PI3K enzyme. Most of insulin metabolic actions are produced through the PI3K pathway and decreasing or blocking this pathway will reduce the insulin physiological effects leading to IR (Kanai et al. 1993; Cheatham et al. 1994).
The formation of advanced glycation end products (AGEs) results from the reactions of excessive sugars with lipids and/or proteins due to exposure of cells to excess glucose that leads to inflammation and other pathological disorders (Bierhaus et al. 2005). The impairments caused by AGEs are largely related to the interaction of AGEs and its receptors (RAGE) that mediate many signaling transduction pathways and proinflammatory responses with increased inflammation, oxidative stress, and cell growth (Schmidt et al. 2000). Diabetic patients with high levels of blood AGEs showed a dramatic increase in the abundance of RAGE (Tanji et al. 2000). AGEs/RAGE interaction stimulates the activation of a diverse array of signaling cascades, including PI3K (Ramasamy et al. 2009). AGEs are among several sources of reactive oxygen species (ROS) that described under diabetic conditions (Vlassara and Uribarri 2014).
Cinnamaldehyde (CIN) is a liquid aldehyde derived from the bark of Cinnamomum trees. Its occurrence is detected in numerous brands of cinnamon cereals, puddings, breads, fruit juices, and cookies (Kar et al. 2003). CIN has several pharmacological effects including antioxidant, antibacterial, anticancer, immunomodulatory, antidiabetic, and anti-inflammatory effects (Youn et al. 2008; Zhang et al. 2008); however, the antidiabetic effect is the most remarkable effect (Cao et al. 2007). Previous reports suggested that CIN may have hypolipidemic and hypoglycemic effects, indicating it can be used as a natural treatment for T2D (Subash et al. 2007; Anand et al. 2010).
In this study, a rat model treated with a low-dose streptozotocin (STZ) following high-fat diet (HFD)/fructose in drinking water was used to mimic T2D.
Materials and methods
Drugs and chemicals
STZ, CIN, Tris (hydroxymethyl) aminomethane, trichloroacetic acid pentobarbital sodium, thiobarbituric acid, reduced glutathione, pyrogallol, Ellman’s reagent [5,5′-dithio-bis(2-nitrobenzoic acid)] (Sigma-Aldrich, St. Louis, MO), cholesterol (El-Goumhouria Co. for trading medicines, Research Lab Fine Chem. Industries, India), and fructose powder (Safety Misr, Cairo, Egypt).
Experimental animals
Male Wistar rats were purchased from Vacsera Medical Center, Cairo, Egypt. Rats with average age of 6–8 weeks were kept in a 12-h light–dark cycle, maintained at 25 °C, and rats with weight of 190 ± 10 g were selected for the experiment after acclimatization for 2 weeks. This study protocol was conducted in accordance with “The Principles of Laboratory Animal Care” (NIH publication No. 85-23, revised 1985) and was confirmed by the Ethical Committee of Faculty of Pharmacy, Mansoura University, Egypt.
Development of T2D and experimental design
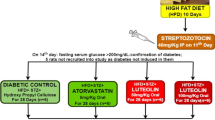
After acclimatization, rats were fed normal pellet diet (NPD) or high-fat diet (HFD) for the initial period of 30 days. The compositions of HFD were as follows: sucrose 20%, lard fat 10%, cholesterol 2.5% and powdered NPD 67.5% (Zhang et al. 2008; Wang et al. 2013; Luo et al. 2015), and 25% fructose in drinking water (Schaalan et al. 2009; Saleh et al. 2014; Kumar et al. 2015). After 30 days dietary feeding, rats fed HFD were injected with freshly prepared STZ (35 mg/kg body weight, i.p.) dissolved in 0.1 mmol/L cold citrate buffer (pH 4.2) (Zhang et al. 2008; Govindaraj and Sorimuthu 2015; Cai et al. 2016). Rats fed NPD were only given the citrate buffer. Blood glucose levels were measured by a glucometer (GlucoSure Star, Apexbio, Taiwan). After 72 h, rats with fasted blood glucose level above 180 mg/dL were considered as diabetic (Wang et al. 2013). The diabetic rats were then randomly divided into two groups: diabetic control group (n = 6) and CIN group (10 mg/kg, CIN was given to animals as a suspension in 0.5% carboxymethyl cellulose (CMC) by oral administration, n = 6) (Subash et al. 2007). The rats fed NPD were divided into two groups: the normal control group (NC; n = 6), which were given vehicle (CMC, 4 mL/kg) by intragastric administration, and the CIN control group, which were given CIN (10 mg/kg as a suspension in 0.5% CMC) (Khare et al. 2016). For another 30 days, animals were allowed to feed on their respective diets till the end of the experiment (60 days).
Food intake, feeding efficiency, and body weight change
Food intake was determined daily by calculating the difference between the daily amount of food served and the remnant amount of food in the cage, then dividing the difference value by the number of rats in the cage (3–4 rats were housed in each cage). Feeding efficiency was recorded for every animal as gram live weight gain per gram food intake as dry matter and it was calculated at the end of the feeding period (Choi et al. 2014). Body weight changes of rats in all groups were recorded weekly during the experimental period and the weight changes (initial weight on day 1 versus final weight on day 60) were calculated.
Oral glucose tolerance test (OGTT)
Rats were given orally a freshly prepared 40% glucose solution after fasting for 14–18 h at a dose of 2 g/kg body weight (Shimoda et al. 1993). After making a cut at 1–2 mm from the tail end of rats, a drop of blood was collected from the tail and then blood glucose was measured at 0 (before glucose administration, it was considered as fasting blood glucose level), 30, 60, 120, and 180 min after the oral glucose load using a glucometer. OGTT was performed at the end of the experiment (after 30 days from the start of CIN treatment).
Insulin tolerance test (ITT)
At the end of CIN treatment period and 1-day after OGTT, rats were fasted for 14–18 h and were injected subcutaneously with human insulin (0.5 IU/kg) (Rubino and Marescaux 2004). After insulin injection, blood drops were collected at 0 (before insulin injection), 30, 60, 120, and 180 min and used for measurement of blood glucose using a glucometer.
Twenty-four hours after performing the ITT, the 14–18-h fasted rats were anesthetized by pentobarbital sodium (40 mg/kg i.v.) and blood was drawn from the retro-orbital plexus for determination of fasting insulin levels, measurement of lipid profile and liver function markers, and flurometric determination of AGEs in the serum. The descending thoracic aorta was removed after opening the thorax, sliced into three segments, one segment was immersed in phosphate-buffered saline (PBS) and put in − 80 °C for preparation of aortic tissue homogenate, the second segment was flash-frozen in liquid nitrogen and then stored at − 80 °C for quantitative RT-PCR, and the last segment was kept for histopathological examination by immersion in 10% buffered formalin.
The liver was also removed, where a part of the left median lobe was immersed in PBS and put in − 80 °C for preparation of hepatic tissue homogenate and another part of left median lobe was flash-frozen in liquid nitrogen and stored at − 80 °C for quantitative RT-PCR and a part of right median lobe was kept for histopathological examination by immersion in 10% buffered formalin.
Determination of fasting serum insulin
Rat insulin ELISA kit (Assay kit Co., Ltd., Sunnyvale, USA) was used to measure the levels of insulin in sera of 14–18 h fasted rats according to the manufacturer’s instructions.
Calculation of IR and β-cell function indices
Homeostasis model assessment of insulin resistance (HOMA-IR) index and homeostasis model assessment of β-cell function (HOMA-β) were calculated according to the following equations (Matthews et al. 1985):
Determination of serum lipid profile
The serum levels of triglycerides (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-C) were colorimetrically measured using commercial kits, TG and TC (Spinreact, Santa Coloma, Spain) and HDL-C (Vitro Scient, Hannover, Germany). Very-low-density lipoprotein cholesterol (VLDL-C) and low-density lipoprotein cholesterol (LDL-C) in rat sera were calculated according to the equations VLDL-C = TG/5; LDL-C = TC − [VLDL + HDL-C] (Friedewald et al. 1972).
Determination of liver function biomarkers in serum
The serum levels of ALT (alanine aminotransferase) and AST (aspartate aminotransferase) were colorimetrically measured using commercial kits (Biomed diagnostics, Badr City, Egypt).
Determination of serum AGEs by quantitative fluorescence spectroscopy
Serum was diluted 500-fold with PBS, 20 μL (sensitivity limit) were used and diluted into 10 mL and then filtrated through Millex-GV filters (0.22 μm Millipore), and fluorescence spectra were recorded at room temperature in triplicate on a spectrofluorophotometer (Shimadzu scientific instruments, Japan). The excitation wavelength was adjusted at 370 nm, and the signal intensity was measured at the emission maximum of 445 nm (Monnier and Cerami 1981).
Preparation of hepatic and aortic homogenates
Hepatic and aortic tissues were weighed and homogenized using Omni-125 handheld homogenizer (Omni international, USA) in PBS as 10% (w/v). The homogenates of liver and aorta were spun at 5000g, 4 °C for 15 min, and the supernatant was freshly used for determination of oxidative biomarkers malondialdehyde (MDA), reduced glutathione (GSH), and superoxide dismutase (SOD). Another part of aortic homogenates was spun at 12000g, for 60 min, 4 °C, and 50 μL from the supernatant were used for RAGE determination.
Measurement of aortic receptor for advanced glycation end products (RAGE) levels
The aortic RAGE was measured using rat RAGE picokine ELISA kit (Boster Biological Technology, Pleasanton, California). Assay was performed according to the protocols recommended by the manufacturer.
Determination of hepatic and aortic levels of oxidative stress-related parameters
Thiobarbituric acid reactive substances were measured for determination of malondialdehyde (MDA); the absorbance was determined at 532 nm and expressed as nmol/g tissue (Ohkawa et al. 1979).
For determination of reduced glutathione (GSH), non-protein sulfhydryl compounds were measured by using trichloroacetic acid-deproteinized tissue supernatant according to the method of Ellman (Ellman 1959).
Additionally, the degree of inhibition of the auto-oxidation of pyrogallol at an alkaline pH by superoxide dismutase (SOD) was used for measuring SOD activity which expressed as U/g tissue, and the change in absorbance was recorded at 420 nm (Marklund and Marklund 1974).
Quantitative real-time polymerase chain reaction (RT-PCR)
RNA from liver and aorta samples was extracted using the trizol method, according to manufacturer’s instructions. One microgram from each sample was reverse transcribed using SensiFast cDNA synthesis kit (Bioline reagents Ltd., UK) into complementary DNA (cDNA) according to the manufacturer’s procedures. RT-PCR was performed using Hot Firepol Evagreen qPCR mix plus kit (Solis Biodyne, Tartu, Estonia) with a thermocycler Rotor Gene Q (Qiagen, Hilden, Germany).
The mRNA levels of hepatic insulin receptor substrate 1 (IRS1), phosphatidylinositol 3-kinase regulatory subunit 1 (PI3K-P85 subunit) and AKT serine/threonine kinase 2 (AKT2), aortic nitric oxide synthase 3 (eNOS), and NADPH oxidase 4 (NOX4) were normalized relative to 18S ribosomal RNA (Rn18S) in the same sample. Rn18S, IRS1, PI3K-P85 subunit, AKT2, eNOS, and NOX4 primers are shown in Table 1.
Measurement of hepatic phosphorylated Akt (serine 473) (P-AKT (ser473)) content
The hepatic P-AKT ser473 was measured using phospho-AKT (Ser 473) ELISA kit (Biovision, Milpitas Blvd., Milpitas, USA). Assay was performed according to the protocol recommended by the manufacturer.
Histopathological examination
Hepatic and aortic specimens were embedded in paraffin wax after fixing in 10% neutral-buffered formalin for 24 h and then sectioned and stained with hematoxylin-eosin (H&E) stain and liver samples were assessed for the presence of steatosis and inflammation; steatosis was determined by the fraction of fat in liver cells: 0 = absent, 1 = ˂ 25%, 2 = 25–50%, 3 = 50–75%, 4 = ˃ 75%, and the inflammation was graded from 0 (normal) to 3 (severe). The analyses were performed microscopically (Leica Imaging Systems, Cambridge, UK). The pathologist performing histopathological analyses was blinded to the group that each sample came from.
Statistical analysis
Data are expressed as mean ± standard error of mean (SEM). The GraphPad Prism software (GraphPad Software Inc. V5.02, San Diego, CA, USA) was used for statistical analyses and graphing, and significant difference between groups was measured by one-way analysis of variance (ANOVA) followed by Tukey-Kramer’s multiple comparisons post hoc test. Statistical significance was calculated at P < 0.05.
Results
Treatment with CIN for 30 days had no significant effect on all parameters involved in the study compared to control rats; therefore, results of CIN control group were omitted throughout the results section for simplicity.
Food intake, feeding efficiency, and body weight change
At the start and end of the experimental period, food intake, feeding efficiency, and weight change for all rats were recorded. As shown in Table 2, both mean food intake and body weight gain were not significantly different among groups.
OGTT and ITT
The change in the area under the curve (AUC) was determined to measure the change in OGTT and ITT. In OGTT, the AUC in the untreated diabetic group was significantly increased by 1.65-fold (P < 0.05, n = 6) when compared to control group. Treatment of diabetic rats with CIN significantly lowered the AUC than those of the control group (P < 0.05, n = 6) by 1.42-fold (Fig. 1a, b).
Effect of cinnamaldehyde (CIN) on oral glucose tolerance test (OGTT) and insulin tolerance test (ITT) of STZ-induced diabetic rats. a A curve showing the changes in blood glucose during the OGTT. b A bar chart showing the area under curve (AUC) of OGTT. c A curve showing the changes in blood glucose during the ITT. d A bar chart showing the AUC of ITT. Data are expressed as mean ± SEM, n = 6. *, #Significantly different from control or T2D group respectively, using one-way ANOVA followed by the Tukey-Kramer multiple comparisons post hoc test. BGL, blood glucose level; T2D, type 2 diabetic group; ANOVA, analysis of variance
In ITT, the AUC was significantly (P < 0.05, n = 6) elevated in diabetic rats by 1.63-fold when compared to the control group. Treatment of diabetic group with CIN significantly reduced the increase in AUC by 1.52-fold when compared to the diabetic group (P < 0.05, n = 6) (Fig. 1c, d).
Fasting blood glucose, fasting serum insulin, HOMA-IR, and HOMA-β
Fasting blood glucose level was significantly elevated by 1.77-fold (P < 0.05, n = 6) in diabetic group in comparison with the control rats. Thirty-day treatment of diabetic rats with CIN resulted in a reduction of the elevated fasting glucose by 1.33-fold when compared to diabetic group (Fig. 2a). Fasting insulin level was also significantly increased by1.81-fold (P < 0.05, n = 6) in diabetic group than the control. CIN significantly (P < 0.05, n = 6) lowered fasting insulin level returning it to its baseline normal levels (Fig. 2b) when compared to diabetic rats.
Effect of cinnamaldehyde (CIN) on fasting blood glucose (a) and insulin levels (b), HOMA-IR (c), and HOMA-β (d) of STZ-induced diabetic rats. Data are expressed as mean ± SEM, n = 6. *, #Significantly different from control or T2D group respectively, using one-way ANOVA followed by the Tukey-Kramer multiple comparisons post hoc test. T2D. type 2 diabetic group; ANOVA, analysis of variance; HOMA-IR index, homeostasis model assessment of insulin resistance index; HOMA-β index, homeostasis model assessment of β-cell function
In addition, diabetic group exhibited reductions in insulin sensitivity and β-cell function revealed by the significant elevation (P < 0.05, n = 6) in HOMA-IR (2.64-fold increase) (Fig. 2c) and decrease in HOMA-β (1.87-fold decrease) (Fig. 2d) indices as compared to control rats. Treatment with CIN significantly reduced diabetes-induced elevation of HOMA-IR index and increased the diabetes-induced reduction in HOMA-β returning both values to their baseline normal levels in comparison with diabetic group (P < 0.05, n = 6).
Serum lipid profile
TC, TG, LDL-C, and VLDL-C levels in diabetic rats were significantly (P < 0.05, n = 6) higher by 1.68-, 1.82-, 3.74-, and 1.84-fold respectively than those of the control group, while the level of HDL-C in the diabetic group was significantly (P < 0.05, n = 6) lower than those of the control group by1.96-fold. CIN markedly reduced TC, TG, LDL-C, and VLDL-C levels and raised the HDL-C concentration restoring them near to normal levels of control rats (Fig. 3).
Effect of cinnamaldehyde (CIN) on the lipid profile parameters of STZ-induced diabetic rats. Data are expressed as mean ± SEM, n = 6. *, #Significantly different from control or T2D group respectively, using one-way ANOVA followed by the Tukey-Kramer multiple comparisons post hoc test. T2D, type 2 diabetic group; ANOVA, analysis of variance; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; VLDL-C, very-low-density lipoprotein cholesterol
Serum levels of ALT and AST
Serum ALT and AST levels in diabetic group were significantly (P < 0.05, n = 6) higher than control rats by 1.95- and 1.55-fold, respectively (P < 0.05, n = 6). Thirty-day oral administration of CIN significantly restored the enzyme levels to near normal compared to diabetic group (Fig. 4).
Effect of cinnamaldehyde (CIN) on serum ALT (alanine aminotransferase) and AST (aspartate aminotransferase) of STZ-induced diabetic rats. Data are expressed as mean ± SEM, n = 6. *, #Significantly different from control or T2D group respectively, using one-way ANOVA followed by the Tukey-Kramer multiple comparisons post hoc test. T2D, type 2 diabetic group; ANOVA, analysis of variance
Serum AGE level and aortic RAGE content
Serum AGE level was significantly higher by 1.16-fold (P < 0.05, n = 6) in diabetic group when compared to control rats. Additionally, aortic RAGE content in the diabetic group was significantly elevated by 6.07-fold (P < 0.05, n = 6) when compared with control rats.
Thirty-day treatment of diabetic rats with CIN restored serum AGE level to the normal baseline levels (Fig. 5a) and significantly (P < 0.05, n = 6) reduced the raised aortic RAGE value by 4.56-fold when compared to diabetic group (Fig. 5b).
Effect of cinnamaldehyde (CIN) on serum AGEs (advanced glycation end products) (a) and aortic RAGE (receptor for advanced glycation end products) (b) of STZ-induced diabetic rats. Data are expressed as mean ± SEM, n = 6. *, #Significantly different from control or T2D group respectively, using one-way ANOVA followed by the Tukey-Kramer multiple comparisons post hoc test. T2D, type 2 diabetic group; ANOVA, analysis of variance
Oxidative stress markers in hepatic and aortic homogenates
Compared with control rats, hepatic MDA contents of the diabetic rats was significantly elevated by 1.99-fold (P < 0.05, n = 6) (Fig. 6a), while hepatic GSH (Fig. 6b) and SOD (Fig. 6c) of diabetic rats was significantly (P < 0.05, n = 6) lower than those of control rats by 2.07- and 2.47-fold, respectively. Thirty-day oral administration of CIN significantly (P < 0.05, n = 6) restored these levels near to normal values as compared to diabetic group.
Effect of cinnamaldehyde (CIN) on oxidative stress parameters: a malondialdehyde (MDA), b reduced glutathione (GSH), and c superoxide dismutase (SOD) in hepatic homogenate of STZ-induced diabetic rats. Data are expressed as mean ± SEM, n = 6. *, #Significantly different from control or T2D group respectively, using one-way ANOVA followed by the Tukey-Kramer multiple comparisons post hoc test. T2D, type 2 diabetic group; ANOVA, analysis of variance
In addition, aortic MDA content was significantly (P < 0.05, n = 6) raised by 2.08-fold (Fig. 7a), whereas GSH (Fig. 7b) and SOD (Fig. 7c) of diabetic group were significantly (P < 0.05, n = 6) lower than control rats by 1.38- and 2.06-fold, respectively. CIN administration significantly (P < 0.05, n = 6) raised GSH content by 1.28-fold and restored SOD activity to normal level when compared to the diabetic group. Meanwhile, CIN produced a non-significant reduction in MDA value as compared to the diabetic rats (P ˃ 0.05, n = 6).
Effect of cinnamaldehyde (CIN) on oxidative stress parameters: a malondialdehyde (MDA), b reduced glutathione (GSH), and c superoxide dismutase (SOD) in aortic homogenate of T2D rats. Data are expressed as mean ± SEM, n = 6. *, #Significantly different from control or T2D group respectively, using one-way ANOVA followed by the Tukey-Kramer multiple comparisons post hoc test. T2D, type 2 diabetic group; ANOVA, analysis of variance
Detection of hepatic IRS1, PI3K-P85 subunit, AKT2 and aortic eNOS, and NOX4 gene expression levels by RT-PCR
The mRNA expression of hepatic IRS1, PI3K-P85 subunit and AKT2 (Fig. 8), and aortic eNOS (Fig. 9a) were significantly (P < 0.05, n = 6) lowered by 11.11-, 20-, 16.66-, and 25-fold respectively in diabetic group as compared to control rats. Meanwhile, mRNA expression of aortic NOX4 (Fig. 9b) was significantly elevated by 19.2-fold (P < 0.05, n = 6) as compared to diabetic rats.
Effect of cinnamaldehyde (CIN) on mRNA expression of insulin receptor substrate 1(IRS1), phosphatidylinositol 3-kinase regulatory subunit1 (PI3K-P85 subunit), and AKT serine/threonine kinase 2 (AKT2) in hepatic tissue of STZ-induced diabetic rats. Data are expressed as mean ± SEM, n = 6. *, #Significantly different from control or T2D group respectively, using one-way ANOVA followed by the Tukey-Kramer multiple comparisons post hoc test. T2D, type 2 diabetic group; ANOVA, analysis of variance. Data are expressed as mean ± SEM, n = 6
Effect of cinnamaldehyde (CIN) on mRNA expression of NADPH oxidase 4 (NOX4) (a) and nitric oxide synthase 3 (eNOS) (b) in aortic tissue of STZ-induced diabetic rats. Data are expressed as mean ± SEM, n = 6. *, #Significantly different from control or T2D group respectively, using one-way ANOVA followed by Tukey-Kramer multiple comparisons post hoc test. T2D, type 2 diabetic group; ANOVA, analysis of variance
CIN administration significantly (P < 0.05, n = 6) elevated hepatic IRS1, AKT2, and aortic eNOS mRNA expression levels by 6.93-, 13.21-, and 14.95-fold respectively and decreased mRNA expression of aortic NOX4 to normal level when compared to diabetic group. In addition, treatment with CIN produced a non-significant change in hepatic PI3K-P85 subunit compared to the diabetic group.
Estimation of hepatic P-AKT (ser473) content
Hepatic P-AKT (ser473) was significantly decreased by 2.31-fold in diabetic rats (P < 0.05, n = 6) when compared with control rats (Fig. 10). Chronic treatment of diabetic rats with CIN significantly (P < 0.05, n = 6) increased hepatic P-AKT (ser473) content by 2.24-fold when compared to diabetic group.
Effect of cinnamaldehyde (CIN) on hepatic phosphorylated AKT (serine 473) (P-AKT ser473) content of STZ-induced diabetic rats. Data are expressed as mean ± SEM, n = 6. *, #Significantly different from control or T2D group respectively, using one-way ANOVA followed by Tukey-Kramer multiple comparisons post hoc test. T2D, type 2 diabetic group; ANOVA, analysis of variance
Histopathological examination of hepatic and aortic specimens
Liver specimens from control rats exhibited normal hepatocytes arranged in two cell thick plates and no necroinflammatory injury or steatosis (score 0) (Fig. 11a), while diabetic rats showed evident fatty change (steatosis) (score 3), hydropic degeneration (ballooning) of hepatocytes, focal lytic necrosis, and moderate inflammatory cellular infiltrates (score 2) (Fig. 11b). Treatment with CIN in diabetic rats caused a dramatic regression of steatosis (score 1) with mild inflammatory cellular infiltrates (score 1) and congested sinusoids (Fig. 11c).
Effect of cinnamaldehyde (CIN) on hepatic and aortic histopathological examination of STZ-induced diabetic rats (hematoxylin and eosin 400×) (n = 5 in each group). Group A: control group showing normal hepatocytes arranged in two cell thick plates with no evidence of steatosis (score 0) or necroinflammatory injury (score 0); group B: diabetic rats showing evident fatty change (steatosis), hydropic degeneration (ballooning) of hepatocytes, focal lytic necrosis, and portal tract expansion by inflammation and fibrosis; group C: diabetic + CIN group showing mild steatosis (score 1), mild inflammatory cellular infiltrate (score 1), and congested sinusoids; group D: control group showing normal appearance with no inflammation, degeneration, or necrosis; group E: diabetic rats showing mild inflammatory reaction, focal injury to endothelium, proliferation of smooth muscles of medial elastic lamina, and no necrosis; group F: diabetic + CIN group showing mild inflammatory reaction and focal injury to endothelium
In addition, aortic specimens from the control group showed normal appearance with no inflammation, degeneration, or necrosis (Fig. 11d), while diabetic rats showed mild inflammatory reaction, focal injury to endothelium, and proliferation of smooth muscles of medial elastic lamina (Fig. 11e). Treatment with CIN in diabetic rats showed mild inflammatory cellular infiltrate with focal injury to the endothelium (Fig. 11f). Histology scores of hepatic tissues are shown in Fig. 12.
Scatter dot plots of the histopathological assessment of hepatic tissue of rats. a Scores of steatosis in control, diabetic, and diabetic + CIN groups. b Scores of inflammation in control, diabetic, and diabetic + CIN groups. The median of each data set is indicated. *Significantly different when compared to control group using the Kruskal-Wallis test followed by Dunn’s multiple comparison test
Discussion
This study was designed to explore the beneficial effects of CIN on insulin signaling pathways in STZ-induced T2D rat model. Consistent with previous studies, a rat model of T2D was established by practical application of the fat + fructose/STZ method (Schaalan et al. 2009; Kumar et al. 2015; Nair et al. 2017; Wong et al. 2017). In this study, T2D was confirmed by impaired glucose and insulin tolerance which reflected by the increased AUCOGTT and AUCITT and the raised levels of fasting glucose, insulin, and HOMA-IR index, which confirmed the IR state. It also showed a decline of β-cell function that is elucidated by a reduction of HOMA-β. Additionally, a marked dyslipidemia, increased serum ALT and AST activity, a raised serum AGE and aortic RAGE levels, elevated MDA, and reduced GSH and SOD in hepatic and aortic homogenates. Moreover, the mRNA expression of hepatic IRS1, PI3K-P85 subunit, AKT2, and aortic eNOS was decreased, while the expression of aortic NOX4 was elevated.
As compared to control group, the average weight of the diabetic rats was not significantly different and this is consistent with a previous study which revealed that a 4-week adaptation period was needed for the HFD group as it began to have a higher growth rate at the sixth week and presented significantly heavier weights at the eighth week (Zhang et al. 2008). Additionally, Reed et al. showed that the weight of the chow-fed group and fat-fed group was not significantly different after STZ injection (Reed et al. 2000) and the study of Srinivasan et al. revealed that intraperitoneal injection of STZ (35 mg/kg) resulted in significant decrease in the body weights of the HFD-fed rats and the body weights remained largely not changed between control NPD-fed group and STZ-treated rats (Srinivasan et al. 2005).
Hyperinsulinemia and hyperglycemia are reported as an outcome of T2D (Nattrass and Bailey 1999). To evaluate CIN effect on glucose homeostasis in rats, OGTT, an important index for islet function evaluation was firstly performed (Buchanan et al. 2002).
In T2D, HOMA-IR ≤ 2 is rated as normal insulin sensitive, HOMA-IR 2–5 is moderate IR, and HOMA-IR ≥ 5 is severe IR. Therefore, this model was authenticated by HOMA-IR calculation (Islam and Loots 2009; Wilson and Islam 2012). T2D also elicited a decline of β-cell function which occurs because of the sustained load on β-cells in an attempt to recompense for IR (Reaven 1995); HOMA-β index is used for evaluation of β-cells basic function that correlates well with other validated physiological methods and a decrease of HOMA-β index is an indication of diminished β-cell function (Hermans et al. 1999; Wallace et al. 2004).
In this study, CIN treatment reduced fasting glucose, insulin levels, OGTTAUC, ITTAUC, HOMA-IR index, and increased HOMA-β index. A previous study showed that CIN lowered fasting blood glucose, fasting serum insulin, and HOMA-IR levels and its effects on decreasing fasting blood glucose; ameliorating insulin sensitivity could be probably mediated by upregulation of the expression of GLUT4 that can eventually improve the insulin activity and enhance the uptake and utilization of glucose in peripheral tissues (Zhang et al. 2008).
Serum lipid abnormalities is an important character of diabetic rats fed a HFD, and these include higher levels of TG and TC, which could be resulted from the increased mobilization of free fatty acids to central blood circulation from peripheral deposits (Veerapur et al. 2012). Hypercholesterolemia and hypertriglyceridemia may be due increasing the absorption of dietary cholesterol from the small intestine and increased formation and absorption of TG in the form of chylomicrons after exogenous consumption of fat-rich diet or decreased peripheral tissues uptake of TG and through raised endogenous production of TG-enriched hepatic VLDL in a diabetic condition (Srinivasan et al. 2005).
Chronic CIN treatment improved lipid profile in diabetic group as CIN significantly decreased TC, TG, LDL-C, and VLDL-C and increased HDL-C levels. These results were consistent with Zhang et al. study in which CIN treatment for 4 weeks displayed significant hypolipidemic effect in HFD/STZ-induced T2D rats (Zhang et al. 2008). Previous studies proved that cinnamon ameliorates the metabolism of glucose and lipids in patients with T2D by enhancing the uptake of glucose and synthesis of glycogen. In the study of Khan et al., it was shown that cinnamon treatment for 40 days in subjects with T2D effectively decreased the fasting blood glucose, TG, LDL-C, and TC (Khan et al. 2003). Similarly, CIN presented valuable antihyperglycemic and hypolipidemic functions in diabetic mice, which lowered the fasting blood glucose and ameliorated disturbance of lipid metabolism (Qin et al. 2004; Blevins et al. 2007).
Serum ALT and AST levels were measured as readout of hepatic function. The increased ALT and AST plasma levels in T2D rats results from the leakage of these enzymes into the blood stream from the cytosol of liver indicating hepatocellular injury (Concepcion et al. 1993; Ohaeri 2001; Subash et al. 2007; Wang et al. 2015; Huang et al. 2017). Cho et al. found a strong link between serum ALT level and HOMA-IR, and it has been revealed as a foreteller of T2D in human subjects (Cho et al. 2007).
Chronic CIN treatment decreased serum ALT and AST levels, and this is consistent with the Subash-Babu et al.’s study which demonstrated that CIN administration to STZ-induced male diabetic rats restored the altered plasma ALT and AST enzymes near to normal (Subash-Babu et al. 2014).
Oxidative stress due to the elevated levels of reactive oxygen species (ROS) results from chronic hyperglycemia which has the potential to alter antioxidant defense, increase lipid peroxidation, and further contribute to the pernicious cycle of IR (Balasubashini et al. 2004; Sepici-Dincel et al. 2007; Bansal et al. 2012). NADPH oxidase (NOX) has been protruded as the primary source of ROS in the cardiovascular tissues (Arozal et al. 2009). NADPH oxidase 4 (NOX4) is a constitutive enzyme that is concerned with controlling the response of oxidative stress (Cornelius et al. 2014), and it considered an oxygen sensor to generate ROS from molecular oxygen (Yong et al. 2013). NOX4 upregulation may motivate oxidative stress and cause dysfunction of mitochondria (Kuroda et al. 2010).
Previous reports demonstrated the increased plasma MDA level and the decreased activity of SOD inT2D patients (Huang et al. 2017), elevated MDA and lowered GSH level and SOD activity in the serum of T2D rats (Cvetkovic et al. 2009) and liver of T2D mice (Bansal et al. 2012), and elevated expression of NOX4 in the heart of diabetic rats and mice (Thandavarayan et al. 2009; Li et al. 2014). Additionally, it was reported that protein expression of renal and vascular NOX subunits in diabetic mice and cardiac NOX activity in diabetic rats were increased (Wenzel et al. 2008; Lakshmanan et al. 2011).
In the present study, CIN treatment increased hepatic and aortic content of GSH and activity of SOD and decreased hepatic MDA and the aortic NOX4 mRNA expression which may result in the decreased oxidative stress. These results elucidated a potent in vivo antioxidant activity of CIN that could be helpful toT2D patients. It has been demonstrated that CIN was more effective in prevention of oxidative injury to the cells which caused by cyadox in isolated rabbit erythrocytes (Farag et al. 2017). Additionally, pretreatment with CIN reduced the generation of ROS in glutamate-induced apoptosis and oxidative stress in PC12 cells as CIN attenuated the altered GSH, MDA levels, and SOD activity (Lv et al. 2017) and mitigated vascular oxidative stress in hypercholestrolemic rabbits (Nour et al. 2018). Moreover, previous studies revealed that NOX4 protein expression was lowered by CIN treatment in LPS-induced cardiac dysfunction in rats (Zhao et al. 2016) and CIN improved the oxidative stress status of the injured brain endothelial cells in human (Li et al. 2016)
The formation of AGEs with accompanying oxidative stress production is considered as a major participator in damage of pancreatic β-cell in T2D (Reed et al. 2000). In serum and tissues of people with T2D, increased concentration of circulating glucose, AGEs precursors, and oxidative stress accelerate AGE formation (Kilhovd et al. 1999).
In the present study, serum AGE level and aortic RAGE content were increased in diabetic group that was compatible with the study of Kihovd et al. (Kilhovd et al. 1999). Additionally, a previous study confirmed the increased mRNA expression of aortic rat RAGE and the increased concentration of aortic NOX and RAGE in T2D rats (Kang et al. 2016). CIN treatment lowered the increased levels of AGEs in serum and aortic RAGE content. It has been shown that CIN reversed the elevated AGE concentration and RAGE protein level in diabetic human renal proximal tubular cells (Huang et al. 2015).
In insulin signaling pathway, insulin induces insulin receptor substrate 1/2, upon that, it stimulates the PI3K pathway which thereafter leads to AKT phosphorylation and glycogen synthase kinase-3 inhibition, and AKT2 might stimulate other transcription factors leading to inhibition of gluconeogenesis and glycogen accumulation in liver (Xin et al. 2012; Cordero-Herrera et al. 2013). On the other hand, IRS1 plays a critical role in coupling signaling from the insulin receptor to PI3K and following eNOS activation (Montagnani et al. 2002). Additionally, PI3K was shown to play a considerable role in insulin signaling pathway associated with nitric oxide (NO) formation (Zeng et al. 2000). Previous studies have proved forthright confirmation for a complete biochemical pathway encompassing the IRS1, PI3K, and eNOS which explain insulin physiological actions in stimulating NO production (Zeng and Quon 1996; Zeng et al. 2000; Montagnani et al. 2002). It has been demonstrated that the expression of eNOS was decreased in aortas and second-order mesenteric arteries of T2D rats (Li et al. 2014; Alameddine et al. 2015).
Therefore, this study discussed CIN impact on IRS1, PI3K-P85 subunit, and AKT2 genes at mRNA expression level as they are participated in insulin receptor signaling pathway (Mokashi et al. 2017). Additionally, the impairment of AKT signaling is closely linked with hepatic insulin resistance (Sylow et al. 2014). Therefore, in order to determine whether CIN improves insulin signaling in diabetic rat liver, AKT activation was assessed. In the present study, CIN significantly activated AKT signaling in the liver of diabetic rats, as demonstrated by increased levels of phosphorylated AKT (serine 473).
CIN treatment increased the expression levels of IRS1, PI3K, and AKT2 genes. This indicates that CIN promotes the upregulation of these genes via insulin signaling pathway (IRS1/PI3K/AKT2 pathway). CIN treatment also increased eNOS expression which may lead to the ameliorated vascular function in T2D rats. This result is consistent with a previous study that elucidated the increased eNOS content in injured human brain microvascular endothelial cells upon CIN treatment (Li et al. 2016) and the study of Yuan et al. (2018) who showed that an induced phosphorylated AKT and its downstream target eNOS in human umbilical vein endothelial cells (Yuan et al. 2018).
In the present study, histopathological examination provided evidence that HFD/fructose/STZ-treatment produced hepatic and aortic lesions in rats because of associated hyperglycemia, hyperinsulinemia, increased oxidative stress, and hyperlipidemia. These results agreed with the previous report which elucidated that the liver samples of T2D rats presented loss of hepatocytes architecture and balloon degeneration of hepatocytes associated with hepatic necrosis (Huang et al. 2017).
In addition, aortic histopathological examination findings was consistent with the previous study which showed that the aorta of T2D rats presented breaking off the endothelial cells and proliferation of the smooth muscle cells in media (Guo et al. 2012).
CIN treatment reduced steatosis and inflammation observed in liver tissue of rats indicating the hepatoprotective effect of CIN against degeneration. Additionally, CIN treatment prevented proliferation of smooth muscles of media of aortic tissue of rats.
In summary, we provided the evidence that CIN has antidiabetic activity through upregulating IRS1/PI3K/AKT2 signaling pathway. Additionally, the beneficial effects of CIN could be partly due to antioxidant activity through suppression of NOX4 expression and attenuation of AGEs and RAGE elevation. In conclusion, CIN could be an adjunct to the treatment of T2D, even though additional researches are needed before the conclusive recommendations.
References
Alameddine A, Fajloun Z, Bourreau J, Gauquelin-Koch G, Yuan M, Gauguier D, Derbre S, Ayer A, Custaud MA, Navasiolava N (2015) The cardiovascular effects of salidroside in the Goto-Kakizaki diabetic rat model. J Physiol Pharmacol 66:249–257
Anand P, Murali KY, Tandon V, Murthy PS, Chandra R (2010) Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem Biol Interact 186:72–81
Arozal W, Watanabe K, Veeraveedu PT, Ma M, Thandavarayan RA, Suzuki K, Tachikawa H, Kodama M, Aizawa Y (2009) Effects of angiotensin receptor blocker on oxidative stress and cardio-renal function in streptozotocin-induced diabetic rats. Biol Pharm Bull 32:1411–1416
Balasubashini MS, Rukkumani R, Viswanathan P, Menon VP (2004) Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother Res 18:310–314
Bansal P, Paul P, Mudgal J, Nayak PG, Pannakal ST, Priyadarsini KI, Unnikrishnan MK (2012) Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice. Exp Toxicol Pathol 64:651–658
Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP (2005) Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl) 83:876–886
Blevins SM, Leyva MJ, Brown J, Wright J, Scofield RH, Aston CE (2007) Effect of cinnamon on glucose and lipid levels in non insulin-dependent type 2 diabetes. Diabetes Care 30:2236–2237
Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP (2002) Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 51:2796–2803
Cai S, Sun W, Fan Y, Guo X, Xu G, Xu T, Hou Y, Zhao B, Feng X, Liu T (2016) Effect of mulberry leaf (Folium Mori) on insulin resistance via IRS-1/PI3K/Glut-4 signalling pathway in type 2 diabetes mellitus rats. Pharm Biol 54:2685–2691
Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296:1655–1657
Cao H, Polansky MM, Anderson RA (2007) Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch Biochem Biophys 459:214–222
Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR (1994) Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol 14:4902–4911
Cho NH, Jang HC, Choi SH, Kim HR, Lee HK, Chan JC, Lim S (2007) Abnormal liver function test predicts type 2 diabetes: a community-based prospective study. Diabetes Care 30:2566–2568
Choi KM, Lee YS, Kim W, Kim SJ, Shin KO, Yu JY, Lee MK, Lee YM, Hong JT, Yun YP, Yoo HS (2014) Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. J Nutr Biochem 25:201–207
Concepcion NM, Pilar MM, Martin A, Jimenez J, Pilar UM (1993) Free radical scavenger and antihepatotoxic activity of Rosmarinus tomentosus. Planta Med 59:312–314
Cordero-Herrera I, Martin MA, Bravo L, Goya L, Ramos S (2013) Cocoa flavonoids improve insulin signalling and modulate glucose production via AKT and AMPK in HepG2 cells. Mol Nutr Food Res 57:974–985
Cornelius C, Koverech G, Crupi R, Di PR, Koverech A, Lodato F, Scuto M, Salinaro AT, Cuzzocrea S, Calabrese EJ, Calabrese V (2014) Osteoporosis and Alzheimer pathology: role of cellular stress response and hormetic redox signaling in aging and bone remodeling. Front Pharmacol 5:120
Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ (2000) Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest 105:311–320
Cvetkovic T, Mitic B, Lazarevic G, Vlahovic P, Antic S, Stefanovic V (2009) Oxidative stress parameters as possible urine markers in patients with diabetic nephropathy. J Diabetes Complicat 23:337–342
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Farag MR, Alagawany M, Tufarelli V (2017) In vitro antioxidant activities of resveratrol, cinnamaldehyde and their synergistic effect against cyadox-induced cytotoxicity in rabbit erythrocytes. Drug Chem Toxicol 40:196–205
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Govindaraj J, Sorimuthu PS (2015) Rosmarinic acid modulates the antioxidant status and protects pancreatic tissues from glucolipotoxicity mediated oxidative stress in high-fat diet: streptozotocin-induced diabetic rats. Mol Cell Biochem 404:143–159
Guo Z, Zhang R, Li J, Xu G (2012) Effect of telmisartan on the expression of adiponectin receptors and nicotinamide adenine dinucleotide phosphate oxidase in the heart and aorta in type 2 diabetic rats. Cardiovasc Diabetol 11:94
Hermans MP, Levy JC, Morris RJ, Turner RC (1999) Comparison of tests of beta-cell function across a range of glucose tolerance from normal to diabetes. Diabetes 48:1779–1786
Huang JS, Lee YH, Chuang LY, Guh JY, Hwang JY (2015) Cinnamaldehyde and nitric oxide attenuate advanced glycation end products-induced the Jak/STAT signaling in human renal tubular cells. J Cell Biochem 116:1028–1038
Huang XL, He Y, Ji LL, Wang KY, Wang YL, Chen DF, Geng Y, OuYang P, Lai WM (2017) Hepatoprotective potential of isoquercitrin against type 2 diabetes-induced hepatic injury in rats. Oncotarget 8:101545–101559
Islam MS, Loots dT (2009) Experimental rodent models of type 2 diabetes: a review. Methods Find Exp Clin Pharmacol 31:249–261
Kahn BB (1998) Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell 92:593–596
Kanai F, Ito K, Todaka M, Hayashi H, Kamohara S, Ishii K, Okada T, Hazeki O, Ui M, Ebina Y (1993) Insulin-stimulated GLUT4 translocation is relevant to the phosphorylation of IRS-1 and the activity of PI3-kinase. Biochem Biophys Res Commun 195:762–768
Kang MK, Chung WB, Hong SK, Kim OR, Ihm SH, Chang K, Seung KB (2016) Effects of candesartan cilexetil and amlodipine orotate on receptor for advanced glycation end products expression in the aortic wall of Otsuka Long-Evans Tokushima Fatty (OETFF) type 2 diabetic rats. Arch Pharm Res 39:565–576
Kar A, Choudhary BK, Bandyopadhyay NG (2003) Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J Ethnopharmacol 84:105–108
Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA (2003) Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 26:3215–3218
Khare P, Jagtap S, Jain Y, Baboota RK, Mangal P, Boparai RK, Bhutani KK, Sharma SS, Premkumar LS, Kondepudi KK, Chopra K, Bishnoi M (2016) Cinnamaldehyde supplementation prevents fasting-induced hyperphagia, lipid accumulation, and inflammation in high-fat diet-fed mice. Biofactors 42:201–211
Kilhovd BK, Berg TJ, Birkeland KI, Thorsby P, Hanssen KF (1999) Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care 22:1543–1548
Kumar SA, Magnusson M, Ward LC, Paul NA, Brown L (2015) Seaweed supplements normalise metabolic, cardiovascular and liver responses in high-carbohydrate, high-fat fed rats. Mar Drugs 13:788–805
Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J (2010) NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A 107:15565–15570
Lakshmanan AP, Watanabe K, Thandavarayan RA, Sari FR, Harima M, Giridharan VV, Soetikno V, Kodama M, Aizawa Y (2011) Telmisartan attenuates oxidative stress and renal fibrosis in streptozotocin induced diabetic mice with the alteration of angiotensin-(1-7) mas receptor expression associated with its PPAR-gamma agonist action. Free Radic Res 45:575–584
Li XW, Hao W, Liu Y, Yang JR (2014) Effect of sequoyitol on expression of NOX4 and eNOS in aortas of type 2 diabetic rats. Yao Xue Xue Bao 49:329–336
Li XD, Gu LW, Ran QS, Zhou P, Zhan XL, Li CH, Jiang TL (2016) Protective effects of three phenylallyl compounds from Guizhi decoction ox-LDL-induced oxidative stress injury of human brain microvascular endothelial cells. Zhongguo Zhong Yao Za Zhi 41:2315–2320
Luo C, Yang H, Tang C, Yao G, Kong L, He H, Zhou Y (2015) Kaempferol alleviates insulin resistance via hepatic IKK/NF-kappaB signal in type 2 diabetic rats. Int Immunopharmacol 28:744–750
Lv C, Yuan X, Zeng HW, Liu RH, Zhang WD (2017) Protective effect of cinnamaldehyde against glutamate-induced oxidative stress and apoptosis in PC12 cells. Eur J Pharmacol 815:487–494
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Mokashi P, Bhatt LK, Khanna A, Pandita N (2017) Swertisin rich fraction from Enicostema littorale ameliorates hyperglycemia and hyperlipidemia in high-fat fed diet and low dose streptozotacin induced type 2 diabetes mellitus in rats. Biomed Pharmacother 96:1427–1437
Monnier VM, Cerami A (1981) Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science 211:491–493
Montagnani M, Ravichandran LV, Chen H, Esposito DL, Quon MJ (2002) Insulin receptor substrate-1 and phosphoinositide-dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol Endocrinol 16:1931–1942
Nair S, Gagnon J, Pelletier C, Tchoukanova N, Zhang J, Ewart HS, Ewart KV, Jiao G, Wang Y (2017) Shrimp oil extracted from the shrimp processing waste reduces the development of insulin resistance and metabolic phenotypes in diet-induced obese rats. Appl Physiol Nutr Metab 42:841–849
Nattrass M, Bailey CJ (1999) New agents for type 2 diabetes. Baillieres Best Pract Res Clin Endocrinol Metab 13:309–329
Nour OAA, Shehatou GSG, Rahim MA, El-Awady MS, Suddek GM (2018) Cinnamaldehyde exerts vasculoprotective effects in hypercholestrolemic rabbits. Naunyn Schmiedebergs Arch Pharmacol 391:1203–1219
Ohaeri OC (2001) Effect of garlic oil on the levels of various enzymes in the serum and tissue of streptozotocin diabetic rats. Biosci Rep 21:19–24
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Paneni F, Costantino S, Cosentino F (2014) Insulin resistance, diabetes, and cardiovascular risk. Curr Atheroscler Rep 16:419
Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y (2004) Cinnamon extract prevents the insulin resistance induced by a high-fructose diet. Horm Metab Res 36:119–125
Ramasamy R, Yan SF, Schmidt AM (2009) RAGE: therapeutic target and biomarker of the inflammatory response—the evidence mounts. J Leukoc Biol 86:505–512
Reaven GM (1995) Pathophysiology of insulin resistance in human disease. Physiol Rev 75:473–486
Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM (2000) A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism 49:1390–1394
Rubino F, Marescaux J (2004) Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 239:1–11
Saleh S, El-Maraghy N, Reda E, Barakat W (2014) Modulation of diabetes and dyslipidemia in diabetic insulin-resistant rats by mangiferin: role of adiponectin and TNF-alpha. An Acad Bras Cienc 86:1935–1948
Schaalan M, El-Abhar HS, Barakat M, El-Denshary ES (2009) Westernized-like-diet-fed rats: effect on glucose homeostasis, lipid profile, and adipocyte hormones and their modulation by rosiglitazone and glimepiride. J Diabetes Complicat 23:199–208
Schmidt AM, Yan SD, Yan SF, Stern DM (2000) The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta 1498:99–111
Sepici-Dincel A, Acikgoz S, Cevik C, Sengelen M, Yesilada E (2007) Effects of in vivo antioxidant enzyme activities of myrtle oil in normoglycaemic and alloxan diabetic rabbits. J Ethnopharmacol 110:498–503
Shimoda I, Koizumi M, Shimosegawa T, Shishido T, Ono T, Sato K, Ishizuka J, Toyota T (1993) Physiological characteristics of spontaneously developed diabetes in male WBN/Kob rat and prevention of development of diabetes by chronic oral administration of synthetic trypsin inhibitor (FOY-305). Pancreas 8:196–203
Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P (2005) Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 52:313–320
Subash BP, Prabuseenivasan S, Ignacimuthu S (2007) Cinnamaldehyde—a potential antidiabetic agent. Phytomedicine 14:15–22
Subash-Babu P, Alshatwi AA, Ignacimuthu S (2014) Beneficial antioxidative and antiperoxidative effect of cinnamaldehyde protect streptozotocin-induced pancreatic beta-cells damage in Wistar rats. Biomol Ther (Seoul) 22:47–54
Sylow L, Kleinert M, Pehmoller C, Prats C, Chiu TT, Klip A, Richter EA, Jensen TE (2014) Akt and Rac1 signaling are jointly required for insulin-stimulated glucose uptake in skeletal muscle and downregulated in insulin resistance. Cell Signal 26:323–331
Tanji N, Markowitz GS, Fu C, Kislinger T, Taguchi A, Pischetsrieder M, Stern D, Schmidt AM, D’Agati VD (2000) Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol 11:1656–1666
Thandavarayan RA, Watanabe K, Ma M, Gurusamy N, Veeraveedu PT, Konishi T, Zhang S, Muslin AJ, Kodama M, Aizawa Y (2009) Dominant-negative p38alpha mitogen-activated protein kinase prevents cardiac apoptosis and remodeling after streptozotocin-induced diabetes mellitus. Am J Physiol Heart Circ Physiol 297:H911–H919
Veerapur VP, Prabhakar KR, Thippeswamy BS, Bansal P, Srinivasan KK, Unnikrishnan MK (2012) Antidiabetic effect of Ficus racemosa Linn. stem bark in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rats: a mechanistic study. Food Chem 132:186–193
Vlassara H, Uribarri J (2014) Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep 14:453
Wallace TM, Levy JC, Matthews DR (2004) Use and abuse of HOMA modeling. Diabetes Care 27:1487–1495
Wang Q, Jiang C, Fang S, Wang J, Ji Y, Shang X, Ni Y, Yin Z, Zhang J (2013) Antihyperglycemic, antihyperlipidemic and antioxidant effects of ethanol and aqueous extracts of Cyclocarya paliurus leaves in type 2 diabetic rats. J Ethnopharmacol 150:1119–1127
Wang F, Zhou Z, Ren X, Wang Y, Yang R, Luo J, Strappe P (2015) Effect of Ganoderma lucidum spores intervention on glucose and lipid metabolism gene expression profiles in type 2 diabetic rats. Lipids Health Dis 14:49
Wenzel P, Schulz E, Oelze M, Muller J, Schuhmacher S, Alhamdani MS, Debrezion J, Hortmann M, Reifenberg K, Fleming I, Munzel T, Daiber A (2008) AT1-receptor blockade by telmisartan upregulates GTP-cyclohydrolase I and protects eNOS in diabetic rats. Free Radic Biol Med 45:619–626
Wilson RD, Islam MS (2012) Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacol Rep 64:129–139
Wong WY, Ward LC, Fong CW, Yap WN, Brown L (2017) Anti-inflammatory gamma- and delta-tocotrienols improve cardiovascular, liver and metabolic function in diet-induced obese rats. Eur J Nutr 56:133–150
Xin G, Honggui L, Hang X, Shihlung W, Hui D, Fuer L, Alex JL, Chaodong W (2012) Glycolysis in the control of blood glucose homeostasis. Acta Pharm Sin B 2:358–367
Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G (2013) Prevalence and control of diabetes in Chinese adults. JAMA 310:948–959
Yong R, Chen XM, Shen S, Vijayaraj S, Ma Q, Pollock CA, Saad S (2013) Plumbagin ameliorates diabetic nephropathy via interruption of pathways that include NOX4 signalling. PLoS One 8:e73428
Youn HS, Lee JK, Choi YJ, Saitoh SI, Miyake K, Hwang DH, Lee JY (2008) Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem Pharmacol 75:494–502
Yuan X, Han L, Fu P, Zeng H, Lv C, Chang W, Runyon RS, Ishii M, Han L, Liu K, Fan T, Zhang W, Liu R (2018) Cinnamaldehyde accelerates wound healing by promoting angiogenesis via up-regulation of PI3K and MAPK signaling pathways. Lab Investig 98:783–798
Zeng G, Quon MJ (1996) Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest 98:894–898
Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, Quon MJ (2000) Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 101:1539–1545
Zhang W, Xu YC, Guo FJ, Meng Y, Li ML (2008) Anti-diabetic effects of cinnamaldehyde and berberine and their impacts on retinol-binding protein 4 expression in rats with type 2 diabetes mellitus. Chin Med J 121:2124–2128
Zhao H, Zhang M, Zhou F, Cao W, Bi L, Xie Y, Yang Q, Wang S (2016) Cinnamaldehyde ameliorates LPS-induced cardiac dysfunction via TLR4-NOX4 pathway: the regulation of autophagy and ROS production. J Mol Cell Cardiol 101:11–24
Author contribution statement
MA, GS, GMS, and HS designed the research. MA and GS conducted experiments and analyzed data. MA and GMS wrote the manuscript. GS, GMS, and HS revised the manuscript. RA performed pathological assessments. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Abdelmageed, M.E., Shehatou, G.S., Abdelsalam, R.A. et al. Cinnamaldehyde ameliorates STZ-induced rat diabetes through modulation of IRS1/PI3K/AKT2 pathway and AGEs/RAGE interaction. Naunyn-Schmiedeberg's Arch Pharmacol 392, 243–258 (2019). https://doi.org/10.1007/s00210-018-1583-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-018-1583-4