Abstract
Cognitive impairment is a common comorbidity of chronic pain, significantly disrupting patients’ quality of life. Despite this comorbidity being clinically recognized, the underlying neuropathological mechanisms remain unclear. Recent preclinical studies have focused on the fundamental mechanisms underlying the coexistence of chronic pain and cognitive decline. Pain chronification is accompanied by structural and functional changes in the neural substrate of cognition. Based on the developments in electrophysiology and optogenetics/chemogenetics, we summarized the relevant neural circuits involved in pain-induced cognitive impairment, as well as changes in connectivity and function in brain regions. We then present the cellular and molecular alternations related to pain-induced cognitive impairment in preclinical studies, mainly including modifications in neuronal excitability and structure, synaptic plasticity, glial cells and cytokines, neurotransmitters and other neurochemicals, and the gut-brain axis. Finally, we also discussed the potential treatment strategies and future research directions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain is a complex experience consisting of sensory, affective, and cognitive dimensions [1]. Chronic pain (CP) is often defined by pain duration, which persists beyond the healing process or lasts longer than 3 months [2]. Cognitive dysfunction is a particularly burdensome comorbidity of CP. Clinical evidence highlights that approximately 20% of CP individuals experience cognitive impairments [3], and those with multisite pain have a higher risk of dementia and faster cognitive decline [4]. Patients with CP are reported to experience impairments in memory, language, attention, processing speed, and visual-spatial function [5, 6]. Despite the comorbidity being clinically well-established, the involved neuropathological mechanisms are difficult to investigate in patients. The commonly used animal models of chronic pain, such as complete Freund adjuvant (CFA)-induced inflammatory pain, spared nerve injury (SNI)-induced neuropathic pain, and colitis-induced visceral pain, have contributed to exploring the neural mechanisms underlying CP-induced cognitive impairment and potential interventions [7].
One hypothesis for the comorbidity between chronic pain and cognitive impairment is that pain involves a complex neural network (called the “pain matrix”), which shares overlapping brain regions with cognitive function [8, 9]. Neuroimaging studies indicated that pain chronification is accompanied by reshuffling of brain activity from sensory to emotional and limbic structures [10]. Structural and functional plasticity changes in the corticolimbic regions contribute to the development of chronic pain, including the media prefrontal cortex (mPFC), anterior cingulate cortex (ACC), hippocampus, and amygdala [11]. Alternation in these regions during chronic pain may also disrupt cognitive processing. Here, we reviewed current preclinical research aimed at modeling comorbid cognitive deficits in chronic pain. We then discuss the possible mechanisms underlying CP-induced cognitive dysfunction in the relevant brain regions, involving neural circuits, neuronal changes, synaptic plasticity, glial cells, neurotransmitters, and other neurochemicals, as well as the gut-brain axis. Finally, we discuss the potential treatment strategies and future directions to enhance our understanding of the mechanisms underlying CP-induced cognitive deficits.
Pain and Cognitive Dysfunction
Chronic pain can disrupt normal cognitive processes, leading to impairments in learning and memory, attention, and cognitive flexibility [12]. We present an overview of preclinical evidence for CP-related cognitive impairment assessed by different cognitive domains (Table 1) and discuss the factors influencing these consequences. Our primary focus has been on discussing cognitive impairments in models of CP with a peripheral origin, specifically neuropathic pain, inflammatory pain, and visceral pain. Certain models, such as chronic unpredictable stress-induced pain and stroke-induced central pain, are excluded due to the challenge of establishing whether chronic pain is the primary cause of cognitive impairments.
Pain and Memory Impairment
Pain negatively impacts memory performance in rodents, including discriminative memory, working memory, and spatial memory, which highly relies on the normal function of the hippocampus and prefrontal cortex (PFC). Memory dysfunction usually occurs approximately 2 to 4 weeks after the injury and can last for at least a year.
Discriminative memory is often assessed by the novel object recognition test (NORT) in pain models. The interval between NORT training and testing phases, known as memory consolidation, allows evaluation of short-term (5 min to 2 h) and long-term (24 h) memory [48]. Our previous research has shown significant impairments in long-term recognition memory in a rodent neuropathic pain model [25, 26]. Interestingly, the results of short-term cognitive impairment in chronic pain are controversial. For instance, some studies noted short-term memory impairment in rodents with SNI lasting at least 1 month [17,18,19,20,21], while others observed no short-term memory impairment in partial sciatic nerve ligation (PSNL) and chronic constriction injury (CCI) models [14, 15, 49, 50]. It is worth noting that no memory dysfunction was evident within a week after pain surgery [13], suggesting that cognitive impairment might be associated with the progression of chronic pain over time. Additionally, the NORT has been used to assess discrimination between dissimilar (recognition memory) and similar (pattern separation) objects in neuropathic pain mice. Guida et al. found that 1-month post-SNI mice struggled with both similar and dissimilar objects in short- and long-term memory tests, while 12-month post-SNI mice showed normal recognition of dissimilar objects but difficulty recognizing similar objects in long-term retention tests [22]. These results indicate that discriminative memory impairment in pain is relevant to pain progression, behavioral paradigms, and task difficulty.
Working memory (WM) is another form of short-term memory that involves planning and performing actions rather than passive information storage [51]. Y-maze is frequently used in preclinical studies to evaluate spatial working memory by measuring spontaneous alternations. Studies have indicated that the spontaneous alternations rate was decreased in the CCI and SNI groups within 2 to 3 weeks after surgery [15, 17, 23, 32, 52]. Other tasks, like food-reinforcement delayed spatial alternation in figure-eight [53] and T-mazes [54], revealed poor spatial working memory performance in mice with neuropathic and inflammatory pain.
Spatial reference memory involves the recall of spatial relationships between different locations or landmarks. The Morris Water Maze (MWM) task assesses long-term memory, with a probe trial conducted at least 24 h after training [48]. Xia et al. revealed that CCI mice initially displayed similar performance to controls in the MWM within 5 days, but exhibited increased escape latency and decreased time in the target quadrant after 21 days [34]. Furthermore, mice with chronic pain took longer to find the platform compared to those with acute pain or no pain, indicating spatial memory impairment during chronic pain [55]. Moreover, Mohammadi et al. demonstrated that inflammatory pain led to spatial memory impairment 7 days after CFA injection and gradually recovered by day 21 [31], which was correlated with the progression of thermal hyperalgesia. These results indicate that persistent painful stimulation is a primary contributor to cognitive pathology.
Pain and Attention Deficits
Clinical research has discovered that individuals with chronic pain often struggle with tasks requiring sustained attention [56]. Likewise, rats in the osteoarthritis group and SNI group exhibited higher error rates and more omissions in the 5-choice serial reaction time task (5-CSRTT) [38, 39]. Importantly, these impairments persisted even with temporary pain relief, suggesting permanent alterations in neurobiological attention mechanisms due to chronic pain [38]. Moazen and colleagues demonstrated that CCI rats exhibited attention deficits in the tasks of both low and high attention needs [40]. In addition, poor performance on visual attention tasks was found in both SNI mice [41] and colitis rats with chronic visceral pain [42].
Pain and Cognitive Flexibility
Cognitive flexibility is an executive function primarily controlled by the PFC. Stephanie et al. used the attentional set-shifting task (ASST) to evaluate cognitive flexibility deficits induced by SNI. The results showed impairment in both male and female mice, with males requiring more trials and making more mistakes [44]. Importantly, short-term analgesia with clonidine did not reverse these deficits, indicating that chronic pain caused persistent deficits in high brain regions [44]. Furthermore, spinal nerve ligation (SNL) rats, osteoarthritis rats, and colitis rats exhibited a preference for the familiar but less profitable options in the gambling tasks [43, 46]. These findings highlight that chronic pain significantly disrupts executive functions.
Methodological Considerations
In considering methodologies for studying the mechanism underlying pain-induced cognitive impairment in preclinical research, several factors should be considered. Firstly, as mentioned above, the transition of pain from the acute phase to the chronic phase is crucial for memory impairment. Therefore, it is important to determine the time points of behavioral assessment. Secondly, task difficulty is a significant factor influencing cognitive assessment [57]. Phelps and colleagues found that rats with nerve damage showed memory deficits in more challenging tasks in the NORT, indicating that pain may consume cognitive resources, leaving limited capacity for complex tasks [58]. Furthermore, it is worth noting the potential impact of the side of pain on behavioral changes, due to the ascending pathways of nociceptive input and lateralization of brain function [59, 60]. For instance, Leite-Almeida et al. revealed that left-sided nerve lesions negatively affected emotional behavior in SNI mice, while right-sided lesions worsened cognitive performance, especially in PFC-dependent tasks [60]. These differences may relate to different PFC activity changes in SNI-L (left) and SNI-R (right) rats [61]. Moreover, emerging evidence suggested sexual dimorphism in cognition, with male mice being more vulnerable to chronic pain-related cognitive deficits [44, 62]. Additionally, differences in animal species (mice vs. rats), chronic pain models, and baseline pain intensity can contribute to variations in behavioral outcomes. Therefore, a comprehensive understanding of the pain-cognition relationship in preclinical studies requires consideration and control of these methodological factors.
Neural Circuits Involved in Pain-Related Cognitive Dysfunction
Brain imaging studies showed that chronic pain engages the transformation of brain activity from the sensory areas to the corticolimbic regions. However, the continuous activation of corticolimbic circuitry may in turn induce their functional and anatomic alterations, and affect cognitive processes [63]. Here, we discuss the brain region and related neural circuits implicated in chronic pain-related cognitive dysfunction (Fig.1).
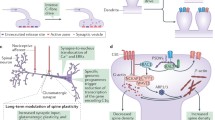
Altered neural circuits in the pain condition that contribute to cognitive deficits. (mPFC, media prefrontal cortex; PrL, prelimbic cortex; IL, infralimbic cortex; ACC, anterior cingulate cortex; Hip, hippocampus; dCA1, dorsal CA1; vCA1, ventral CA1; DG, dentate gyrus; VTA, ventral tegmental area; LHb, lateral habenula; BLA, basolateral amygdala; NAc, nucleus accumbens; MD, mediodorsal thalamus)
Hippocampal-Related Circuits
The hippocampus is a key region of the limbic system involved in learning and memory, emotion, and sensory-motor integration. Neuroimaging studies revealed reduced hippocampal grey matter volume [64], altered shape [65], and disrupted functional connectivity in chronic pain patients [66]. Cognitive dysfunction in CP patients correlated with hippocampal structural and functional abnormalities [67], and those with persistent back pain exhibited a large decrease in hippocampal connectivity with mPFC [68].
Similar to clinical research, a recent study showed that SNI reduced the activity of hippocampal dCA1 neurons and dCA1-mPFC connectivity, and optogenetic activation of dCA1-mPFC projections reversed pain and memory deficits [69]. In the inflammatory pain models, the inhibited ventral hippocampus CA1 (vCA1)-infralimbic cortex (IL) pathway contributed to pain-related cognitive deficits [70]. Moreover, Cardoso-Cruz et al. found chronic neuropathic pain disrupted neuronal activity and theta oscillations across the dCA1-vCA1 axis during cognitive tasks, which was associated with changes in the dopaminergic balance of intrahippocampal networks [54, 71]. Hence, both the interconnectivity and extra circuitry of hippocampal subregions are disrupted by chronic pain. Neuroimaging reported a decrease in medial dorsal thalamus (MD) connectivity with mPFC and hippocampus in patients with CP, which correlates with negative emotions [72]. This leads us to speculate that MD, as an important relay of pain pathway, may be related to hippocampal dysfunction during chronic pain, although the specific mechanisms remain unknown.
mPFC-Related Circuits
The mPFC is a hub that integrates information about pain and cognition [73]. Structural and functional changes in the mPFC contribute to cognitive impairment in chronic pain. Brain imaging has shown a decreased mPFC grey matter volume in individuals with CP [64], which is closely correlated with working memory performance in patients [74]. In addition, functional MRI (fMRI) revealed that patients with chronic low back pain showed significantly less activation in the cingulate-frontal-parietal cognitive/attention network during attention-demanding tasks [75].
Animal research by Ji and colleagues demonstrated that the deactivation of the mPFC contributes to the cognitive comorbidity of pain. Chronic pain-induced hyperactivity in the basolateral amygdala (BLA) led to mPFC deactivation through glutamate-driven synaptic inhibition, causing decision-making deficits [76]. Similarly, Cardoso-Cruz et al. found that nerve lesions impaired spatial memory performance by disrupting information flowing and altering oscillation patterns in the mPFC–dCA1 circuit [53]. They further demonstrated that optogenetic inhibition of PL (prelimbic cortex)-mPFC glutamatergic neurons reversed neuropathic pain-induced WM deficits by restoring mPFC-dCA1 interaction [77] and local neuronal firing activity [78]. Furthermore, it is known that the mPFC-MD-hippocampus interactions are involved in spatial memory [79], while chronic inflammatory pain reduced mPFC-MD activity during a spatial alternation WM task, leading to spatial working memory impairment [80]. In addition, chronic pain enhanced functional connectivity between PL-mPFC to NAc (nucleus accumbens), and its optogenetic inhibition restored pain-induced working memory deficits [81]. These results suggest that chronic pain disrupts the neural network connections of the mPFC and significantly impairs the cognitive process. Neuronal excitability changes [82] and neurotransmitter imbalances [83] may be the reasons for the mPFC inactivation.
ACC-Related Circuits
The ACC plays a critical role in pain perception, unpleasantness of pain, memory, and attention [84]. EEG and neuroimaging data showed activation of the ACC in both acute and chronic pain conditions [85, 86], which aligns with increased activity of pyramidal cells in the ACC in mice with neuropathic pain [87]. fMRI studies found that the activity evoked by pain and attention-demanding tasks is typically located in similar regions of the cingulate cortex, indicating that pain-induced ACC activity may influence cognitive process [88].
ACC receives nociceptive information from the thalamus and brain regions involved in the regulation of emotional states, such as the amygdala and insular cortex during pain [89]. It has been reported that the BLA and ACC form an interconnected neural circuit involved in decision-making [90]. Cao et al. found chronic visceral pain impaired theta oscillation synchronization between the BLA and ACC, contributing to execution deficits in visceral hypersensitivity (VH) rats [91]. Moreover, they found that reduced ACC L-lactate release suppressed neuronal theta synchrony, and optogenetic astrocytic activation restored BLA-ACC desynchronization and improved decision-making performance via lactate release in VH rats [47]. Furthermore, enhancing oligodendrocyte myelination restored ACC network function and improved cognitive behaviors in VH rats [92]. Importantly, it is essential to further investigate distinct ACC circuits that likely contribute to the development and maintenance of pain-induced cognitive dysfunction and their maladaptive changes during pain chronicity.
VTA-Related Circuits
The ventral tegmental area (VTA) is an important part of the mesolimbic dopamine (DA) system and participates in pain-related depression [93], anxiety [94], as well as reward and motivation [95]. Studies showed that reduced VTA-to-dentate gyrus (DG) dopamine projections negatively affected neurogenesis and spatial memory formation during chronic pain [34]. The inhibited activity of VTA dopaminergic neurons caused by chronic pain may be regulated by lateral habenula (LHb). Alemi et al. demonstrated that LHb hyperactivity increased local GABAergic inhibition on VTA dopamine neurons, resulting in working memory impairment in CFA rats, which could be reversed by optogenetic inhibition of LHb [96]. It is worth noting that hyperactivity of LHb glutamatergic neurons induced endoplasmic reticulum stress, inflammatory responses, and dopaminergic neuronal damage in the VTA [97]. The disruption of VTA decreased dendritic spine density in the PFC and hippocampus and impaired cognitive function after surgery, which may be due to the decreased release of DA [97]. In summary, these limited findings highlight that dysfunctions of VTA and DA projections during pain have potential effects on cognitive aspects, and related molecular and cellular mechanisms need further investigation.
Cellular Mechanisms Involved in Pain-Related Cognitive Dysfunction
Chronic pain-induced cellular changes serve as the foundation for functional and structural alterations in brain regions and circuits. Alterations in neuronal plasticity and glial activation in the corticolimbic structures contribute to the cognitive deficits induced by chronic pain.
Neurons
Morphological and Electrophysiological Changes
The hippocampus and mPFC undergo significant morphological and functional changes in response to chronic pain (Table 2). Dendritic morphology, a key factor influencing grey matter volume in neuroimaging [101], is altered in neuropathic pain mice. This includes reduced dendritic complexity, decreased spine density, and neuronal atrophy in CA1 and CA3 pyramidal neurons [15, 23, 98], along with decreased intrinsic excitability in CA1 pyramidal neurons of spared nerve injury (SNI) mice [69, 90]. In contrast, DG granule cells displayed an increase in the length, complexity of dendrites, and total spines’ density, while mushroom spines that are responsible for the “storage of memories” were decreased [23]. These maladaptive changes in neurons are associated with decreased excitatory synaptic transmission, reduced BDNF levels, and neuroinflammation in the hippocampus induced by persistent pain [15, 50, 98].
Metz et al. observed changes in mPFC layer 2/3 (L2/3) pyramidal neurons after SNI, including increased basal dendrites complexity, spine density, NMDA/AMPK currents [99], and neuronal excitability [73, 100]. It has been found that chronic pain significantly increased noradrenergic innervation within the mPFC L2/3 neuron, which inhibited the HCN-mediated current and induced L2/3 pyramidal hyperactivity [102]. Conversely, layer 5 (L5) pyramidal neurons in the mPFC exhibited reduced complexity, shorter apical dendrites, and decreased neuronal excitability in neuropathic pain [100]. Shiers et al. reported shortened axon initial segments (AIS) in infralimbic (IL) layer 5/6 neurons in SNI mice, impacting neuronal excitability and presynaptic inputs [45]. The hypoactivity of L5 pyramidal neurons can be attributed to enhanced local GABAergic inhibition, reduced glutamatergic activation, and decreased cholinergic modulation during pain [82, 103,104,105]. Moreover, studies showed that the glutamatergic inputs from both the hippocampus and thalamus to mPFC L5 pyramidal neurons were weakened after SNI, which likely contributes to the mPFC deactivation in neuropathic pain and may impair PFC-dependent cognitive tasks [104]. Functional studies highlight the crucial role of PFC L5 pyramidal neurons in short-term memory [106]. Optogenetic activation of mPFC L5 pyramidal neurons can reverse object recognition memory deficits in Alzheimer’s disease (AD) mice [106]. Similarly, optogenetic activation of the neural circuit from dCA1 to mPFC L5 pyramidal neurons alleviated neuropathic pain behaviors and improved novel object recognition ability in SNI mice [69]. Based on the layer-specific alterations in the mPFC induced by pain, further research can explore the role of different layers and their outputs in pain and related comorbidities using layer-specific transgenic mice. In addition to pyramidal neurons, neuropathic pain increased spine density on mPFC interneurons, thus enhancing inhibitory inputs and suppressing glutamate signaling during working memory tasks [107]. These maladaptive changes induced by chronic pain collectively deactivate the mPFC and disrupt cognitive processes.
Synaptic Plasticity
Synaptic plasticity refers to the activity-dependent modification of synaptic strength [108]. Chronic pain significantly impacts synaptic plasticity in the hippocampus and can manifest as a reduction in synapses. Studies have found reduced expression of synaptic proteins in the hippocampus during chronic pain such as postsynaptic density protein 95 (PSD95), synapsin 1 (SYN1), vesicular glutamate transporters (vGLUTs), and Arc [24,25,26, 50], as well as decreased dendritic spine density in hippocampal pyramidal neurons, indicating a reduction in excitatory synapses [15, 20]. The resulting synaptic loss can disrupt information processing and transmission within neural networks associated with cognition. Moreover, chronic pain induced glutamatergic synaptic hypofunction, as evidenced by impaired excitatory postsynaptic currents (EPSCs) and reduced NMDA/AMPA currents in hippocampal pyramidal neurons [25, 98]. Long-term potentiation (LTP) and long-term depression (LTD) are forms of synaptic plasticity that have been widely studied in the context of learning and memory [25, 89]. As shown in Table 3, LTP at the lateral entorhinal cortex (LEC)-DG and CA3-CA1 synapses are diminished after high-frequency stimulation in neuropathic pain models, which may be disrupted by the impairment of excitatory synaptic transmission in hippocampal pyramidal neurons. However, no significant alteration was observed in LTD at hippocampal synapses under pain conditions [110].
In addition, Zhuo and colleagues have proposed that enhanced LTP and suppressed LTD of excitatory synaptic transmission in ACC result in a dysregulation of synaptic tone and hyperactivity in ACC neurons, which contributes to chronic pain and its related emotional changes [89, 112]. Behaviorally, postsynaptic-LTP at ACC synapses encodes hyperalgesia and unpleasantness of pain, while presynaptic-LTP mediates pain-induced anxiety [89, 113]. Moreover, the restoration of ACC LTP and LTD has been shown to rescue peripheral pain hypersensitivity [113, 114]. Chronic pain is considered a result of plastic changes in the corticolimbic system, with synaptic plasticity as a common mechanism for pain and cognition [9]. Further investigation is needed to comprehend how changes in ACC LTP and LTD influence synaptic activity, neural circuit function, and their potential involvement in the cognitive impairment induced by chronic pain.
Intracellular and Extracellular Structural Changes
Both intracellular and extracellular structural alternations contribute to the maladaptive brain plasticity and cognitive deficits associated with pain. Microtubules (MTs) are essential structures for stable neuronal morphology and function, with the Tau protein, an MT-associated cytoskeletal element known to contribute to neuronal atrophy and dysfunction in neurodegenerative disorders, like AD [115]. Clinical evidence suggests that chronic pain increases the risk for dementia and AD [116]. In animal research, trigeminal neuralgia (TN) induced overexpression of phosphorylated tau protein and amyloid-β 1–42 in the cortex and hippocampus [117]. Guerreiro et al. first demonstrated that SNI triggered memory deficits and AD-related neuropathological changes, characterized by Tau hyperphosphorylation accumulation and neuronal atrophy in the hippocampus [118]. Genetic ablation and degradation of Tau effectively halted the hippocampal deficits induced by SNI, confirming the involvement of Tau in the memory decline associated with pain [118]. In addition, MT dynamic equilibrium also contributes to the memory decline in pain. You et al. found that SNI increased levels of stable microtubules in the hippocampus, and treatment of microtubule destabilizer improved hippocampal LTP and alleviated memory deficits in SNI rats [13].
The extracellular matrix (ECM) is a complex extracellular network that modulates neuronal plasticity and connectivity [119]. Chronic pain-induced changes in hippocampal neuronal structure, LTP, ECM microarchitecture, and ECM components and enzymes, such as increased levels of MMP8 [120]. Interestingly, normalizing these ECM imbalances reversed the memory decline, neuronal structure changes, and LTP disruption induced by chronic pain, which highlighted the involvement of extracellular mechanisms of pain-related brain plasticity [120].
Glial Cells
Increased inflammatory molecules, disruption of the brain-blood barrier (BBB), infiltration of immune cells, and glial activation are the hallmarks of neuroinflammation. These factors contribute to the establishment and persistence of central sensitization in pain [121].
Microglia
Microglia, as the innate immune cells in the CNS, are significantly increased and activated in supraspinal regions in the pain condition [27, 122]. Minocycline, a non-selective microglial inhibitor, alleviated pain-related cognitive dysfunction by inhibiting pro-inflammatory cytokine expression and oxidative stress and restoring synaptic plasticity in the hippocampus [98, 123]. High-mobility group box 1 (HMGB1) is a proinflammatory molecule involved in microglial activation and blockade of HMGB1 in the hippocampus effectively alleviated cognitive deficits induced by neuropathic pain [14]. Microglia can transform into either pro-inflammatory (M1) or neuroprotective (M2) phenotypes based on their activation mode. The hippocampus and mPFC exhibited increased M1-polarized microglia in the pain models that aggregated inflammatory response [49, 124]. Modulation of microglial polarization towards the M2 phenotype is beneficial for inhibiting neuroinflammation and improving behavioral outcomes [125]. Moreover, studies have reported that microglia-mediated excessive synaptic pruning contributed to synaptic loss and cognitive impairment in neurologic diseases [126]. Therefore, inhibition of microglia-mediated neuroinflammation is an essential target to improve behavioral abnormality following chronic pain.
Inflammatory cytokines and chemokines are released by activated glial cells, immune cells, and damaged neurons. Studies have shown the overexpression of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-6, in the hippocampus, PFC, and cerebrospinal fluid in pain models [55, 127,128,129]. These increased cytokines can also be detected in plasma samples from chronic pain patients [129]. Inhibiting TNF-α, IL-1β, and IL-6 or genetically downregulating their receptors could prevent microglia activation and maladaptive synaptic plasticity in the hippocampus and rescue cognitive impairment caused by pain [27, 33, 35].
Interestingly, certain studies have indicated that the presence of pro-inflammatory cytokines and reactive microgliosis appeared to be confined to the contralateral hippocampus of the injury side [130, 131]. However, accumulating evidence also suggested that chronic pain-induced neuroinflammation in the bilateral hippocampus and mPFC [98, 127]. It is worth noting that supraspinal neuroinflammation induced by pain originates not only from the CNS in situ but also from peripheral inflammation [132]. For example, chronic pain disrupted BBB permeability and increased leukocyte infiltration into the CNS [133]. Mai et al. found that the plasma chemokine CXCL12 mediated circulating monocyte recruitment into the perivascular space, contributing to hippocampal neuroinflammation and cognitive impairment in the condition of neuropathic pain [134]. Given that nociceptive information is primarily conveyed to the contralateral hemisphere, neuroinflammation tends to be more robust in the brain regions contralateral to the injury site [135].
Astrocyte
Astrocytes serve critical functions in CNS homeostasis and neuronal metabolites [136]. Some studies reported reduced astrocyte numbers and atrophy in the hippocampus and mPFC, possibly due to the neurotoxic effects of noxious inputs [137,138,139]. Conversely, others found reactive astrocyte activation in these cognitive regions as a consequence of neuroinflammation [134, 140, 141]. These discrepancies may be related to the dynamic changes in astrocytes during different pain progression stages. Moreover, chronic pain induced alternations in the lactate metabolism of astrocytes, leading to disruptions in the supply of energy to the brain. Reduced lactate release from dysfunctional astrocytes decreased the excitability of dCA1 pyramidal neurons in SNI mice, leading to hippocampus-dependent memory deficits [69]. ACC astrogliosis in VH rats impaired L-lactate release under high activity demand conditions such as decision-making, which then suppressed ACC neuronal activity [47]. These studies enhance our understanding of astro-neuron interactions in brain regions during pain conditions. Future research should investigate astrocyte metabolic and functional changes and their interactions with other cells during comorbid pain and memory deficits.
Molecular Mechanisms of Pain-Related Cognitive Dysfunction
A multitude of molecules within the CNS play a role in the development of pain-induced cognitive deficits. In the following section, we provide a summary of current research on the role of these molecules in the comorbidity of pain and cognitive disorders, including neurotransmitters and their receptors, neuropeptides, neurotrophic factors, the endocannabinoid system, and the gut-brain axis.
Neurotransmitters and Their Receptors
Glutamate
As a major excitatory neurotransmitter in the CNS, glutamate plays an important role in the cognitive process. Glutamate acts on the postsynaptic ionotropic glutamate receptors, including N-methyl-D-aspartate receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), kainate, and metabotropic glutamate receptors (mGluRs) [142]. Dysfunction of glutamate and its receptors, NMDARs hypofunction in particular, is a key mechanism contributing to the cognitive deficits observed in chronic pain.
NMDA receptor is an important mediator of LTP and excitatory synaptic transmission [143]. In a previous study, we found reduced hippocampal glutamate concentrations and downregulation of NMDA receptor subunits NR1 and NR2B in SNI rats, along with disruption of hippocampal glutamatergic transmission and LTP [25]. The administration of d-serine, an endogenous NMDAR agonist, successfully restored hippocampal glutamatergic function and synaptic plasticity and alleviated cognitive decline induced by neuropathic pain [25]. Other studies also reported reduced hippocampal NMDARs and memory deficits in pain conditions [50, 144]. Furthermore, NMDA-mediated currents in CA1 pyramidal cells were attenuated, while AMPA function remained unchanged under neuropathic pain conditions [111]. These findings highlight the significance of NMDARs downregulation and hypofunction in the hippocampus in promoting cognitive impairment induced by CP. Moreover, reduced glutamate level in the mPFC is associated with emotional and cognitive dysregulation in chronic pain patients [145]. In SNI mice, Guida et al. found disrupted glutamatergic synapse homeostasis in the mPFC, including decreased extracellular glutamate levels and increased NR2B subunits [20]. They found that administration of NMDARs agonist d-Asp solution restored glutamate neurotransmission to physiological levels in the Mpfc and alleviated pain-induced cognitive deficits in mice [146].
LTP is triggered by Ca2+ influx through NMDARs but expressed by an increased abundance of AMPARs at the postsynapses [147]. It is widely accepted that upregulation of AMPARs-mediated LTP in ACC underlies the unpleasant experience of chronic pain [89, 148, 149]. However, decreased AMPAR subunits GluR1-3 were found in the hippocampus after PSNL, possibly due to increased AMPAR internalization and degradation in the neuroinflammatory context. This may be involved in the disruption of hippocampal synaptic plasticity [14, 144]. In the mature brain, most AMPARs consist of Ca2+-impermeable GluR1/GluR2 subunits that maintain low cytoplasmic Ca2+, while GluR2-lacking AMPARs are permeable to Ca2+ and dramatically alter synaptic function. Liu et al. found that neonatal repetitive noxious stimuli increased GluR2-lacking AMPARs in hippocampal neurons, which can result in elevated Ca2+ influx, dendritic spine dysfunction, and ultimately contribute to pain, spatial learning, and memory deficits in adulthood [150].
It also evidenced that negative modulation of mGluRs alleviates cognitive decline. mGluRs are widely distributed on the synaptic boutons that control excitatory synaptic function by regulating glutamate release. Blockade of mGluR1 overcame cognitive deficits by attenuating local GABAergic inhibition and restoring the inactivation of mPFC neurons during pain [151]. Inhibition of mGluR5 ameliorated pain-related cognitive impairment and restored LTP disruption due to its correction of glutamate homeostasis [18]. Additionally, the mGluR7 negative modulator normalized increased mGluR7 expression in the hippocampus and cognitive deficits in SNI mice, which might be related to mGluR7’s inhibitory effect on glutamate [17]. Therefore, these findings highlight the significance of proper functioning of the glutamatergic system for effective cognitive function.
GABA
GABA (γ-aminobutyric acid) serves as the primary inhibitory neurotransmitter in the CNS, acting on ionotropic GABAA and GABAC receptors, and metabotropic GABAB receptors. The GABAergic system is crucial for CNS function, particularly in learning and memory processes [152]. In chronic pain models, elevated GABA levels in the hippocampus [22, 32], and mPFC [153] contribute to an E/I imbalance, resulting in cognitive impairment. In the supraspinal region, GABA predominantly interacts with GABAA receptors to mediate rapid inhibitory synaptic transmission [154]. Blocking GABAA receptors in the mPFC can restore neuronal activity in the pain model, providing a potential strategy for ameliorating chronic pain-induced cognitive deficits [151]. Studies also linked memory impairment to enhanced tonic inhibition via GABAA-α5 receptors [155, 156]. Cai et al. found a remarkable increase in the expression of the GABAA-α5 receptors on the parvalbumin and somatostatin interneurons in the hippocampus in SNI rats, and antagonizing of these receptors improved cognitive impairment, highlighting the role of E/I neurotransmission imbalance in pain-induced cognitive dysfunction [157].
Monoamines
Monoamine neurotransmitters, including dopamine (DA), norepinephrine (NE), and serotonin (5-HT), are associated with pain and memory formation. Thus, it is imperative to discuss their roles in the comorbidity of chronic pain and cognitive decline.
The hippocampus and PFC mainly receive NE from the locus coeruleus (LC) [158]. The LC-NE system plays a significant role in arousal, cognition, pain processing, and stress response [159]. Chronic pain induces LC-NE dysfunction and increases the release of NE, contributing to pain facilitation and cognitive/emotional disorders [155, 156]. Peripheral nerve injury elevated NE concentration in the PFC and impaired cognitive function [40, 157]. Selective depletion of norepinephrine in the LC alleviated attention deficits in chronic pain [40]. However, chronic pain-induced LC-NE system dysfunction is complex and likely based on downstream neuron function and location [116]. The decreased noradrenergic tone was found in the hippocampus in models of chronic pain [32, 109]. Microinjection of a β2 receptor agonist into dCA1 and activation of LC-dCA1 noradrenergic projections restored memory deficits in SNI mice, suggesting the role of the noradrenergic system in pain-induced cognitive dysfunction [160]. Stimulation of β-adrenoceptors with isoproterenol restored hippocampal LTP damage in chronic pain models [110]. Furthermore, studies demonstrated that NE can effectively alleviate neuroinflammation and microglial activation in neuropathic pain mice and AD mice [161,162,163]. Therefore, further research on the dysfunction of the LC-NE system in chronic pain, especially its targeted brain regions and cell types, may generate new ideas in the treatment of pain and related comorbidities.
Dopamine is a critical neurotransmitter in hippocampal-dependent memory processes [164]. Studies highlighted the significance of the hippocampal dopaminergic system in cognitive deficits associated with pain. Cardoso-Cruz et al. found that activation of dopamine D2/D3 receptors restored the impaired neural activity and dorsoventral connectivity in the hippocampus, ultimately improving working memory in SNI mice [71]. Conversely, blocking D2 receptors led to a significant decrease in theta-oscillation-mediated connectivity in the hippocampus and worsened spatial working memory deficits [54]. The dopamine released by the LC plays a crucial role in the dorsal hippocampus, influencing selective attention and spatial object recognition processes through D1/D5 receptors [165]. However, the effects of chronic pain on the dopaminergic system and its role in cognitive dysfunction are not fully understood.
5-HT plays a regulatory role in pain perception, cognition, and emotion [166]. Mice with chronic pain exhibited increased 5-HT levels in the hippocampus [55, 109], with potentially detrimental effects on hippocampal neurogenesis [167]. Jayarajan et al. reported that 5-HT6 receptor antagonists normalized neuropathic pain-induced memory deficits, possibly by attenuation of stress response in pain [168]. The widespread and diverse receptors underlie the complex function of 5-HT in the CNS. Subsequent research can focus on the potential role of 5-HT and its receptors in pain and cognitive processes.
Neuropeptides
Several studies have focused on the role of neuropeptides in cognitive processes. Our previous study found that the glucagon-like-peptide-1(GLP-1)/GLP-1 receptors axis was inhibited in the hippocampus of neuropathic pain mice [26], and its activation ameliorated chronic pain-induced cognitive impairment by regulating hippocampal neuroinflammation and synaptic impairment [26, 140]. Orexin (Orx) is an excitatory peptide that contributes to arousal, appetite, and cognition. Pretreatment of Orx1 in the hippocampal effectively alleviated spatial memory impairment induced by orofacial pain [169], while blocking Orx1Rs in the BLA aggregated learning and memory dysfunction in migraine rats [170]. In addition, oxytocin (OXT), a classic hypothalamic neuropeptide for social memory and emotion, has been found to reduce hyperalgesia [171] and anxiety [172] induced by chronic pain. OXT treatment also alleviated cognitive impairments by decreasing hippocampal microglial activation and synaptic defects in a mouse model of sepsis-associated encephalopathy [173]. Collectively, neuropeptides show promise as effective treatments for chronic pain-induced cognitive deficits.
Neurotrophic Factors
Brain-derived neurotrophic factor (BDNF) is widely expressed in the CNS and plays an essential role in synaptic plasticity. In neuropathic pain mice, reduced BDNF levels in the hippocampus impaired synaptic plasticity [98]. Mature BDNF binds to the tropomyosin-receptor-kinase B (TrkB) receptor with high affinity, participating in mechanisms related to learning and memory, including LTP, neurogenesis, and synaptic efficiency and formation. In contrast, pro-BDNF exerts the opposing effect [172]. Enrichment environment mediated BDNF/TrkB signaling to ameliorate memory decline and enhance synaptic plasticity deficits in nerve-injured mice [24]. Peripheral inflammatory pain increased the microglia-dependent proBDNF/BDNF ratio, leading to hippocampal neuron death and spatial memory impairment in rats [31]. Furthermore, recent research demonstrated that the activation of BDNF release from the VTA to the DG improved memory decline in neuropathic pain mice via restoring hippocampal neurogenesis [34]. This study establishes a mechanistic link, relying on neural circuits and BDNF signaling to modulate neurogenesis, between chronic pain and cognitive deficits.
Endocannabinoid System
The endocannabinoid (eCB) system, consisting of cannabinoid receptors (CB1R and CB2R) and endogenous endocannabinoids (anandamide, AEA; 2-arachidonoyl glycerol, 2-AG), has been extensive studies in the field of pain, cognition, and emotion [174,175,176]. Palmitoylethanolamide (PEA), an eCB-like compound, alleviated neuropathic pain-related nociceptive, emotional, and cognitive behaviors by improving glutamatergic synapse homeostasis in the mPFC [20]. Additionally, PEA treatment restored impaired synaptic plasticity and neurogenesis in the hippocampus of SNI mice [19]. Recent findings indicated that inhibition of AEA breakdown improved pain-related behaviors, enhanced LTP in the LEC-DG pathway, and normalized monoamine levels in the hippocampus via CB1 receptors following osteoarthritis [109]. These findings highlight the beneficial role of the endocannabinoid system in maintaining synaptic plasticity and homeostasis in the CNS during pain. Furthermore, both CB1R and CB2R agonists show potential in alleviating nociceptive and anxiety-like behaviors, whereas only CB1R agonists improved memory impairment in the osteoarthritis pain model [177]. These diverse effects may be attributed to the different distribution and cellular locations of the two cannabinoid receptors. In the condition of pain, CB1R is expressed throughout the CNS, with CB2R being less abundant in the brain [178]. The eCB system holds the potential to serve as a multi-target therapeutic approach for pain management.
Microbiota Gut-Brain Axis
The microbiota gut-brain axis has been demonstrated to play a significant role in pain modulation and cognitive dysfunction. It refers to bidirectional communication between the gut and the brain through immunological, neural, and hormonal signals, and the gut microbiota is now considered a key gastrointestinal factor [179]. Hua et al. found fecal microbiota transplantation (FMT) from the Sham group improved allodynia and cognitive performance in SNI mice by normalizing eCB signaling in the PFC, suggesting a link between gut microbiota, endocannabinoids, and neurological changes in chronic neuropathic pain (CNP) [180]. Gut dysbiosis in CNP may disrupt eCB signaling, exacerbating neuroinflammation and brain energy imbalances, ultimately resulting in cognitive dysfunction [180]. Evidence also highlighted the role of short-chain fatty acids (SCFAs) in cognitive function by regulating BBB permeability, immune response, and CNS maturation [181]. Studies found that chronic postsurgical pain (CPSP) mice with cognitive impairment had altered gut SCFAs-producing bacteria, and FMT from CPSP rats transmitted cognitive impairment. SCFAs supplement improved cognitive impairment in CPSP mice by enhancing histone acetylation and synaptic transmission [182]. Moreover, the gut-microbiota-brain axis via the subdiaphragmatic vagus nerve modulated neuroinflammation, and alleviated inflammatory pain and comorbid spatial working memory impairment in the CFA mice [183]. These results showed that gut microbiota dysbiosis might be an important etiology of cognitive impairment in chronic pain patients, and its neural mechanism needs further investigation.
Potential Intervention Strategies for Chronic Pain–Induced Cognitive Impairment
Effective pain analgesia and management may alleviate its associated cognitive impairment. Reports indicate that the chronic pain-induced gray matter decrease and cognitive network disruption can be partly recovered when chronic pain is treated successfully [68, 184]. However, the commonly used analgesic medications, such as gabapentin and opioids, have been shown to evoke or exacerbate existing cognitive impairment [185, 186]. The cognitive impairment induced by CP is marginally addressed in the clinic. Therefore, some potential treatments for cognitive impairment in CP patients are needed.
rTMS and tDCS
Repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) are innovative tools in neurology and psychiatry for CNS diseases. They are able to modulate neuronal activities in stimulated areas, inducing functional changes in connected distant regions. It has been reported that rTMS stimulation on the dorsolateral prefrontal cortex (DLPFC) produced cognitive-enhancing effects in patients with depression, mild cognitive impairment, AD, and Parkinson’s disease [187,188,189,190]. Low-frequency magnetic stimulation improved cognitive behavior by enhancing structural synaptic plasticity in the hippocampus of aging mice [191]. Additionally, tDCS on DLPFC enhances response speed and accuracy in cognitive tasks [192]. These findings suggest that rTMS and tDCS are potential treatments for cognitive impairment in chronic pain patients by targeting the prefrontal cortex and hippocampus, and modulating dysfunctional neural networks.
Anti-inflammation and Neuroprotection
Anti-inflammation and neuroprotection are key focuses in treating cognitive dysfunction induced by pain. Preclinical research indicated that anti-TNF-α, IL-1β, or IL-6 agents and their receptor antagonists may be effective treatments to reduce inflammation and exert neuroprotective effects [27, 33, 35]. Minocycline is widely used to inhibit microglia activation and has shown promising clinical applications in pain and cognition [193, 194]. Erythropoietin (EPO), as an immune modulator factor, improved recognition memory impairment through reducing hippocampal microglial expression, IL-1β production, hippocampal apoptosis, and necroptosis induced by inflammatory pain [195]. Metformin alleviated pain-related cognitive impairment and restored functional and morphological changes in the brain of neuropathic mice [44]. The neuropeptide GLP-1 receptor agonist exendin-4 suppresses neuroinflammation, protecting neuronal plasticity in mice with neuropathic pain [26]. Moreover, treatments with NMDAR agonists d-serine and d-Asp can restore synaptic transmission and synaptic plasticity in the brain [25, 146, 196]. SCFA treatment improved gut microbiota, alleviated neuroinflammation and microglia activation in the cortex and hippocampus, and restored synaptic transmission in pain [182, 197]. Other targets, like BDNF, and eCB-like compound PAE, are potential treatments for cognitive impairments in CP patients. However, translating these preclinical findings into clinically useful medicines remains a challenge.
Conclusion and Future Directions
Clinical evidence has consistently demonstrated that patients suffering from chronic pain often experience cognitive impairments, which facilitates preclinical research to investigate the underlying mechanisms of CP-induced cognitive dysfunction. For this purpose, animal models seem necessary even though some findings from experimental animals may not be translated to bed-side. In this review, we have summarized methodological considerations for generating these models. Moreover, we proposed that chronic pain shares overlapping neuroanatomical substrates with cognitive processing, which may change the structure and function of these regions and lead to cognitive deficits. We discussed the mechanisms at molecular, cellular, and neural circuit levels that contribute to the comorbid cognitive deficits in chronic pain, primarily focusing on the hippocampus and mPFC (Fig. 2).
However, several important issues remain less studied. Firstly, the most currently used animal models of pain are chronic neuropathic and inflammatory pain, with less attention given to other prevalent types of clinical pain like chronic visceral pain, headaches, and musculoskeletal pain. Expanding investigations to include these different pain conditions can provide a more comprehensive understanding of the relationship between pain and cognitive deficits and accurately model the pain experiences in humans. Moreover, the current preclinical research on pain-induced cognitive dysfunction is largely restricted to the hippocampus and mPFC, with much less exploration of other relevant brain regions like ACC, amygdala, NAc, and LC. Despite extensive research on the ACC’s role in pain and emotion disorders [84], there is still limited research on its direct mechanisms underlying pain-related memory impairments. Understanding local molecular and cellular changes in these brain regions and specific circuits by which pain disrupts cognitive processes is important in future research on pain-induced cognitive deficits, which provides guidance for clinical drug targets and electro-physical therapies.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Phelps CE, Navratilova E, Porreca F (2021) Cognition in the chronic pain experience: preclinical insights. Trends Cogn Sci 25(5):365–376. https://doi.org/10.1016/j.tics.2021.01.001
Treede RD, Rief W, Barke A et al (2015) A classification of chronic pain for ICD-11. Pain. 156(6):1003–1007. https://doi.org/10.1097/j.pain.0000000000000160
Nahin RL, DeKosky ST (2020) Comorbid pain and cognitive impairment in a nationally representative adult population: prevalence and associations with health status, health care utilization, and satisfaction with care. Clin J Pain 36(10):725–739. https://doi.org/10.1097/AJP.0000000000000863
Zhao W, Zhao L, Chang X, Lu X, Tu Y (2023) Elevated dementia risk, cognitive decline, and hippocampal atrophy in multisite chronic pain. Proc Natl Acad Sci USA 120(9):e2215192120. https://doi.org/10.1073/pnas.2215192120
Kazim MA, Strahl A, Moritz S, Arlt S, Niemeier A (2023) Chronic pain in osteoarthritis of the hip is associated with selective cognitive impairment. Arch Orthop Trauma Surg 143(4):2189–2197. https://doi.org/10.1007/s00402-022-04445-x
Corti EJ, Gasson N, Loftus AM (2021) Cognitive profile and mild cognitive impairment in people with chronic lower back pain. Brain Cogn 151:105737. https://doi.org/10.1016/j.bandc.2021.105737
Cunha AM, Pereira-Mendes J, Almeida A, Guimarães MR, Leite-Almeida H (2020) Chronic pain impact on rodents’ behavioral repertoire. Neurosci Biobehav Rev 119:101–127. https://doi.org/10.1016/j.neubiorev.2020.09.022
Tracey I, Mantyh PW (2007) The cerebral signature for pain perception and its modulation. Neuron 55(3):377–391. https://doi.org/10.1016/j.neuron.2007.07.012
McCarberg B, Peppin J (2019) Pain pathways and nervous system plasticity: learning and memory in pain. Pain Med 20(12):2421–2437. https://doi.org/10.1093/pm/pnz017
Hashmi JA, Baliki MN, Huang L et al (2013) Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 136(9):2751–2768. https://doi.org/10.1093/brain/awt211
Yang S, Chang MC (2019) Chronic pain: structural and functional changes in brain structures and associated negative affective states. Int J Mol Sci 20(13):3130. https://doi.org/10.3390/ijms20133130
Moriarty O, McGuire BE, Finn DP (2011) The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol 93(3):385–404. https://doi.org/10.1016/j.pneurobio.2011.01.002
You Z, Zhang S, Shen S et al (2018) Cognitive impairment in a rat model of neuropathic pain: role of hippocampal microtubule stability. Pain 159(8):1518–1528. https://doi.org/10.1097/j.pain.0000000000001233
Hisaoka-Nakashima K, Ohata K, Yoshimoto N et al (2022) High-mobility group box 1-mediated hippocampal microglial activation induces cognitive impairment in mice with neuropathic pain. Exp Neurol 355:114146. https://doi.org/10.1016/j.expneurol.2022.114146
Tyrtyshnaia AA, Manzhulo IV, Konovalova SP, Zagliadkina AA (2020) Neuropathic pain causes a decrease in the dendritic tree complexity of hippocampal CA3 pyramidal neurons. Cells Tissues Organs 208(3-4):89–100. https://doi.org/10.1159/000506812
Wang Y, Li CM, Han R et al (2020) PCC0208009, an indirect IDO1 inhibitor, alleviates neuropathic pain and co-morbidities by regulating synaptic plasticity of ACC and amygdala. Biochem Pharmacol 177:113926. https://doi.org/10.1016/j.bcp.2020.113926
Palazzo E, Romano R, Luongo L et al (2015) MMPIP, an mGluR7-selective negative allosteric modulator, alleviates pain and normalizes affective and cognitive behavior in neuropathic mice. Pain 156(6):1060. https://doi.org/10.1097/j.pain.0000000000000150
Boccella S, Marabese I, Iannotta M et al (2019) Metabotropic glutamate receptor 5 and 8 modulate the ameliorative effect of ultramicronized palmitoylethanolamide on cognitive decline associated with neuropathic pain. Int J Mol Sci 20(7):1757. https://doi.org/10.3390/ijms20071757
Boccella S, Cristiano C, Romano R et al (2019) Ultra-micronized palmitoylethanolamide rescues the cognitive decline-associated loss of neural plasticity in the neuropathic mouse entorhinal cortex-dentate gyrus pathway. Neurobiol Dis 121:106–119. https://doi.org/10.1016/j.nbd.2018.09.023
Guida F, Luongo L, Marmo F et al (2015) Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice. Mol Brain 8:47. https://doi.org/10.1186/s13041-015-0139-5
Tajerian M, Leu D, Zou Y et al (2014) Brain neuroplastic changes accompany anxiety and memory deficits in a model of complex regional pain syndrome. Anesthesiology 121(4):852–865. https://doi.org/10.1097/ALN.0000000000000403
Guida F, Iannotta M, Misso G et al (2022) Long-term neuropathic pain behaviors correlate with synaptic plasticity and limbic circuit alteration: a comparative observational study in mice. Pain 163(8):1590–1602. https://doi.org/10.1097/j.pain.0000000000002549
Tyrtyshnaia A, Manzhulo I (2020) Neuropathic pain causes memory deficits and dendrite tree morphology changes in mouse hippocampus. J Pain Res 13:345–354. https://doi.org/10.2147/JPR.S238458
Wang XM, Pan W, Xu N, Zhou ZQ, Zhang GF, Shen JC (2019) Environmental enrichment improves long-term memory impairment and aberrant synaptic plasticity by BDNF/TrkB signaling in nerve-injured mice. Neurosci Lett 694:93–98. https://doi.org/10.1016/j.neulet.2018.11.049
Xiong B, Zhang W, Zhang L et al (2020) Hippocampal glutamatergic synapses impairment mediated novel-object recognition dysfunction in rats with neuropathic pain. Pain 161(8):1824–1836. https://doi.org/10.1097/j.pain.0000000000001878
Zhang LQ, Zhang W, Li T et al (2021) GLP-1R activation ameliorated novel-object recognition memory dysfunction via regulating hippocampal AMPK/NF-kappaB pathway in neuropathic pain mice. Neurobiol Learn Mem 182:107463. https://doi.org/10.1016/j.nlm.2021.107463
Gui WS, Wei X, Mai CL et al (2016) Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol Pain 12:174480691664678. https://doi.org/10.1177/1744806916646784
Jang JH, Song EM, Do YH et al (2021) Acupuncture alleviates chronic pain and comorbid conditions in a mouse model of neuropathic pain: the involvement of DNA methylation in the prefrontal cortex. PAIN. 162(2):514. https://doi.org/10.1097/j.pain.0000000000002031
Ferdousi MI, Calcagno P, Sanchez C et al (2023) Characterization of pain-, anxiety-, and cognition-related behaviors in the complete Freund’s adjuvant model of chronic inflammatory pain in Wistar–Kyoto rats. Front Pain Res 4:1131069. https://doi.org/10.3389/fpain.2023.1131069
Negrete R, García Gutiérrez MS, Manzanares J, Maldonado R (2017) Involvement of the dynorphin/KOR system on the nociceptive, emotional and cognitive manifestations of joint pain in mice. Neuropharmacology 116:315–327. https://doi.org/10.1016/j.neuropharm.2016.08.026
Mohammadi M, Manaheji H, Maghsoudi N, Danyali S, Baniasadi M, Zaringhalam J (2020) Microglia dependent BDNF and proBDNF can impair spatial memory performance during persistent inflammatory pain. Behav Brain Res 390:112683. https://doi.org/10.1016/j.bbr.2020.112683
Boccella S, Guida F, Iannotta M et al (2021) 2-Pentadecyl-2-oxazoline ameliorates memory impairment and depression-like behaviour in neuropathic mice: possible role of adrenergic alpha2- and H3 histamine autoreceptors. Mol Brain 14(1):28. https://doi.org/10.1186/s13041-020-00724-z
Ren WJ, Liu Y, Zhou LJ et al (2011) Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacol 36(5):979–992. https://doi.org/10.1038/npp.2010.236
Xia SH, Hu SW, Ge DG et al (2020) Chronic pain impairs memory formation via disruption of neurogenesis mediated by mesohippocampal brain-derived neurotrophic factor signaling. Biol Psychiatry 88(8):597–610. https://doi.org/10.1016/j.biopsych.2020.02.013
Hisaoka-Nakashima K, Moriwaki K, Yoshimoto N et al (2022) Anti-interleukin-6 receptor antibody improves allodynia and cognitive impairment in mice with neuropathic pain following partial sciatic nerve ligation. Int Immunopharmacol 112:109219. https://doi.org/10.1016/j.intimp.2022.109219
Saffarpour S, Janzadeh A, Rahimi B, Ramezani F, Nasirinezhad F (2021) Chronic nanocurcumin treatment ameliorates pain-related behavior, improves spatial memory, and reduces hippocampal levels of IL-1β and TNFα in the chronic constriction injury model of neuropathic pain. Psychopharmacology 238(3):877–886. https://doi.org/10.1007/s00213-020-05739-x
Du J, Deng Y, Qiu Z et al (2021) Curcumin alleviates chronic pain and improves cognitive impairment via enhancing hippocampal neurogenesis in sciatic nerve constriction rats. J Pain Res 14:1061–1070. https://doi.org/10.2147/JPR.S299604
Pais-Vieira M, Lima D, Galhardo V (2009) Sustained attention deficits in rats with chronic inflammatory pain. Neurosci Lett 463(1):98–102. https://doi.org/10.1016/j.neulet.2009.07.050
Higgins GA, Silenieks LB, Van Niekerk A et al (2015) Enduring attentional deficits in rats treated with a peripheral nerve injury. Behav Brain Res 286:347–355. https://doi.org/10.1016/j.bbr.2015.02.050
Moazen P, Torabi M, Azizi H, Fathollahi Y, Mirnajafi-Zadeh J, Semnanian S (2020) The locus coeruleus noradrenergic system gates deficits in visual attention induced by chronic pain. Behav Brain Res 387:112600. https://doi.org/10.1016/j.bbr.2020.112600
Low LA, Millecamps M, Seminowicz DA et al (2012) Nerve injury causes long-term attentional deficits in rats. Neurosci Lett 529(2):103–107. https://doi.org/10.1016/j.neulet.2012.09.027
Millecamps M, Etienne M, Jourdan D, Eschalier A, Ardid D (2004) Decrease in non-selective, non-sustained attention induced by a chronic visceral inflammatory state as a new pain evaluation in rats. Pain 109(3):214–224. https://doi.org/10.1016/j.pain.2003.12.028
Pais-Vieira M, Mendes-Pinto MM, Lima D, Galhardo V (2009) Cognitive impairment of prefrontal-dependent decision-making in rats after the onset of chronic pain. Neuroscience 161(3):671–679. https://doi.org/10.1016/j.neuroscience.2009.04.011
Shiers S, Pradhan G, Mwirigi J et al (2018) Neuropathic pain creates an enduring prefrontal cortex dysfunction corrected by the type ii diabetic drug metformin but not by gabapentin. J Neurosci 38(33):7337–7350. https://doi.org/10.1523/JNEUROSCI.0713-18.2018
Shiers S, Mwirigi J, Pradhan G et al (2020) Reversal of peripheral nerve injury-induced neuropathic pain and cognitive dysfunction via genetic and tomivosertib targeting of MNK. Neuropsychopharmacol 45(3):524–533. https://doi.org/10.1038/s41386-019-0537-y
Cowen SL, Phelps CE, Navratilova E et al (2018) Chronic pain impairs cognitive flexibility and engages novel learning strategies in rats. Pain 159(7):1403. https://doi.org/10.1097/j.pain.0000000000001226
Wang J, Tu J, Cao B et al (2017) Astrocytic l -lactate signaling facilitates amygdala-anterior cingulate cortex synchrony and decision making in rats. Cell Rep 21(9):2407–2418. https://doi.org/10.1016/j.celrep.2017.11.012
Morellini F (2013) Spatial memory tasks in rodents: what do they model? Cell Tissue Res 354(1):273–286. https://doi.org/10.1007/s00441-013-1668-9
Tyrtyshnaia A, Manzhulo I, Kipryushina Y, Ermolenko E (2019) Neuroinflammation and adult hippocampal neurogenesis in neuropathic pain and alkyl glycerol ethers treatment in aged mice. Int J Mol Med 43(5):2153–2163. https://doi.org/10.3892/ijmm.2019.4142
Tyrtyshnaia A, Bondar A, Konovalova S, Manzhulo I (2021) Synaptamide improves cognitive functions and neuronal plasticity in neuropathic pain. Int J Mol Sci 22(23):12779. https://doi.org/10.3390/ijms222312779
Cowan N (2008) What are the differences between long-term, short-term, and working memory? Prog Brain Res 169:323–338. https://doi.org/10.1016/S0079-6123(07)00020-9
Zhang GF, Zhou ZQ, Guo J et al (2021) Histone deacetylase 3 in hippocampus contributes to memory impairment after chronic constriction injury of sciatic nerve in mice. Pain 162(2):382–395. https://doi.org/10.1097/j.pain.0000000000002056
Cardoso-Cruz H, Lima D, Galhardo V (2013) Impaired spatial memory performance in a rat model of neuropathic pain is associated with reduced hippocampus-prefrontal cortex connectivity. J Neurosci 33(6):2465–2480. https://doi.org/10.1523/JNEUROSCI.5197-12.2013
Cardoso-Cruz H, Dourado M, Monteiro C, Galhardo V (2018) Blockade of dopamine D2 receptors disrupts intrahippocampal connectivity and enhances pain-related working memory deficits in neuropathic pain rats. Eur J Pain 22(5):1002–1015. https://doi.org/10.1002/ejp.1186
Ding X, Gao X, Wang Z et al (2021) Preoperative chronic and acute pain affects postoperative cognitive function mediated by neurotransmitters. J Mol Neurosci 71(3):515–526. https://doi.org/10.1007/s12031-020-01673-x
Oosterman JM, Derksen LC, van Wijck AJ, Kessels RP, Veldhuijzen DS Executive and attentional functions in chronic pain: does performance decrease with increasing task load? Pain Res Manag 17:159–165. https://doi.org/10.1155/2012/962786
Lier EJ, van Rijn CM, de Vries M, van Goor H, Oosterman JM (2022) The interaction between pain and cognition: on the roles of task complexity and pain intensity. Scand J Pain 22(2):385–395. https://doi.org/10.1515/sjpain-2021-0119
Phelps CE, Navratilova E, Porreca F (2021) Chronic pain produces reversible memory deficits that depend on task difficulty in rats. J Pain 22(11):1467–1476. https://doi.org/10.1016/j.jpain.2021.04.016
Cerqueira JJ, Almeida OFX, Sousa N (2008) The stressed prefrontal cortex. Left? Right! Brain Behav Immun 22(5):630–638. https://doi.org/10.1016/j.bbi.2008.01.005
Leite-Almeida H, Cerqueira JJ, Wei H et al (2012) Differential effects of left/right neuropathy on rats’ anxiety and cognitive behavior. Pain 153(11):2218. https://doi.org/10.1016/j.pain.2012.07.007
Leite-Almeida H, Guimarães MR, Cerqueira JJ et al (2014) Asymmetric c-Fos expression in the ventral orbital cortex is associated with impaired reversal learning in a right-sided neuropathy. Mol Pain 10:1744–8069. https://doi.org/10.1186/1744-8069-10-41
Won S, Park K, Lim H, Lee SJ (2020) Sexual dimorphism in cognitive disorders in a murine model of neuropathic pain. Behav Brain Funct 16(1):1. https://doi.org/10.1186/s12993-019-0164-0
Apkarian AV, Hashmi JA, Baliki MN (2011) Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain 152(3 Suppl):S49–S64. https://doi.org/10.1016/j.pain.2010.11.010
Mutso AA, Radzicki D, Baliki MN et al (2012) Abnormalities in hippocampal functioning with persistent pain. J Neurosci 32(17):5747–5756. https://doi.org/10.1523/JNEUROSCI.0587-12.2012
Reckziegel D, Abdullah T, Wu B et al (2020) Hippocampus shape deformation: potential diagnostic biomarker for chronic back pain in women. Pain. https://doi.org/10.1097/j.pain.0000000000002143
Yu W, Wu X, Chen Y et al (2021) Pelvic pain alters functional connectivity between anterior cingulate cortex and hippocampus in both humans and a rat model. Front Syst Neurosci:15. https://doi.org/10.3389/fnsys.2021.642349
Tan Y, Zhou C, He L (2022) Altered structural and functional abnormalities of hippocampus in classical trigeminal neuralgia: a combination of DTI and fMRI study. Journal of Healthcare Engineering 2022:e8538700. https://doi.org/10.1155/2022/8538700
Mutso AA, Petre B, Huang L et al (2014) Reorganization of hippocampal functional connectivity with transition to chronic back pain. J Neurophysiol 111(5):1065–1076. https://doi.org/10.1152/jn.00611.2013
Han S, Ren J, Li Z, Wen J, Jiang B, Wei X (2023) Deactivation of dorsal CA1 pyramidal neurons projecting to medial prefrontal cortex contributes to neuropathic pain and short-term memory impairment. Pain Published online October 27. https://doi.org/10.1097/j.pain.0000000000003100
Shao S, Zheng Y, Fu Z et al (2023) Ventral hippocampal CA1 modulates pain behaviors in mice with peripheral inflammation. Cell Rep 42(1):112017. https://doi.org/10.1016/j.celrep.2023.112017
Cardoso-Cruz H, Dourado M, Monteiro C, Matos MR, Galhardo V (2014) Activation of dopaminergic D2/D3 receptors modulates dorsoventral connectivity in the hippocampus and reverses the impairment of working memory after nerve injury. J Neurosci 34(17):5861–5873. https://doi.org/10.1523/JNEUROSCI.0021-14.2014
Iwabuchi SJ, Drabek MM, Cottam WJ et al (2023) Medio-dorsal thalamic dysconnectivity in chronic knee pain: a possible mechanism for negative affect and pain comorbidity. Eur J Neurosci 57(2):373–387. https://doi.org/10.1111/ejn.15880
Kummer KK, Mitrić M, Kalpachidou T, Kress M (2020) The medial prefrontal cortex as a central hub for mental comorbidities associated with chronic pain. Int J Mol Sci 21(10):3440. https://doi.org/10.3390/ijms21103440
Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T (2008) Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain–cognition interaction. Brain. 131(12):3222–3231. https://doi.org/10.1093/brain/awn229
Mao CP, Zhang QL, Bao FX, Liao X, Yang XL, Zhang M (2014) Decreased activation of cingulo-frontal-parietal cognitive/attention network during an attention-demanding task in patients with chronic low back pain. Neuroradiology. 56(10):903–912. https://doi.org/10.1007/s00234-014-1391-6
Ji G, Sun H, Fu Y et al (2010) Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci 30(15):5451–5464. https://doi.org/10.1523/JNEUROSCI.0225-10.2010
Cardoso-Cruz H, Paiva P, Monteiro C, Galhardo V (2019) Bidirectional optogenetic modulation of prefrontal-hippocampal connectivity in pain-related working memory deficits. Sci Rep 9(1):10980. https://doi.org/10.1038/s41598-019-47555-0
Cardoso-Cruz H, Paiva P, Monteiro C, Galhardo V (2019) Selective optogenetic inhibition of medial prefrontal glutamatergic neurons reverses working memory deficits induced by neuropathic pain. Pain. 160(4):805–823. https://doi.org/10.1097/j.pain.0000000000001457
Griffin AL (2015) Role of the thalamic nucleus reuniens in mediating interactions between the hippocampus and medial prefrontal cortex during spatial working memory. Front Syst Neurosci 9:29. https://doi.org/10.3389/fnsys.2015.00029
Cardoso-Cruz H, Sousa M, Vieira JB, Lima D, Galhardo V (2013) Prefrontal cortex and mediodorsal thalamus reduced connectivity is associated with spatial working memory impairment in rats with inflammatory pain. Pain. 154(11):2397–2406. https://doi.org/10.1016/j.pain.2013.07.020
Cardoso-Cruz H, Laranjeira I, Monteiro C, Galhardo V (2022) Altered prefrontal-striatal theta-band oscillatory dynamics underlie working memory deficits in neuropathic pain rats. Eur J Pain 26(7):1546–1568. https://doi.org/10.1002/ejp.1982
Kelly CJ, Huang M, Meltzer H, Martina M (2016) Reduced glutamatergic currents and dendritic branching of layer 5 pyramidal cells contribute to medial prefrontal cortex deactivation in a rat model of neuropathic pain. Front Cell Neurosci 10:133. https://doi.org/10.3389/fncel.2016.00133
Kang D, Hesam-Shariati N, McAuley JH et al (2021) Disruption to normal excitatory and inhibitory function within the medial prefrontal cortex in people with chronic pain. Eur J Pain 25(10):2242–2256. https://doi.org/10.1002/ejp.1838
Journée SH, Mathis VP, Fillinger C, Veinante P, Yalcin I (2023) Janus effect of the anterior cingulate cortex: Pain and emotion. Neurosci Biobehav Rev 153:105362. https://doi.org/10.1016/j.neubiorev.2023.105362
Christmann C, Koeppe C, Braus DF, Ruf M, Flor H (2007) A simultaneous EEG–fMRI study of painful electric stimulation. NeuroImage 34(4):1428–1437. https://doi.org/10.1016/j.neuroimage.2006.11.006
Davis KD, Moayedi M (2013) Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol 8(3):518–534. https://doi.org/10.1007/s11481-012-9386-8
Zhu DY, Cao TT, Fan HW et al (2022) The increased in vivo firing of pyramidal cells but not interneurons in the anterior cingulate cortex after neuropathic pain. Mol Brain 15(1):12. https://doi.org/10.1186/s13041-022-00897-9
Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ (1997) Functional MRI of pain- and attention-related activations in the human cingulate cortex. J Neurophysiol 77(6):3370–3380. https://doi.org/10.1152/jn.1997.77.6.3370
Bliss TVP, Collingridge GL, Kaang BK, Zhuo M (2016) Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 17(8):485–496. https://doi.org/10.1038/nrn.2016.68
Floresco SB, Ghods-Sharifi S (2006) Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb Cortex 17(2):251–260. https://doi.org/10.1093/cercor/bhj143
Cao B, Wang J, Mu L, Poon DCH, Li Y (2016) Impairment of decision making associated with disruption of phase-locking in the anterior cingulate cortex in viscerally hypersensitive rats. Exp Neurol 286:21–31. https://doi.org/10.1016/j.expneurol.2016.09.010
Hasan M, Lei Z, Akter M et al (2023) Chemogenetic activation of astrocytes promotes remyelination and restores cognitive deficits in visceral hypersensitive rats. iScience. 26(1):105840. https://doi.org/10.1016/j.isci.2022.105840
Markovic T, Pedersen CE, Massaly N et al (2021) Pain induces adaptations in ventral tegmental area dopamine neurons to drive anhedonia-like behavior. Nat Neurosci 24(11):1601–1613. https://doi.org/10.1038/s41593-021-00924-3
Abdul M, Yan HQ, Zhao WN et al (2022) VTA-NAc glutaminergic projection involves in the regulation of pain and pain-related anxiety. Front Mol Neurosci 15:1083671. https://doi.org/10.3389/fnmol.2022.1083671
Navratilova E, Porreca F (2014) Reward and motivation in pain and pain relief. Nat Neurosci 17(10):1304–1312. https://doi.org/10.1038/nn.3811
Alemi M, Pereira AR, Cerqueira-Nunes M, Monteiro C, Galhardo V, Cardoso-Cruz H (2023) Role of glutamatergic projections from lateral habenula to ventral tegmental area in inflammatory pain-related spatial working memory deficits. Biomedicines 11(3):820. https://doi.org/10.3390/biomedicines11030820
Xin J, Shan W, Li J, Yu H, Zuo Z (2022) Activation of the lateral habenula-ventral tegmental area neural circuit contributes to postoperative cognitive dysfunction in mice. Adv Sci (Weinh) 9(22):e2202228. https://doi.org/10.1002/advs.202202228
Liu Y, Zhou LJ, Wang J et al (2017) TNF-α differentially regulates synaptic plasticity in the hippocampus and spinal cord by microglia-dependent mechanisms after peripheral nerve injury. J Neurosci 37(4):871–881. https://doi.org/10.1523/jneurosci.2235-16.2017
Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M (2009) Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci USA 106(7):2423–2428. https://doi.org/10.1073/pnas.0809897106
Mitrić M, Seewald A, Moschetti G et al (2019) Layer- and subregion-specific electrophysiological and morphological changes of the medial prefrontal cortex in a mouse model of neuropathic pain. Sci Rep 9(1):9479. https://doi.org/10.1038/s41598-019-45677-z
Zatorre RJ, Fields RD, Johansen-Berg H (2012) Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 15(4):528–536. https://doi.org/10.1038/nn.3045
Cordeiro Matos S, Zamfir M, Longo G, Ribeiro-da-Silva A, Séguéla P (2018) Noradrenergic fiber sprouting and altered transduction in neuropathic prefrontal cortex. Brain Struct Funct 223(3):1149–1164. https://doi.org/10.1007/s00429-017-1543-7
Zhang Z, Gadotti VM, Chen L, Souza IA, Stemkowski PL, Zamponi GW (2015) Role of prelimbic GABAergic circuits in sensory and emotional aspects of neuropathic pain. Cell Rep 12(5):752–759. https://doi.org/10.1016/j.celrep.2015.07.001
Kelly CJ, Martina M (2018) Circuit-selective properties of glutamatergic inputs to the rat prelimbic cortex and their alterations in neuropathic pain. Brain Struct Funct 223(6):2627–2639. https://doi.org/10.1007/s00429-018-1648-7
Radzicki D, Pollema-Mays SL, Sanz-Clemente A, Martina M (2017) Loss of M1 receptor dependent cholinergic excitation contributes to mPFC deactivation in neuropathic pain. J Neurosci 37(9):2292–2304. https://doi.org/10.1523/JNEUROSCI.1553-16.2017
Sun Q, Zhang J, Li A et al (2022) Acetylcholine deficiency disrupts extratelencephalic projection neurons in the prefrontal cortex in a mouse model of Alzheimer’s disease. Nat Commun 13(1):998. https://doi.org/10.1038/s41467-022-28493-4
Papadogiannis A, Dimitrov E (2022) A possible mechanism for development of working memory impairment in male mice subjected to inflammatory pain. Neuroscience 503:17–27. https://doi.org/10.1016/j.neuroscience.2022.09.007
Citri A, Malenka RC (2008) Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacol 33(1):18–41. https://doi.org/10.1038/sj.npp.1301559
Kędziora M, Boccella S, Marabese I et al (2023) Inhibition of anandamide breakdown reduces pain and restores LTP and monoamine levels in the rat hippocampus via the CB1 receptor following osteoarthritis. Neuropharmacology 222:109304. https://doi.org/10.1016/j.neuropharm.2022.109304
Kodama D, Ono H, Tanabe M (2007) Altered hippocampal long-term potentiation after peripheral nerve injury in mice. Eur J Pharmacol 574(2-3):127–132. https://doi.org/10.1016/j.ejphar.2007.07.054
ProfXG L (2013) Magnesium L-threonate prevents and restores memory deficits associated with neuropathic pain by inhibition of TNF-α. Pain Physician 16(5):E563–E575. https://doi.org/10.36076/ppj.2013/16/E563
Zhuo M (2006) Molecular mechanisms of pain in the anterior cingulate cortex. J Neurosci Res 84(5):927–933. https://doi.org/10.1002/jnr.21003
Koga K, Descalzi G, Chen T et al (2015) Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 85(2):377–389. https://doi.org/10.1016/j.neuron.2014.12.021
Li XH, Matsuura T, Xue M et al (2021) Oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional anxiety by inhibiting presynaptic long-term potentiation. Cell Rep 36(3):109411. https://doi.org/10.1016/j.celrep.2021.109411
Spillantini MG, Goedert M (2013) Tau pathology and neurodegeneration. Lancet Neurol 12(6):609–622. https://doi.org/10.1016/S1474-4422(13)70090-5
Cao S, Fisher DW, Yu T, Dong H (2019) The link between chronic pain and Alzheimer’s disease. J Neuroinflammation 16(1):204. https://doi.org/10.1186/s12974-019-1608-z
Ren C, Chen M, Mu G, Peng S, Liu X, Ou C (2021) NLRP3 Inflammasome mediates neurodegeneration in rats with chronic neuropathic pain. Shock: Injury, Inflammation, Sepsis: Lab Clin Approach 56(5):840–849. https://doi.org/10.1097/SHK.0000000000001832
Guerreiro SR, Guimarães MR, Silva JM et al (2022) Chronic pain causes Tau-mediated hippocampal pathology and memory deficits. Mol Psychiatry 27(11):4385–4393. https://doi.org/10.1038/s41380-022-01707-3
Tajerian M, Clark JD (2015) The role of the extracellular matrix in chronic pain following injury. Pain 156(3):366. https://doi.org/10.1097/01.j.pain.0000460323.80020.9d
Tajerian M, Hung V, Nguyen H et al (2018) The hippocampal extracellular matrix regulates pain and memory after injury. Mol Psychiatry 23(12):2302–2313. https://doi.org/10.1038/s41380-018-0209-z
Ji RR, Nackley A, Huh Y, Terrando N, Maixner W (2018) Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 129(2):343–366. https://doi.org/10.1097/ALN.0000000000002130
Barcelon EE, Cho WH, Jun SB, Lee SJ (2019) Brain microglial activation in chronic pain-associated affective disorder. Front Neurosci 13:213. https://doi.org/10.3389/fnins.2019.00213
Xu N, Tang XH, Pan W et al (2017) Spared nerve injury increases the expression of microglia m1 markers in the prefrontal cortex of rats and provokes depression-like behaviors. Front Neurosci:11. https://doi.org/10.3389/fnins.2017.00209
Wang X, Jiang Y, Li J et al (2021) DUSP1 promotes microglial polarization toward M2 phenotype in the medial prefrontal cortex of neuropathic pain rats via inhibition of MAPK pathway. ACS Chem Neurosci 12(6):966–978. https://doi.org/10.1021/acschemneuro.0c00567
Xu X, Xiao X, Yan Y, Zhang T (2021) Activation of liver X receptors prevents emotional and cognitive dysfunction by suppressing microglial M1-polarization and restoring synaptic plasticity in the hippocampus of mice. Brain Behav Immun 94:111–124. https://doi.org/10.1016/j.bbi.2021.02.026
Zhai Q, Zhang Y, Ye M et al (2023) Reducing complement activation during sleep deprivation yields cognitive improvement by dexmedetomidine. Br J Anaesth 131(3):542–555. https://doi.org/10.1016/j.bja.2023.04.044
Chen C, Liu A, Lu Q et al (2022) HDAC6 inhibitor ACY-1215 improves neuropathic pain and its comorbidities in rats of peripheral nerve injury by regulating neuroinflammation. Chem Biol Interact 353:109803. https://doi.org/10.1016/j.cbi.2022.109803
Dong Y, Xu Z, Huang L, Zhang Y, Xie Z (2016) Peripheral surgical wounding may induce cognitive impairment through interlukin-6-dependent mechanisms in aged mice. Med Gas Res 6(4):180. https://doi.org/10.4103/2045-9912.196899
Koch A, Zacharowski K, Boehm O et al (2007) Nitric oxide and pro-inflammatory cytokines correlate with pain intensity in chronic pain patients. Inflamm Res 56(1):32–37. https://doi.org/10.1007/s00011-007-6088-4
del Rey A, Yau HJ, Randolf A et al (2011) Chronic neuropathic pain-like behavior correlates with IL-1β expression and disrupts cytokine interactions in the hippocampus. Pain 152(12):2827–2835. https://doi.org/10.1016/j.pain.2011.09.013
Fiore NT, Austin PJ (2019) Peripheral nerve injury triggers neuroinflammation in the medial prefrontal cortex and ventral hippocampus in a subgroup of rats with coincident affective behavioural changes. Neuroscience 416:147–167. https://doi.org/10.1016/j.neuroscience.2019.08.005
Austin PJ, Fiore NT (2019) Supraspinal neuroimmune crosstalk in chronic pain states. Curr Opin Physio 11:7–15. https://doi.org/10.1016/j.cophys.2019.03.008
Brooks TA, Hawkins BT, Huber JD, Egleton RD, Davis TP (2005) Chronic inflammatory pain leads to increased blood-brain barrier permeability and tight junction protein alterations. Am J Phys Heart Circ Phys 289(2):H738–H743. https://doi.org/10.1152/ajpheart.01288.2004
Mai CL, Tan Z, Xu YN et al (2021) CXCL12-mediated monocyte transmigration into brain perivascular space leads to neuroinflammation and memory deficit in neuropathic pain. Theranostics 11(3):1059–1078. https://doi.org/10.7150/thno.44364
Taylor AMW, Mehrabani S, Liu S, Taylor AJ, Cahill CM (2017) Topography of microglial activation in sensory- and affect-related brain regions in chronic pain. J Neurosci Res 95(6):1330–1335. https://doi.org/10.1002/jnr.23883
Li T, Chen X, Zhang C, Zhang Y, Yao W (2019) An update on reactive astrocytes in chronic pain. J Neuroinflammation 16(1):140. https://doi.org/10.1186/s12974-019-1524-2
Fiore NT, Austin PJ (2018) Glial-cytokine-neuronal adaptations in the ventral hippocampus of rats with affective behavioral changes following peripheral nerve injury. Neuroscience 390:119–140. https://doi.org/10.1016/j.neuroscience.2018.08.010
Zhang Y, Feng J, Ou C, Zhou X, Liao Y (2023) AQP4 mitigates chronic neuropathic pain-induced cognitive impairment in mice. Behav Brain Res 440:114282. https://doi.org/10.1016/j.bbr.2022.114282
Alvarado S, Tajerian M, Millecamps M, Suderman M, Stone LS, Szyf M (2013) Peripheral Nerve injury is accompanied by chronic transcriptome-wide changes in the mouse prefrontal cortex. Mol Pain 9:1744–8069. https://doi.org/10.1186/1744-8069-9-21
Cui SS, Feng XB, Zhang BH, Xia ZY, Zhan LY (2020) Exendin-4 attenuates pain-induced cognitive impairment by alleviating hippocampal neuroinflammation in a rat model of spinal nerve ligation. Neural Regen Res 15(7):1333–1339. https://doi.org/10.4103/1673-5374.272620
Asgharpour-Masouleh N, Rezayof A, Alijanpour S, Delphi L (2023) Pharmacological activation of mediodorsal thalamic GABA-A receptors modulates morphine/cetirizine-induced changes in the prefrontal cortical GFAP expression in a rat model of neuropathic pain. Behav Brain Res 438:114213. https://doi.org/10.1016/j.bbr.2022.114213
Dauvermann MR, Lee G, Dawson N (2017) Glutamatergic regulation of cognition and functional brain connectivity: insights from pharmacological, genetic and translational schizophrenia research. Br J Pharmacol 174(19):3136–3160. https://doi.org/10.1111/bph.13919
Zhuo M (2009) Plasticity of NMDA receptor NR2B subunit in memory and chronic pain. Mol Brain 2(1):4. https://doi.org/10.1186/1756-6606-2-4
Jang JH, Kim YK, Jung WM et al (2019) Acupuncture improves comorbid cognitive impairments induced by neuropathic pain in mice. Front Neurosci:13. https://doi.org/10.3389/fnins.2019.00995
Naylor B, Hesam-Shariati N, McAuley JH et al (2019) Reduced glutamate in the medial prefrontal cortex is associated with emotional and cognitive dysregulation in people with chronic pain. Front Neurol:10. https://doi.org/10.3389/fneur.2019.01110
Palazzo E, Luongo L, Guida F et al (2016) d-Aspartate drinking solution alleviates pain and cognitive impairment in neuropathic mice. Amino Acids 48(7):1553–1567. https://doi.org/10.1007/s00726-016-2205-4
Derkach VA, Oh MC, Guire ES, Soderling TR (2007) Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci 8(2):101–113. https://doi.org/10.1038/nrn2055
Liu SB, Zhang MM, Cheng LF, Shi J, Lu JS, Zhuo M (2015) Long-term upregulation of cortical glutamatergic AMPA receptors in a mouse model of chronic visceral pain. Mol Brain 8(1):76. https://doi.org/10.1186/s13041-015-0169-z
Zhuo M (2016) Neural mechanisms underlying anxiety-chronic pain interactions. Trends Neurosci 39(3):136–145. https://doi.org/10.1016/j.tins.2016.01.006
Liu X, Li S, Zhang W et al (2022) PRG-1 prevents neonatal stimuli-induced persistent hyperalgesia and memory dysfunction via NSF/Glu/GluR2 signaling. iScience 25(9):104989. https://doi.org/10.1016/j.isci.2022.104989
Ji G, Neugebauer V (2011) Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABAA receptors. J Neurophysiol 106(5):2642–2652. https://doi.org/10.1152/jn.00461.2011
Zhang W, Xiong BR, Zhang LQ et al (2021) The role of the GABAergic system in diseases of the central nervous system. Neuroscience 470:88–99. https://doi.org/10.1016/j.neuroscience.2021.06.037
Medeiros P, de Freitas RL, Boccella S et al (2020) Characterization of the sensory, affective, cognitive, biochemical, and neuronal alterations in a modified chronic constriction injury model of neuropathic pain in mice. J Neurosci Res 98(2):338–352. https://doi.org/10.1002/jnr.24501
Luo C, Kuner T, Kuner R (2014) Synaptic plasticity in pathological pain. Trends Neurosci 37(6):343–355. https://doi.org/10.1016/j.tins.2014.04.002
Wang DS, Zurek AA, Lecker I et al (2012) Memory deficits induced by inflammation are regulated by α5-subunit-containing GABAA receptors. Cell Rep 2(3):488–496. https://doi.org/10.1016/j.celrep.2012.08.022
Zhang W, Xiong BR, Zhang LQ et al (2020) Disruption of the GABAergic system contributes to the development of perioperative neurocognitive disorders after anesthesia and surgery in aged mice. CNS Neurosci Ther 26(9):913–924. https://doi.org/10.1111/cns.13388
Cai X, Qiu L, Wang C et al (2022) Hippocampal inhibitory synapsis deficits induced by α5-containing GABAA receptors mediate chronic neuropathic pain–related cognitive impairment. Mol Neurobiol 59(10):6049–6061. https://doi.org/10.1007/s12035-022-02955-8
Mello-Carpes PB, da Silva de Vargas L, Gayer MC, Roehrs R, Izquierdo I (2016) Hippocampal noradrenergic activation is necessary for object recognition memory consolidation and can promote BDNF increase and memory persistence. Neurobiol Learn Mem 127:84–92. https://doi.org/10.1016/j.nlm.2015.11.014
Benarroch EE (2009) The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology 73(20):1699–1704. https://doi.org/10.1212/WNL.0b013e3181c2937c
Han S, Jiang B, Ren J et al Impaired lactate release in dorsal CA1 astrocytes contributed to nociceptive sensitization and comorbid memory deficits in rodents. Anesthesiology. https://doi.org/10.1097/ALN.0000000000004756
Tsetsenis T, Badyna JK, Li R, Dani JA (2022) Activation of a locus coeruleus to dorsal hippocampus noradrenergic circuit facilitates associative learning. Front Cell Neurosci 16. https://doi.org/10.3389/fncel.2022.887679
Evans AK, Ardestani PM, Yi B, Park HH, Lam RK, Shamloo M (2020) Beta-adrenergic receptor antagonism is proinflammatory and exacerbates neuroinflammation in a mouse model of Alzheimer’s Disease. Neurobiol Dis 146:105089. https://doi.org/10.1016/j.nbd.2020.105089
Zou HL, Li J, Zhou JL, Yi X, Cao S (2021) Effects of norepinephrine on microglial neuroinflammation and neuropathic pain. Ibrain 7(4):309–317. https://doi.org/10.1002/ibra.12001
Speranza L, di Porzio U, Viggiano D, de Donato A, Volpicelli F (2021) Dopamine: The neuromodulator of long-term synaptic plasticity, reward and movement control. Cells 10(4):735. https://doi.org/10.3390/cells10040735
Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER (2016) Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci 113(51):14835–14840. https://doi.org/10.1073/pnas.1616515114
Hao S, Shi W, Liu W, Chen QY, Zhuo M (2023) Multiple modulatory roles of serotonin in chronic pain and injury-related anxiety. Frontiers in Synaptic. Neuroscience:15. https://doi.org/10.3389/fnsyn.2023.1122381
Song NN, Jia YF, Zhang L et al (2016) Reducing central serotonin in adulthood promotes hippocampal neurogenesis. Sci Rep 6(1):20338. https://doi.org/10.1038/srep20338
Jayarajan P, Nirogi R, Shinde A et al (2015) 5-HT6 receptor antagonist attenuates the memory deficits associated with neuropathic pain and improves the efficacy of gabapentinoids. Pharmacol Rep 67(5):934–942. https://doi.org/10.1016/j.pharep.2015.03.013
Kooshki R, Abbasnejad M, Esmaeili-Mahani S, Raoof M (2017) The modulatory role of orexin 1 receptor in CA1 on orofacial pain-induced learning and memory deficits in rats. Basic Clin Neurosci 8(3):213–222. https://doi.org/10.18869/nirp.bcn.8.3.213
Askari-Zahabi K, Abbasnejad M, Kooshki R, Esmaeili-Mahani S (2021) Orexin one receptors within the basolateral amygdala are involved in the modulation of cognitive deficits associated with a migraine-like state in rats. Neurol Res 43(12):1087–1097. https://doi.org/10.1080/01616412.2021.1949687
Nishimura H, Yoshimura M, Shimizu M et al (2022) Endogenous oxytocin exerts anti-nociceptive and anti-inflammatory effects in rats. Commun Biol 5(1):907. https://doi.org/10.1038/s42003-022-03879-8
Cappoli N, Tabolacci E, Aceto P, Dello Russo C (2020) The emerging role of the BDNF-TrkB signaling pathway in the modulation of pain perception. J Neuroimmunol 349:577406. https://doi.org/10.1016/j.jneuroim.2020.577406
Jiang J, Zou Y, Xie C et al (2023) Oxytocin alleviates cognitive and memory impairments by decreasing hippocampal microglial activation and synaptic defects via OXTR/ERK/STAT3 pathway in a mouse model of sepsis-associated encephalopathy. Brain Behav Immun 114:195–213. https://doi.org/10.1016/j.bbi.2023.08.023
Cristino L, Bisogno T, Di Marzo V (2020) Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol 16(1):9–29. https://doi.org/10.1038/s41582-019-0284-z
Mecca CM, Chao D, Yu G et al (2021) Dynamic change of endocannabinoid signaling in the medial prefrontal cortex controls the development of depression after neuropathic pain. J Neurosci 41(35):7492–7508. https://doi.org/10.1523/JNEUROSCI.3135-20.2021
De Gregorio D, McLaughlin RJ, Posa L et al (2019) Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 160(1):136–150. https://doi.org/10.1097/j.pain.0000000000001386
La Porta C, Bura SA, Llorente-Onaindia J et al (2015) Role of the endocannabinoid system in the emotional manifestations of osteoarthritis pain. Pain 156(10):2001–2012. https://doi.org/10.1097/j.pain.0000000000000260
Finn DP, Haroutounian S, Hohmann AG, Krane E, Soliman N, Rice AS (2021) Cannabinoids, the endocannabinoid system and pain: a review of preclinical studies. Pain 162(Suppl 1):S5–S25. https://doi.org/10.1097/j.pain.0000000000002268
Lin B, Wang Y, Zhang P, Yuan Y, Zhang Y, Chen G (2020) Gut microbiota regulates neuropathic pain: potential mechanisms and therapeutic strategy. J Headache Pain 21(1):103. https://doi.org/10.1186/s10194-020-01170-x
Hua D, Li S, Li S et al (2022) Gut microbiome and plasma metabolome signatures in middle-aged mice with cognitive dysfunction induced by chronic neuropathic pain. Front Mol Neurosci:14. https://doi.org/10.3389/fnmol.2021.806700
Subramaniam CB, Bowen JM, Gladman MA, Lustberg MB, Mayo SJ, Wardill HR (2020) The microbiota-gut-brain axis: an emerging therapeutic target in chemotherapy-induced cognitive impairment. Neurosci Biobehav Rev 116:470–479. https://doi.org/10.1016/j.neubiorev.2020.07.002
Li Z, Sun T, He Z et al (2022) SCFAs ameliorate chronic postsurgical pain–related cognition dysfunction via the ACSS2-HDAC2 axis in rats. Mol Neurobiol 59(10):6211–6227. https://doi.org/10.1007/s12035-022-02971-8
Yue C, Luan W, Gu H et al (2023) The role of the gut–microbiota–brain axis via the subdiaphragmatic vagus nerve in chronic inflammatory pain and comorbid spatial working memory impairment in complete Freund’s adjuvant mice. J Psychiatr Res 166:61–73. https://doi.org/10.1016/j.jpsychires.2023.09.003
Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A (2009) Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci 29(44):13746–13750. https://doi.org/10.1523/JNEUROSCI.3687-09.2009
Suto T, Eisenach JC, Hayashida K, Ichiro (2014) Peripheral nerve injury and gabapentin, but not their combination, impair attentional behavior via direct effects on noradrenergic signaling in the brain. Pain® 155(10):1935–1942. https://doi.org/10.1016/j.pain.2014.05.014
Pask S, Dell’Olio M, Murtagh FEM, Boland JW (2020) The effects of opioids on cognition in older adults with cancer and chronic noncancer pain: a systematic review. J Pain Symptom Manag 59(4):871–893.e1. https://doi.org/10.1016/j.jpainsymman.2019.10.022
Alcalá-Lozano R, Morelos-Santana E, Cortés-Sotres JF, Garza-Villarreal EA, Sosa-Ortiz AL, González-Olvera JJ (2018) Similar clinical improvement and maintenance after rTMS at 5 Hz using a simple vs. complex protocol in Alzheimer’s disease. Brain Stimul 11(3):625–627. https://doi.org/10.1016/j.brs.2017.12.011
Boggio PS, Fregni F, Bermpohl F et al (2005) Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson’s disease and concurrent depression. Mov Disord 20(9):1178–1184. https://doi.org/10.1002/mds.20508
Martin DM, McClintock SM, Forster JJ, Lo TY, Loo CK (2017) Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: a systematic review and meta-analysis of individual task effects. Depress Anxiety 34(11):1029–1039. https://doi.org/10.1002/da.22658
Turriziani P (2012) Enhancing memory performance with rTMS in healthy subjects and individuals with mild cognitive impairment: the role of the right dorsolateral prefrontal cortex. Front Hum Neurosci:6. https://doi.org/10.3389/fnhum.2012.00062
Ma J, Zhang Z, Kang L et al (2014) Repetitive transcranial magnetic stimulation (rTMS) influences spatial cognition and modulates hippocampal structural synaptic plasticity in aging mice. Exp Gerontol 58:256–268. https://doi.org/10.1016/j.exger.2014.08.011
Dedoncker J, Brunoni AR, Baeken C, Vanderhasselt MA (2016) A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: influence of stimulation parameters. Brain Stimul 9(4):501–517. https://doi.org/10.1016/j.brs.2016.04.006
Shin DA, Kim TU, Chang MC (2021) Minocycline for controlling neuropathic pain: a systematic narrative review of studies in humans. J Pain Res 14:139–145. https://doi.org/10.2147/JPR.S292824
Takazawa T, Horiuchi T, Orihara M et al (2023) Prevention of postoperative cognitive dysfunction by minocycline in elderly patients after total knee arthroplasty: a randomized, double-blind, placebo-controlled clinical trial. Anesthesiology 138(2):172–183. https://doi.org/10.1097/ALN.0000000000004439
Rahmani N, Mohammadi M, Manaheji H et al (2022) Carbamylated erythropoietin improves recognition memory by modulating microglia in a rat model of pain. Behav Brain Res 416:113576. https://doi.org/10.1016/j.bbr.2021.113576
D’Aniello A, Luongo L, Romano R et al (2017) d-Aspartic acid ameliorates painful and neuropsychiatric changes and reduces β-amyloid Aβ1-42 peptide in a long lasting model of neuropathic pain. Neurosci Lett 651:151–158. https://doi.org/10.1016/j.neulet.2017.04.041
Zhou F, Wang X, Han B et al (2021) Short-chain fatty acids contribute to neuropathic pain via regulating microglia activation and polarization. Mol Pain 17:1744806921996520. https://doi.org/10.1177/1744806921996520
Acknowledgements
We thank Figdraw (www.figdraw.com) for expert assistance in the pattern drawing.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81974170) and the Natural Science Foundation of Hubei Province (2021CFB341).
Author information
Authors and Affiliations
Contributions
Each author’s contribution to this manuscript is as follows: W.Z. and X.T. conceived the idea. S.H. wrote the initial draft. J.W., W.Z., and X.T. revised the manuscript and figures. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, S., Wang, J., Zhang, W. et al. Chronic Pain–Related Cognitive Deficits: Preclinical Insights into Molecular, Cellular, and Circuit Mechanisms. Mol Neurobiol 61, 8123–8143 (2024). https://doi.org/10.1007/s12035-024-04073-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-024-04073-z