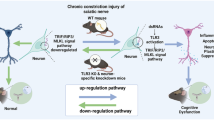
Abstract
Background
Memory deficit is a common cognitive comorbid in patients with neuropathic pain that need better treatment. Recent research revealed that nanocurcumin has an antinociceptive action and a protective effect against memory disorders, suggesting its possible effectiveness for the treatment of neuropathic pain and its comorbidity.
Methods
Adult male albino Wistar rats (n = 32) were randomly divided into four experimental groups: CCI+ nanocurcumin, CCI + vehicle, sham + nanocurcumin, and sham + vehicle. Neuropathic pain induced by a chronic constriction injury of the sciatic nerve. Nanocurcumin or vehicle was injected intraperitoneally for 10 days. Behavioral assessment achieved to evaluate pain threshold in the von Frey test and radiant heat test, also spatial learning and memory examined by the Morris water maze (MWM) test. To explore the possible relation, IL-1β, and TNF-α levels of the hippocampus measured by enzyme-linked immunosorbent assay (ELISA).
Results
Our data showed that CCI caused neuropathic pain-related behaviors and spatial learning and memory disorders in rats. Chronic treatment with nanocurcumin significantly increased pain threshold (P < 0.001; F = 27.63, F = 20.58), improved spatial memory (P < 0.01; F = 47.37), and decreased the hippocampal levels of IL-1β (P < 0.001; F = 33.57) and TNF-α (P < 0.01; F = 7.25) in CCI rats.
Conclusion
Chronic nanocurcumin can ameliorate pain-related behavior, improve spatial learning and memory deficits, and is associated with the reduction of IL-1β and TNF-α levels in the hippocampus in CCI rats. Nanocurcumin may be potentially providing a therapeutic alternative for the treatment of neuropathic pain and its memory impairment comorbidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropathic pain is a frequent complication that results in from damage or disease disturbing the nervous system and commonly signifies as allodynia and hyperalgesia. Cognitive disorders such as memory abnormality are the common comorbidities in patients suffering from chronic neuropathic pain that affects their daily activities and declines the patient’s quality of life (Colloca et al. 2017; Sumitani et al. 2018; Rosenberger et al. 2020; Scholz et al. 2019).
Memory deficit is a common cognitive comorbid in neuropathic pain patients that need better treatment. Many kinds of literature studied the mechanisms of neuropathic pain and the relation between neuropathic pain and memory abnormality and determined similarity underlying between them (Saffarpour et al. 2017; Jayarajan et al. 2015; Wang et al. 2019). Previous data suggested that chronic neuropathic pain affected working memory, short-term memory; inhibits long-term potentiation; and decreases excitatory synapses in rodent’s hippocampus (Gui et al. 2016a; Rahn et al. 2013; Qian et al. 2019; Bilbao et al. 2018). Also, clinical studying revealed that the volume of the hippocampus and hippocampus-prefrontal cortex connection reduced in patients suffered from chronic neuropathic pain (Boadas-Vaello et al. 2017; Yalcin et al. 2014). It suggested enhanced expression of the proinflammatory cytokines like TNF-α and IL-1β in the hippocampus involved in memory disorder related to chronic pain in rats (Gui et al. 2016a; Mai et al. 2019; Ren et al. 2011).
The immunity dysregulation and neuroinflammation are the frequent reasons for causing neurological disorders such as neuropathic pain and cognitive comorbidities. Neuropathic pain can develop secondary to the chronic compression and epineurial ischemia of the peripheral nerves that result in neuroinflammation. Neuroinflammation associated with the activation of glial cells and releasing various proinflammatory cytokines, as well as IL-1β and TNF-α, which have been involved directly in the processing of neuropathic pain. Recent assays reported that the overproduction of IL-1 β and TNF-α in supraspinal regions like the hippocampus associated with behavioral symptoms of neuropathic pain and memory deficits following peripheral nerve injury, whereas the blockade of IL-1β signaling and inhibition of TNF-α alleviated nerve injury-induced neuropathic pain and its comorbidity. Thus, it considers that immune modulation can be a therapeutic target for neuropathic pain and its memory complications (Gui et al. 2016a; Sommer et al. 2018; Liu et al. 2017; Vo et al. 2009; Nasirinezhad and Saffarpour 2009).
Curcumin is a dietary polyphenolic pigment, extracted from Curcuma longa has a wide range of beneficial effects. Previous studies have shown anti-inflammatory, antioxidant, and neuroprotective properties of curcumin, which all involved in the process of neuropathic pain (Yavarpour-Bali et al. 2019; Wright and Rizzolo 2017; Zhao et al. 2014). Recent assays suggest that curcumin has an analgesic effect in different pain animal models (Gerard et al. 2015; Zhang et al. 2018; Zhu et al. 2014a; Liu et al. 2016; Zhu et al. 2014b).Also, it has reported the protective effect of curcumin on memory loss. Some curcumin derivative improved cognitive impairment and enhanced memory in clinical situation and rodent models of Alzheimer’s disease (Agrawal et al. 2018; Reddy et al. 2016, 2018).
However, curcumin is a dietary spice with the pharmacological properties, poor bioavailability, and in vivo metabolic stability restricts its utilization in the clinic. Therefore, the strategy has been dedicated to improving the bioavailability of curcumin is by forming curcumin nanoparticles. Nanoscale drug delivery systems can enhance the drug bioavailability and targeting, control drug release, and decrease drug toxic side effects. New approaches include assessing the efficacy and action mechanisms of nanocurcumin in various diseases; for this purpose, nanoparticle-encapsulated curcumin has developed, and its effect on the treatment of some diseases such as cancer, cognition disorders, Alzheimer’s disease, pain, and diabetic neuropathy have studied (Mirzaie et al. 2019; Flora et al. 2013).
Although the specific mechanism of nanocurcumin action is not fully known, it seems that its possible usefulness in treating pain symptoms and memory deficit associated with neuropathic pain. Therefore, this assay planned to find out whether chronic administration of nanocurcumin could ameliorate neuropathic pain symptoms and improve memory impairments in the sciatic nerve injury model of rats and illuminate the relative IL-1β and TNF-α level alteration in the hippocampus.
Experimental procedures
Animals
Male albino Wistar rats weighing 250–300 g purchased from the laboratory animal breeding center of the Iran University of Medical Sciences. The rats kept in a temperature and humidity standard room (22 ± 2 °C) on a 12-h light/dark cycle with food and water available ad libitum. Female animals have hormonal fluctuations, and behavioral complexity results in the estrus cycle, as well as the progesterone and estrogen levels, are varying during the estrus cycle. As stated in the variation of the female sex hormones from cycle to cycle, and their impact on pain sensation, in this study, male rats were used.
The animals were randomly assigned to one of the four experimental groups: CCI + nanocurcumin, CCI + vehicle, sham + nanocurcumin, and sham + vehicle (n = 8 per group, 32 rats totally). Caregivers were blended to the grouping. All behavioral tests blindly achieved between 10:00 AM and 02:00 PM for each experimental group. The experiments adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (IASP) and were approved by the Ethical Committee of the University (97-4-32-13617).
CCI surgery of sciatic nerve
The CCI model proposed by Bennett and Xie (Bennett and Xie 1988) achieved to cause neuropathic pain signs. In brief, the adult male rats anesthetized by using ketamine (100 mg/kg) and xylazine (15 mg/kg) intraperitoneally, and an incision shaped in the left thigh. After the sciatic nerve appeared, four loose sutures around the nerve struck by using a 4-0 chromic tread. The sutures placed at an interval of 1 mm and only constricted the nerve while circulation through the epidural vasculature was not interrupted. In the sham surgery, the sciatic nerve appeared, and no further manipulation performed.
Drugs and treatment
Nanocurcumin (nanomicellar form of curcumin, the average size of 10 nm) soft gelatin capsules (SinaCurcumin, 40 mg) were used in this study and purchased from Minoo Pharmaceuticals Company. Nanocurcumin excreted from the capsule using an insulin syringe and immediately injected intraperitoneally (40 mg/kg) in sham and CCI treatment groups. Vehicle groups received olive oil (40 mg/kg) intraperitoneally. The doses of the drugs have been selected based on previous studies (Zhao et al. 2014; Flora et al. 2013; Zhao et al. 2017).
Experimental design
The day of surgical procedure (CCI or a sham operation) was considered the zero day of the experiment. Behavioral pain assessment carried out 7, 14, 21, and 28 days after surgery for the ipsilateral hind paws in all experimental groups. The pain behavior assessed for contralateral hind paw using these tests, but the results were not significant between groups, and we did not show them in the “Results” sections. The treatment with nanocurcumin or vehicle started on day 7 until day 16 (totally 10 days) after the surgical procedure (CCI or a sham operation).The rats in the treatment groups received 40 mg/kg of nanocurcumin or olive oil (vehicle) daily for 10 consecutive days intraperitoneally. At the end of the medication, the Morris water maze (MWM) test performed to evaluate the spatial learning and memory from day 18 to day 21 after surgery. On the day 28 experiment, the probe test again achieved to assess the long-term memory. Lastly, the rats (sham-operated or CCI) scarified, and the brains were rapidly removed and dissected on an ice-chilled glass plate, the right hippocampus contralateral to the peripheral injury, based on previous studies obtained (Tyrtyshnaia et al. 2019), and stored at − 80 °C for ELISA assessment.
Behavioral tests
von Frey test
Rats separately put in a Plexiglas box with a wire mesh bottom and let to habituate for 30 min. Tactile allodynia was evaluated by the von Frey filaments (4.56, 4.74, 4.93, 5.07, 5.18, 5.46 and 5.88 g) according to previous protocol (Chaplan et al. 1994). Filaments were arranged in ascending order and applied upright to the surface of the mid-plantar hind paw using the up and down method. The withdrawal responses were measured by sequentially increasing and decreasing the stimulus strength and the mean withdrawal threshold assessed by the Dixon nonparametric test.
Radiant heat test
Reactions to noxious heat were examined using paw withdrawal test (Hargreaves apparatus, 7370, Ugo Basile, Comerio, Italy) (Hargreaves et al. 1988). Considering, rats were put in a Plexiglas box with a glass floor and let to habituate for 20 min. A radiant heat source underneath the glass floor radiated at the plantar hind paw, and simultaneously a chronometer was turned on. The interval between heat radiation and the reaction of the rats, such as hind paw lifting, computed as the withdrawal latencies. To prevent tissue damage, the duration of the heat radiation was set at 25 s. The trial was performed three times for each rat at least 1 min apart and the average values calculated for statistical analysis.
MWM test
The hippocampus-dependent spatial learning and memory was checked out by the standard MWM tests, as described previously (Bian et al. 2012). In this test, learning considered to the shorter latencies to escape and decrease the length of the path to find the target platform. The rats trained to reach the submerged platform for three consecutive days with four trials per 18–20 days after the sciatic nerve injury. The platform was hidden in the southeast quadrant of the tank. In all trials, the rats swam until they come to rest on the platform for the 30 s or until 60 s had passed. The distance moved, latency to reach the platform (escape latency), and the velocity was recorded by a tracking system. Reference memory tracked as time elapsed in the target region by a probe trial 24 h after the last acquisition trial (day 21) and 1 week later (28 days after the surgery). The platform was removed at the time of the probe trial to evaluate the reference memory for the previous platform location.
ELISA procedure
IL-1β assay by ELISA
The levels of IL-1 beta were evaluated based on the protocol of the purchased kit (Biorbyt, Cat. No: orb219820, Sensitivity 15 pg/ml). In summary, at the end of the experiment (day 21 and or 28), three animals of each group randomly selected, sacrificed, and their hippocampus from brain isolated freshly and lysed by the lysis buffer prepared in the kit. All the steps have done according to the protocol provided by the company. Briefly, the required number of well for blank, standard, and sample were selected and prepared in the plate, and 0.1 ml of samples and standards were added to the wells and incubated in 37 °C for 90 min. Afterward, added 0.1 ml biotinylated antibodies, and incubated the plate at 37 °C for 60 min. Then, washed plate three times with 0.01 M TBS. A total of 0.1 ml of prepared ABC working solution was added to the wells and incubated at 37 °C for 30 min. The plate washed with 0.01 M TBS for 5 times, and then 90 μl of prepared TMB color developing agent was added into each well and the plate incubated at 37C for 15–20 min; then, 0.1 ml TMB stop solution was added into each well and after 30 min read at 450 nm. After adding the stop solution, the color of the solution was changed into yellow immediately.
TNF-α assay by ELISA
At the end of the experiment period (day 21 and or 28), TNF-α was determined by using ELISA Kit (Diaclon, Cat. No 865.000.096, sensitivity 15 pg/ml) according to the standard manual. Briefly, three animals of each group randomly selected, decapitated and their hippocampus from brain isolated freshly and lysed by the lysis buffer, the supernatant separated, then added 100 μl of each sample and standard diluent in duplicate to wells and then 50 μl of diluted biotinylated anti-rat TNF-α added to all wells. The wells covered with a plastic plate cover incubated at room temperature (18 to 25 °C). Three hours later, the plate was washed 3 times by 0.3 ml of 1 × washing solution into each well. Then, 100 μl of the streptavidin-HRP solution was added into all wells and incubated at room temperature for 30 min. After 3 times washing, 100 μl of TMB substrate solution added into all wells for 10–15 min at room temperature also avoid direct exposure to light by wrapping the plate in aluminum foil. The absorbance value of each well read at 610 nm immediately after adding 100 μl of H2SO4 stop reagent into all wells.
Data and statistical analysis
Differences between the groups were calculated using repeated measures 2-way ANOVA with a post hoc test (Bonferroni’s test) to analyze pain-related behavior and the difference among time effect and groups in training test of the water maze. The mean comparisons for the time spent in the target quadrant and ELISA data were calculated by one-way ANOVA with post hoc test (Tukey’s test) with SPSS 21 in experimental groups. Data presented as mean ± SEM. P < 0.05 considered as significant.
Results
Effect of CCI and chronic nanocurcumin treatment on pain-related behavior
CCI induced mechanical allodynia and thermal hyperalgesia in rats which took long for at least 28 days, as compared with sham-operated rats (Fig. 1a and b, respectively).
The effect of chronic nanocurcumin administration after induction of pain by CCI model on mechanical allodynia (a) and thermal hyperalgesia (b). Nanocurcumin or vehicle treatment started 1 day after surgery and continued for 10 days. •••P < 0.001 indicated the difference between CCI + vehicle vs sham + vehicle. ***P < 0.001 indicated the difference between CCI + nanocurcumin vs CCI + vehicle. Data analyzed by two-way ANOVA with a post hoc (Bonferroni’s test) and expressed as mean ± SEM of 8 animals per group
In vehicle-treated groups, 7,14, 21, and 28 days after the surgery, the CCI rats, presented lower mechanical withdrawal threshold (5.6 ± 0.14 g,3.5 ± 0.37 g, 7.3 ± 0.31 g, 9 ± 0.25 g, respectively) compared with the sham group (14.8 ± 0.11 g, 13.5 ± 0.32 g, 14.9 ± 0.01 g, 14.8 ± 0.13 g, respectively). At the same times, also in vehicle-treated groups, the mean thermal withdrawal latency (10.64 ± 0.14 s,8.46 ± 0.1 8 s,11.49 ± 0.14 s, 13.34 ± 0.12 s) in CCI rats were less than the sham group (20 ± 0.25 s, 19.5 ± 0.13 s, 18 ± 0.32 s, 20 ± 0.26 s, respectively). These results indicated the meaningful reduction in the mechanical force(P < 0.001, F = 27.63) and duration of radiation (P < 0.001, F = 20.58) required to elicit paw withdrawal to the von Frey filament and noxious heat, respectively, in the hind paw ipsilateral to the chronic constriction injury of sciatic nerve.
Chronic nanocurcumin treatment alleviated pain-related behavior in CCI rats. After 10 days treatment, nanocurcumin treatment increased the mechanical allodynia (P < 0.001) and thermal hyperalgesia (P < 0.001) thresholds in CCI rats compared to CCI vehicle group. Mean mechanical withdrawal threshold at days 7, 14, 21, and 28 after surgery for the CCI group that received the nanocurcumin were 6 ± 0.6 g, 8.96 ± 0.5 g, 10 ± 0.58 g, and 11.5 ± 0.62 g, respectively. The means of thermal withdrawal latency in nanocurcumin-treated rats, in 7, 14, 21, and 28 days after CCI surgery were 11.21 ± 0.65 s, 15.25 ± 0.5 s, 16 ± 0.6 s, and 16.78 ± 0.6 s, respectively. There was no significant difference in the mechanical and thermal thresholds between the sham-treated vehicle and sham-treated nanocurcumin groups.
Effect of CCI and chronic nanocurcumin treatment on spatial learning and memory performance in MWM test
As shown in Fig.2, spatial learning ability decreased in CCI rats treated with vehicle. ANOVA analysis showed that CCI-treated vehicle rats have the more latencies to escape(48.65 ± 1.67 s, 44.5 ± 1.5 s, 22.5 ± 1.58 s) and the increase in the length of the path to find the platform (550.6 ± 18.16 cm, 467.3 ± 18.58 cm, 300.6 ± 17.76 cm) in 3 consecutive days of learning-training phase compared to the sham-treated vehicle group. Means of escape latency, in the training phase for the sham group treated by vehicle, were 28.15 ± 3.1 s, 20.5 ± 2.45 s, and 9.8 ± 2.7 s, respectively. Also, the sham animals treated by vehicle moved 362.16 ± 45.16 cm, 208.1 ± 23 cm, and 100.5 ± 20 cm, respectively, to find the hidden platform.
Performance of animals measured by the latency (a, b), distance (c, d), and velocity (e) to reach the hidden platform in the training days of the Morris water maze test from day 18 to day 21(days 1, 2, and 3) in the experimental groups. **P < 0.01 and ***P < 0.001 indicate the difference between groups. Data analyzed by repeated measures 2-way ANOVA with a post hoc test (Bonferroni’s test) and expressed as mean ± SEM of 8 animals per group
However, post hoc analysis determined that the nanocurcumin-treated rats presented a significant decrease in the latency (P < 0.01, F = 16.27) and path length (P < 0.01, F = 15.32) to reach the platform in 3 consecutive days of the learning-training phase compared to CCI rats treated with vehicle (Fig. 2b and Fig. 2d). The means of latency time were 28 ± 2.3 s, 22.5 ± 2 s, and 11.2 ± 2 s; and the means of distance moved were 280.25 ± 17 cm, 189.5 ± 17 cm,98 ± 16.8 cm for nanocurcumin-treated rats in the learning-training phase, respectively. Swimming speeds were similar among all groups (Fig. 2E).
Our results showed that, the CCI rats treated with vehicle spent less time navigating in the target quadrant of the MWM. When pain threshold spontaneously increased, CCI rats spent more time in the target zone so the rate of the probe trial time was significantly (P < 0.05, F = 47) different between the 21st and (10.8 ± 0.7 s) and 28th days (13.5 ± 0.8 s) after the injury (Fig. 3a). Chronic nanocurcumin treatment reversed this effect (Fig. 3b). CCI rats treated by nanocurcumin significantly spent more time navigating in the target quadrant in days 21(14.62 ± 0.84 s) and 28 (17.80 ± 0.8 s) compared with CCI vehicle-treated rats (P < 0.01, F = 47.37). There was no significant difference in the learning and memory results between the sham-treated vehicle and sham-treated nanocurcumin groups.
Results of probe trials in the Morris water maze test in CCI rats (a) and in the CCI + nanocurcumin vs CCI + vehicle groups (b) at day 21 and day 28 after surgery. Probe trials (memory retrieval) as indicated by time spent in the target zone after the training days. *P < 0.05 and **P < 0.01 indicate the difference between groups. Data analyzed by one-way ANOVA with a post hoc test (Tukey’s test) and expressed as mean ± SEM of 8 animals per group
Effect of CCI and chronic nanocurcumin treatment on IL-1β and TNF-α levels in the hippocampus
ELISA’s result analysis is shown in Fig. 4 and Fig. 5. In vehicle-treated rats, CCI surgery caused an increase in IL-1β (P < 0.001, F = 33.57) and TNF-α (P < 0.001, F = 7.25) levels in the hippocampus, 21 and 28 days following surgery in comparing the sham group. The mean level of IL-1β in the hippocampus of CCI rats treated by the vehicle was 2.6 ± 0.25 pg/ml and 2 ± 0.2 pg/ml in days 21 and 28 after surgery and in the sham vehicle-treated rats were 0.57 ± 0.13 pg/ml and 0.5 ± 0.13 pg/ml, respectively. Also, The mean level of TNF-α in the hippocampus of CCI rats treated by the vehicle was 158 ± 5.02 pg/ml and 120 ± 5.1 pg/ml in days 21 and 28 after surgery and in the sham vehicle-treated rats were 99.5 ± 4.15 pg/ml and 103 ± 3.18 pg/ml, respectively.
ELISA evaluation of interleukin 1-beta (Il-1β) in the hippocampus 21 and 28 days after chronic constriction injury (CCI) surgery (a) and chronic treatment by nanocurcumin (b). ***P < 0.001 indicates the difference between groups. Data analyzed by one-way ANOVA with a post hoc test (Tukey’s test) and expressed as mean ± SEM of 3 animals per group
ELISA evaluation of tumor necrosis alpha (TNF-α) in the hippocampus 21 and 28 days after chronic constriction injury (CCI) surgery (a) and chronic treatment by nanocurcumin (b). **P < 0.01, ***P < 0.001 indicate the difference between groups. Data analyzed by one-way ANOVA with a post hoc test (Tukey’s test) and expressed as mean ± SEM of 3 animals per group
ANOVA and post hoc analyses revealed that, after 10 days nanocurcumin treatment, IL-1β level (P < 0.001, F = 33.57) and TNF-α level (P < 0.01, F = 7.25) in the hippocampus of CCI rats significantly decreased compared to CCI vehicle-treated rats. The mean level of IL-1β in the hippocampus of CCI rats treated by nanocurcumin was 0.85 ± 0.15 pg/ml and 0.9 ± 0.1 pg/ml, and TNF-α levels were 107.37 ± 3.1 pg/ml and 90 ± 4.75 pg/ml in days 21 and 28 after surgery, respectively.
Discussion
The present data demonstrated that chronic nanocurcumin treatment is associated with reduced neuropathic pain-related behavior, improved memory deficits, and altered the level of IL-1β and TNF-α in the hippocampus, in the sciatic nerve injury model of rats.
We achieved the CCI model to induce chronic neuropathic pain signs because it can replicate clinical symptoms in neuropathic patients and often used as a typical animal model of neuropathic pain (Bennett and Xie 1988; Austin et al. 2012). In the current study, the nociceptive behavior raised 2 weeks after surgery and reduced gradually up to week 4, which is in agreement with previous results. Data determined chronic constriction injury of the sciatic nerve resulting in marked and prolonged pain-related symptoms that peaked 2 weeks after surgery and hierarchically decreased up to week 7. Therefore in the CCI model, nociceptive behaviors are partially reversible (Khangura et al. 2017; Keay et al. 2004).
Additionally, the current study suggests that concomitant with the decrease in the pain threshold, the function of the spatial learning and memory disrupted in CCI rats. We used MWM test that is a validated behavioral test for the evaluation of learning and memory in mice and rats (Tomas Pereira and Burwell 2015). We showed a slower learning rate and the spatial reference memory deficits during the probe trial of the MWM test after sciatic nerve injury. These changes in cognitive performance should not be related to motor dysfunction, as there are no significant differences in swimming speeds after peripheral nerve injury. Substantial studies observed the learning and memory disorders associated with neuropathic pain symptoms (Wang et al. 2019; Jia et al. 2017; Gui et al. 2016b; Galvez-Sánchez et al. 2019).
Furthermore, in the current study, the CCI rats displayed the spatial reference memory deficits in the course of probe trial on day 21 after surgery, but the result of day 28 showed increased pain threshold accompanied by higher levels of memory retention. This data is consistent with other reports which addressed the CCI rats performed concurrent pain-related and memory deficit behaviors with a significant time effect (Saffarpour et al. 2017; Jayarajan et al. 2015; Wright and Rizzolo 2017).
Some data determined that pain relief can alter cognitive deficits such as memory disorders, associated with chronic pain (Rahn et al. 2013; Galvez-Sánchez et al. 2019). However, other studies show that neuropathic pain and cognitive comorbidities are poorly responsive to common analgesic drugs like opioids and non-steroidal anti-inflammatory drugs. Also, the medications that always prolonged used for the treatment of neuropathic pain may lead to cognitive deficits (Vo et al. 2009; Wright and Rizzolo 2017). Therefore, studies on new therapeutic agents using botanical medicine or dietary has become one of the most important strategies to alleviate neuropathic pain and improve cognitive deficits comorbidity.
In the present assay, we evaluated the therapeutic effect of nanocurcumin in the neuropathic pain condition.
We showed the antinociceptive property of nanocurcumin associated with its memory improvement effect in peripheral neuropathic pain situation. These results identified that chronic nanocurcumin treatment increased withdrawal threshold and latencies in behavioral pain-related exams and shortened escape latencies and increased time in the target quadrant crossings in the water maze task. These data suggested that nanocurcumin treatment can ameliorate neuropathic pain symptoms and improve spatial learning and memory deficit comorbidities. Independent studies have previously reported the antinociceptive effect of nanocurcumin in HIV-gp120 and diabetes-induced neuropathic pain (Zhao et al. 2017), as well as the efficacy of nanocurcumin on memory deficits in Alzheimer’s disease (Yavarpour-Bali et al. 2019; Agrawal et al. 2018; Reddy et al. 2018). Current data described that nanocurcumin, when chronically administered in neuropathic rats, could synchronically alleviate the pain-related behavior and memory disorder induced by sciatic nerve injury, so the antinociceptive effect of nanocurcumin appears to parallel with its efficacy on memory improvement in CCI model of neuropathic pain. However, the mechanisms underlying the antinociceptive and memory improvement actions of nanocurcumin are poorly cleared.
In this assay, the relation between the hippocampal IL-1β and TNF-α simultaneously with the alteration in the retrieval memory process was investigated in the CCI rats. The hippocampus is a critical supraspinal region involved in learning, memory, and pain perception. Chronic pain causes biochemical, anatomical, and functional changes in the hippocampus (Boadas-Vaello et al. 2017; Yalcin et al. 2014; Sepideh and Farinaz 2020). Previous data revealed that CCI disrupted the hippocampal learning and memory function with a significant time effect (Qian et al. 2019; Bilbao et al. 2018; Gerard et al. 2015). Our results confirmed that accompanying the memory deficits, CCI associated with the enhancement in IL-1β and TNF-α in the hippocampus.
Pro- and anti-inflammatory cytokines, such as TNF-α and IL-1β, has been shown to actively participate in the pathogenesis of neuropathic pain (Sommer et al. 2018). Recent work reported the overproduction of IL-1β in the pain perception and memory formation regions of the brain-induced behavioral signs of neuropathic pain and memory deficits following peripheral nerve injury (Gui et al. 2016b). Other studies identified the correlation between neuropathic pain-induced memory deficit and the level of TNF-α in the hippocampus of the rats. In accordance with other data, our results showed enhanced expression of the proinflammatory cytokines like TNF-α and IL-1β in the hippocampus related to memory disorder induced by sciatic nerve injury in rats.
The present study suggested that chronic administration of nanocurcumin was accompanied by the alteration in the level of IL-1β and TNF-α in the hippocampus after peripheral nerve injury. We found that the repetitive use of nanocurcumin attended by the decrease in the hippocampal levels of IL-1β and TNF-α in combination with increasing pain threshold and improving spatial memory in CCI rats. The antinociceptive effect of nanocurcumin may increase the pain threshold in CCI rats and attenuated pain-induced memory deficits. Additionally, the therapeutic efficacy of nanocurcumin on neuropathic pain and its memory impairment comorbidity may relate to the reduction of inflammatory cytokines such as TNF-α and IL-1β in the hippocampus. However, nanocurcumin and curcumin effects on cytokine levels, and its pathways have not well investigated, some data reported that systemic and repetitive administration of curcumin could alleviate chronic neuropathic pain, possibly via inhibiting the production of chemokines and proinflammatory cytokines in the nervous system. These data supported the inhibitory role of curcumin on the production of inflammatory mediators in the central nervous system (Xie et al. 2019).
Conclusion
In conclusion, we showed that chronic nanocurcumin treatment associated with the reduction of TNF-α, IL-1β in the hippocampus, and relatively decreased pain hypersensitivity and improves memory disorder in CCI rats. Since nanocurcumin has antinociceptive and anti-inflammatory properties, the improvement in pain-induced memory deficits by itself in the CCI rats may be preferable to these equities. It seems that nanocurcumin potentially providing a therapeutic alternative for the treatment of chronic neuropathic pain and its memory impairment comorbidity.
Abbreviations
- ABC:
-
Avidin-biotin-peroxidase complex
- CCI:
-
Chronic constriction injury
- ELISA:
-
Enzyme-linked immunosorbent assay
- IL-1β:
-
Interleukin 1 beta
- MWM:
-
Morris water maze
- TBS:
-
Tris-buffered saline
- TMB:
-
3,3,5,5-tetramethylbenzidine
- TNF-α:
-
Tumor necrosis factor alpha
References
Agrawal M, Saraf S, Saraf S, Antimisiaris SG, Chougule MB, Shoyele SA, Alexander A (2018) Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J Control Release 281:139–177
Austin PJ, Wu A, Moalem-Taylor G (2012) Chronic constriction of the sciatic nerve and pain hypersensitivity testing in rats. J Vis Exp 61
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33(1):87–107
Bian Y, Pan Z, Hou Z, Huang C, Li W, Zhao B (2012) Learning, memory, and glial cell changes following recovery from chronic unpredictable stress. Brain Res Bull 88(5):471–476
Bilbao A, Falfán-Melgoza C, Leixner S, Becker R, Singaravelu SK, Sack M, Sartorius A, Spanagel R, Weber-Fahr W (2018) Longitudinal structural and functional brain network alterations in a mouse model of neuropathic pain. Neuroscience 387:104–115
Boadas-Vaello P, Homs J, Reina F, Carrera A, Verdú E (2017) Neuroplasticity of supraspinal structures associated with pathological pain. Anat Rec (Hoboken) 300(8):1481–1501
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1):55–63
Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN (2017) Neuropathic pain. Nature reviews Disease primers 3:17002–17002
Flora G, Gupta D, Tiwari A (2013) Preventive efficacy of bulk and nanocurcumin against lead-induced oxidative stress in mice. Biol Trace Elem Res 152(1):31–40
Galvez-Sánchez CM, Duschek S, Del Paso GAR (2019) Psychological impact of fibromyalgia: current perspectives. Psychol Res Behav Manag 12:117–127
Gerard E, Spengler RN, Bonoiu AC, Mahajan SD, Davidson BA, Ding H, Kumar R, Prasad PN, Knight PR, Ignatowski TA (2015) Chronic constriction injury-induced nociception is relieved by nanomedicine-mediated decrease of rat hippocampal tumor necrosis factor. Pain 156(7):1320–1333
Gui WS, Wei X, Mai CL, Murugan M, Wu LJ, Xin WJ, Zhou LJ, Liu XG (2016a) Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol Pain 12:174480691664678
Gui WS, Wei X, Mai CL, Murugan M, Wu LJ, Xin WJ, Zhou LJ, Liu XG (2016b) Interleukin-1beta overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol Pain 12:174480691664678
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32(1):77–88
Jayarajan P, Nirogi R, Shinde A, Goura V, Babu VA, Yathavakilla S, Bhyrapuneni G (2015) 5-HT6 receptor antagonist attenuates the memory deficits associated with neuropathic pain and improves the efficacy of gabapentinoids. Pharmacol Rep 67(5):934–942
Jia T et al (2017) Nanoparticle-encapsulated curcumin inhibits diabetic neuropathic pain involving the P2Y12 receptor in the dorsal root ganglia. Front Neurosci 11:755
Keay KA, Monassi CR, Levison DB, Bandler R (2004) Peripheral nerve injury evokes disabilities and sensory dysfunction in a subpopulation of rats: a closer model to human chronic neuropathic pain? Neurosci Lett 361(1–3):188–191
Khangura RK, Bali A, Kaur G, Singh N, Jaggi AS (2017) Neuropathic pain attenuating effects of perampanel in an experimental model of chronic constriction injury in rats. Biomed Pharmacother 94:557–563
Liu S, Li Q, Zhang MT, Mao-Ying QL, Hu LY, Wu GC, Mi WL, Wang YQ (2016) Curcumin ameliorates neuropathic pain by down-regulating spinal IL-1β via suppressing astroglial NALP1 inflammasome and JAK2-STAT3 signalling. Sci Rep 6:28956–28956
Liu Y, Zhou LJ, Wang J, Li D, Ren WJ, Peng J, Wei X, Xu T, Xin WJ, Pang RP, Li YY, Qin ZH, Murugan M, Mattson MP, Wu LJ, Liu XG (2017) TNF-α differentially regulates synaptic plasticity in the hippocampus and spinal cord by microglia-dependent mechanisms after peripheral nerve injury. J Neurosci 37(4):871–881
Mai CL et al (2019) Differential regulation of GSK-3β in spinal dorsal horn and in hippocampus mediated by interleukin-1beta contributes to pain hypersensitivity and memory deficits following peripheral nerve injury. Mol Pain 15:1744806919826789
Mirzaie Z, Ansari M, Kordestani SS, Rezaei MH, Mozafari M (2019) Preparation and characterization of curcumin-loaded polymeric nanomicelles to interference with amyloidogenesis through glycation method. Biotechnol Appl Biochem 66(4):537–544
Nasirinezhad F, Saffarpour S (2009) Involvement of NMDA receptors in antinociceptive effect of ascorbic acid in a neuropathic pain model. RJMS 15(0):193–204
Qian Y et al (2019) The role of CaMKII in neuropathic pain and fear memory in chronic constriction injury in rats. Int J Neurosci 129(2):146–154
Rahn EJ, Guzman-Karlsson MC, David Sweatt J (2013) Cellular, molecular, and epigenetic mechanisms in non-associative conditioning: implications for pain and memory. Neurobiol Learn Mem 105:133–150
Reddy PH, Manczak M, Yin X, Grady MC, Mitchell A, Kandimalla R, Kuruva CS (2016) Protective effects of a natural product, curcumin, against amyloid β induced mitochondrial and synaptic toxicities in Alzheimer’s disease. J Investig Med 64(8):1220–1234
Reddy PH, Manczak M, Yin X, Grady MC, Mitchell A, Tonk S, Kuruva CS, Bhatti JS, Kandimalla R, Vijayan M, Kumar S, Wang R, Pradeepkiran JA, Ogunmokun G, Thamarai K, Quesada K, Boles A, Reddy AP (2018) Protective effects of Indian spice curcumin against amyloid-β in Alzheimer’s disease. J Alzheimers Dis 61(3):843–866
Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG (2011) Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacology 36(5):979–992
Rosenberger DC et al (2020) Challenges of neuropathic pain: focus on diabetic neuropathy. Journal of neural transmission (Vienna, Austria: 1996) 127(4):589–624
Saffarpour S, Shaabani M, Naghdi N, Farahmandfar M, Janzadeh A, Nasirinezhad F (2017) In vivo evaluation of the hippocampal glutamate, GABA and the BDNF levels associated with spatial memory performance in a rodent model of neuropathic pain. Physiol Behav 175:97–103
Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, Benoliel R, Cohen M, Cruccu G, Davis KD, Evers S, First M, Giamberardino MA, Hansson P, Kaasa S, Korwisi B, Kosek E, Lavandʼhomme P, Nicholas M, Nurmikko T, Perrot S, Raja SN, Rice ASC, Rowbotham MC, Schug S, Simpson DM, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ, Barke A, Rief W, Treede RD (2019) The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain 160(1):53–59
Sepideh S, Farinaz N (2020) The CA1 hippocampal serotonin alterations involved in anxiety-like behavior induced by sciatic nerve injury in rats. Scand J Pain (0):000010151520200037
Sommer C, Leinders M, Üçeyler N (2018) Inflammation in the pathophysiology of neuropathic pain. Pain 159(3):595–602
Sumitani M, Sakai T, Matsuda Y, Abe H, Yamaguchi S, Hosokawa T, Fukui S (2018) Executive summary of the clinical guidelines of pharmacotherapy for neuropathic pain: second edition by the Japanese Society of Pain Clinicians. J Anesth 32(3):463–478
Tomas Pereira I, Burwell RD (2015) Using the spatial learning index to evaluate performance on the water maze. Behav Neurosci 129(4):533–539
Tyrtyshnaia A, Manzhulo I, Kipryushina Y, Ermolenko E (2019) Neuroinflammation and adult hippocampal neurogenesis in neuropathic pain and alkyl glycerol ethers treatment in aged mice. Int J Mol Med 43(5):2153–2163
Vo T, Rice AS, Dworkin RH (2009) Non-steroidal anti-inflammatory drugs for neuropathic pain: how do we explain continued widespread use? Pain 143(3):169–171
Wang XM, Zhang GF, Jia M, Xie ZM, Yang JJ, Shen JC, Zhou ZQ (2019) Environmental enrichment improves pain sensitivity, depression-like phenotype, and memory deficit in mice with neuropathic pain: role of NPAS4. Psychopharmacology 236(7):1999–2014
Wright ME, Rizzolo D (2017) An update on the pharmacologic management and treatment of neuropathic pain. Jaapa 30(3):13–17
Xie W, Xie W, Kang Z, Jiang C, Liu N (2019) Administration of curcumin alleviates neuropathic pain in a rat model of brachial plexus avulsion. Pharmacology 103(5–6):324–332
Yalcin I, Barthas F, Barrot M (2014) Emotional consequences of neuropathic pain: insight from preclinical studies. Neurosci Biobehav Rev 47:154–164
Yavarpour-Bali H, Ghasemi-Kasman M, Pirzadeh M (2019) Curcumin-loaded nanoparticles: a novel therapeutic strategy in treatment of central nervous system disorders. Int J Nanomedicine 14:4449–4460
Zhang L, Ding X, Wu Z, Wang M, Tian M (2018) Curcumin alleviates pain and improves cognitive impairment in a rat model of cobra venom-induced trigeminal neuralgia. J Pain Res 11:1095–1104
Zhao X, Wang C, Zhang JF, Liu L, Liu AM, Ma Q, Zhou WH, Xu Y (2014) Chronic curcumin treatment normalizes depression-like behaviors in mice with mononeuropathy: involvement of supraspinal serotonergic system and GABAA receptor. Psychopharmacology 231(10):2171–2187
Zhao S, Yang J, Han X, Gong Y, Rao S, Wu B, Yi Z, Zou L, Jia T, Li L, Yuan H, Shi L, Zhang C, Gao Y, Li G, Liu S, Xu H, Liu H, Liang S (2017) Effects of nanoparticle-encapsulated curcumin on HIV-gp120-associated neuropathic pain induced by the P2X3 receptor in dorsal root ganglia. Brain Res Bull 135:53–61
Zhu Q et al (2014a) Antinociceptive effects of curcumin in a rat model of postoperative pain. Sci Rep 4:4932–4932
Zhu X, Li Q, Chang R, Yang D, Song Z, Guo Q, Huang C (2014b) Curcumin alleviates neuropathic pain by inhibiting p300/CBP histone acetyltransferase activity-regulated expression of BDNF and cox-2 in a rat model. PLoS One 9(3):e91303
Acknowledgments
The authors appreciate Mr. A. Razhaghdoust who presented the nanocurcumin from Exire Nano Sina Co. as a generous gift to them.
Funding
This experiment received grant from the Physiology Research Center of Iran University of Medical Science (Grant number: 97-4-32-13617).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
The goal of this study was to investigate the effects of chronic nanocurcumin treatment on neuropathic pain-related behaviors and spatial learning and memory performance in the CCI model of rats and illuminate the relative IL-1β and TNF-α level alteration in the hippocampus.
Rights and permissions
About this article
Cite this article
Saffarpour, S., Janzadeh, A., Rahimi, B. et al. Chronic nanocurcumin treatment ameliorates pain-related behavior, improves spatial memory, and reduces hippocampal levels of IL-1β and TNFα in the chronic constriction injury model of neuropathic pain. Psychopharmacology 238, 877–886 (2021). https://doi.org/10.1007/s00213-020-05739-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05739-x