Abstract
Nowadays, there is an increasing concern regarding traumatic brain injury (TBI) worldwide since substantial morbidity is observed after it, and the long-term consequences that are not yet fully recognized. A number of cellular pathways related to the secondary injury in brain have been identified, including free radical production (owing to mitochondrial dysfunction), excitotoxicity (regulated by excitatory neurotransmitters), apoptosis, and neuroinflammatory responses (as a result of activation of the immune system and central nervous system). In this context, non-coding RNAs (ncRNAs) maintain a fundamental contribution to post-transcriptional regulation. It has been shown that mammalian brains express high levels of ncRNAs that are involved in several brain physiological processes. Furthermore, altered levels of ncRNA expression have been found in those with traumatic as well non-traumatic brain injuries. The current review highlights the primary molecular mechanisms participated in TBI that describes the latest and novel results about changes and role of ncRNAs in TBI in both clinical and experimental research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As defined by the experts, traumatic brain injury (TBI) is one of the injuries resulting from the mechanical external forces, which leads to the brief loss of consciousness or change in mental status [1]. It has been reported that near 50 million TBI cases occur each year; therefore, about 50% of worldwide population suffers from TBI throughout their lives [2]. TBI has been taken into account as the main cause of disability and mortality in the young adults in the UK [3]. Additionally, it was shown that TBI occurrence rate is much higher in non-high-income countries [2]. It imposes a great financial burden on healthcare system every year [2]. It is characterized by primary injury from an external mechanical force, and subsequent second injury refers to pathological changes that finally contribute to neuron cell death [4, 5].
Moreover, non-coding RNAs (ncRNAs) have been shown to be one of the families of RNAs, which cannot to be translated into proteins; however, they constitute the main part of the human transcriptome [6]. They categorized into two main groups based on their functions: housekeeping ncRNAs like transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), as well as small nuclear RNAs (snRNAs), and regulatory ncRNAs, such as PIWI-interacting RNAs (piRNAs), microRNAs (miRNAs), and long non-coding RNAs (lncRNAs); nonetheless, despite the lack of comprehension knowledge about their physiological roles, other ncRNAs were once thought as transcriptional noise [7, 8]. Nonetheless, reports demonstrated that they maintain pivotal physiological as well as pathological roles via modulating translation [9, 10]. High levels of ncRNAs are expressed in mammalian brains, whereas emerging evidence claimed that acute as well as chronic central nervous system (CNS) damages can change the expression profiles of ncRNAs and their functions [11,12,13,14,15,16,17].
The present study provides a comprehensive review concerning the present knowledge on the role and function of ncRNAs in TBI.
MicroRNAs and Brain Trauma
MiRNAs are known as a group of ncRNAs which primarily involve in regulating post-transcriptional via and stimulating messenger RNA (mRNA) degradation [18]. For achieving post-transcriptional regulation, RNA-induced silencing complex (RISC) promotes interactions of mature miRNAs with a 3′ untranslated region of specific mRNAs [19]. Tremendous advancements in bioinformatic technology contributed to understating the pivotal role of miRNAs in TBI [20,21,22]. Emerging reports are in the support of the fact that miRNAs can be considered as biomarkers in a number of diseases related to neurology, including, but not limited to, Parkinson and TBI owing to its consistent human body fluids [23, 24].
NF-κB signaling pathways are taken into account as one of the leading signaling pathways which participated in inflammation, and scientists have found out that their activation is closely associated with complications of TBI [25]. The role of NF-κB signaling pathways and their inhibitors in TBI has been at the center of focus of recent studies. Great amount of attempts has been put forth to use these pathway inhibitors to reduce neuroinflammatory condition and ameliorate the consequences of TBI. For example, an in vivo study demonstrated that metformin exerted neuroprotective effects on TBI through prohibiting the NF-κB and MAPK signaling pathways [26]. Another experimental study declared that apocynin can protect the cerebral neuron against autophagy and enhance the memory and learning capabilities in rat model of TBI via prohibiting the TLR4/NF-κB signaling pathway [27]. Additionally, it was shown that NF-κB activation contributed to higher apoptosis and neuroinflammation in those with TBI [28].
Zhang et al. [29] designed a study to assess their hypothesis that miR-146a may be involved in progressing TBI. For that purpose, they established a TBI mouse model through the controlled cortical impact (CCI) injury. Researchers assessed these chemokine values via ELISAs but applied behavioral assays to evaluate the effect of miR-146a mimics on the neurological function. The investigators used western blot and qRT-PCR to determine the protein levels and the expression values of RNAs. The authors found enhanced levels of miR-146a in serum and brain and serum and its mimic reduced IL-6, tumor necrosis factor α (TNF-α) values, and IL-1β in brain through prohibiting NF-κB signaling pathways. Its mimic also ameliorated the brain injuries and enhanced the long-term learning and memory capabilities in mouse with TBI. In summer, miR-146a has a pivotal role in TBI-induced injuries by activation of the NF-κB signaling pathway [29].
Researchers also introduced calcium/calmodulin-dependent serine protein kinase (CASK) as one of the unique membrane-associated guanylate kinase (MAGUK) proteins which contains numerous domains that are involved in protein interaction. A high amount of CASK can be found in the central nervous system [30, 31]. A variety of functions is attributed to CASK, such as regulation of gene expression, neural development, and synapse formation [30,31,32]. It has been found out that synaptic transmission has a close correlation with several neurological functions like memory and learning so that its loss is associated with cognitive impairment [33, 34].
The alpha-synuclein (SNCA) protein has been known as one of the key components in Lewy bodies of those suffering from Parkinson disease and plays a mandatory contribution to this disease. According to the documents, its expression is modulated by miR-153 [35] and is highly expressed in presynaptic terminals [36,37,38,39,40]. SNCA upregulation is able to stimulate synaptic impairments including prohibition of neurotransmitter secretion and decrease in synaptic vesicle density [41, 42]. The levels of alpha-synuclein in the cerebrospinal fluid (CSF) have been shown to be enhanced in those with TBI, and this enhancement is attributed to secretion of the soluble alpha-synuclein from the damaged glial cells and neurons, as well as the cause of synaptic impairment and disrupt transportation in those with TBI [43, 44]. An experimental research revealed a transient enhance of alpha-synuclein in subcortical axons in an aged mouse model of TBI; nonetheless, this enhancement disappeared 4 months following TBI [45].
Liu et al. [46] made an effort to characterize dynamic miRNA expression profile in the ipsilateral hippocampus of TBI rat model. The investigators explored 156 miRNAs of which 10 were dysregulated significantly and continuously following TBI. Online program analyses demonstrated that these 10 miRNAs can regulate 107 genes, and thereafter, several related biological processes were explored using bioinformatic and gene ontology analyses. These miRNAs may have the potential to initiate explored biological processes. The overexpression of three of these 10 miRNAs (miR-144, miR-340-5p, and miR-153) following TBI established using qRT-PCR and downregulation of their targeted proteins, namely CASK, SNCA, and nuclear factor erythroid 2–related factor 2 (NRF2), has been also demonstrated. Collectively, miR-144, miR-340-5p, and miR-153 have been shown to have a crucial role in neurological complications following TBI and can be considered as the valuable target for neurological complications after TBI [46].
In addition, dual-specificity phosphatase 1 (DUSP1) has been known as a well-recognized phosphatase that participates in the activation of mitogen-activated protein kinase phosphatase 1 (MKP-1) [47]. According to the findings, DUSP1 has the potential to moderate the mitogen-activated protein kinase (MAPK) signaling, thereby modulating cell death and proliferation or rapid growth [48, 49]. MAPK-p38 is thought to maintain a pivotal role in cellular signal transduction pathways [50]. Emerging evidence revealed that MAPK-p38 participated in several cellular processes including transferring signals to the nucleus, modulating gene expression, and cell differentiation and proliferation [51]. A study revealed the prognostic value of MAPK family in osteosarcoma and DUSP1 capacity as a valuable candidate for this malignant tumor treatment [52].
Qi and Wang [53] designed an in vivo experiment to identify the contribution of miR-429 to microglia-induced inflammation after TBI. RT-PCR was employed to determine the miR-429 expression in microglia and LPS-activated microglia in TBI. A TBI model of mice was established for evaluating the impact of miR-429 prohibition on neurological damages and inflammatory responses. LPS and TBI were accompanied by overexpression of inflammatory cytokines, miR-429, MAPK-p38, and phosphorylated NF-κB in microglia. The prohibition of miR-429 led to improvements in neurological dysfunction of mice with TBI. Bioinformatic tests revealed that DUSP1 can be prohibited by miR-429, leading to phosphorylated NF-κB and MAPK-p38 inhibition. In summary, miR-429 is involved in pro-inflammatory responses of the activated microglia via affecting DUSP1. MiR-429 prohibition is associated with lower inflammatory cytokine production of microglia and neurological consequences following TBI [53].
Bcl2 family contains different proteins, which are involved in neuronal death following injuries, such as pro-survival proteins (for example, BclxL and Bcl2), BH3-only domain proteins (for example, PUMA, Noxa, Bim, and Bid), and pro-apoptotic multidomain (for example, Bak and Bax) [54]. BH3-only proteins provoke neuron death via inducing mitochondrial outer membrane permeability as well as secretion of cytochrome c and apoptosis-inducing factor 1 (AIF-1) [55, 56]. Moreover, BH3-only proteins were shown to be involved in neural loss following CNS damage [57], such as TBI [58, 59]. Conversely, Akt (protein kinase B) was shown to stimulate neuronal survival via phosphorylating pro-apoptotic proteins, especially forkhead box O3 (FoxO3) and glycogen synthase kinase 3β (GSK3β), which are proved to be initiators of the expression of BH3-only protein [60,61,62]. It has been shown that the prohibition of Akt signaling pathways contributes to FoxO3a activation and increases its proapoptotic target proteins, like Bim and PUMA expression [63, 64].
Sabirzhanov et al. [65] designed a study to examine whether modifications in expressing some miRNAs following TBI are able to activate cell death signaling pathways or not, which contribute to neuronal destruction and neurological impairments. A TBI model of mice was established using CCI. MiRNA arrays and qPCR were employed to identify the alteration in the expression of miRNAs until 72 h following TBI (Table 1). They found out that miRNA-711 was overexpressed in the cortex of TBI mice, and this overexpression was initiated 1 h, peaked 6 h, and lasted for 3 days following the injury. This overexpression was also detected in the in vitro TBI model. The investigators utilized miR-711 mimics to clarify the role of miRNA-711 in neural cell deaths. MiRNA-711 mimics applied were associated with enhanced neural cell deaths and caspase-3 activity in a concentration-dependent fashion. The inhibition of miRNA-711 significantly overexpressed Akt and its phosphorylated form, as well as GSK3 and its phosphorylated form. MiRNA-711 inhibition also reduced apoptosis markers including FoxO3a, cytochrome c, and AIF-1. In addition, authors observed that miRNA-711 inhibition was associated with enhanced Akt expression, reduced apoptosis and cortical lesion size, neuronal cell destruction in the hippocampus and cortex, and cognitive and motor dysfunctions. In conclusion, overexpression of miRNA-711 has a substantial role in neural destruction following TBI, through prohibition of Akt signaling pathways, indicating its potential as a valuable candidate for TBI treatment [65]. Additionally, a recent research demonstrated that overexpressed miR-219a-5p, which has been found in the neuronal cell injury model, prohibits cullin domain 1 (CACUL1) and cyclin-A2 (CCNA2) expression and regulates p53 and Akt signaling pathway, leading to an increase in levels of caspase-3 and promoting neural cell apoptosis [66]. In conclusion, since miR-219a-5p maintains its vital contribution to TBI development and pathophysiology, it has the potential to be considered as a valuable target for TBI treatment (Fig. 1) [66].
The role of several miRNAs, which act as novel biomarkers for TBI, and the mechanism behind miR-219a-5p–induced neural cell apoptosis. Overexpression of several miRNAs, including miR-219a-5p, miR-103a-3p, miR-422a, miR-520d-3p, miR-302d-3p, miR-518f-3p, and miR-627, was found in TBI, and they are potentially applied as one of the valuable biomarkers to diagnose TBI. In the neuronal cell injury model, overexpressed miR-219a-5p prohibited CACUL1 and CCNA2 expression and regulated p53/Bcl-2 and Akt/Foxo3a signaling pathways, leading to enhanced neuronal cell apoptosis. This figure was adapted from Yan et al. [66]
Osteopontin, also known as SPP1, is proved to be participated in inflammation, cancer development, and drug resistance [67]. SPP1 is capable to alter adhesion and rapid growth capacity of synovial cells by binding to the respective receptors over the synovial cells’ surface [68]. Upregulation of SPP1 in the synovial fluid, articular cartilage, and synovium of those who are suffering from osteoarthritis was observed, and positive associations were found between SPP1 expression values and the severity of osteoarthritis [69, 70]. Bioinformatic assays revealed that miR-433 is a potent upstream mediator of SPP1 [71]. MiR-433 is a miRNA that has been demonstrated to be participated in some diseases. Enhanced expression of miR-433 expression appeared in three models of cardiac fibrosis [72]. MiR-433 was shown to be able to prohibit breast cancer cell proliferation via decreasing Rap1a expression and modulating the MAPK signaling pathway [73]. Besides, it has been unveiled that miR-433 has a crucial role in several malignancies including glioma and esophageal cancer [74, 75].
Han et al. [76] sought to evaluate SPP1 expression in tissues of tibial callus and heterotopic ossification of those with tibial fracture and TBI and assessed its interaction with miR-433. The TBI group was composed of 26 patients suffering from tibial fracture and brain injury, and the control group was composed of 26 patients suffering from only simple tibial fracture. Callus, heterotopic ossification tissues, and plasma of both groups were gathered to identify the SPP1 mRNA expression, protein, and miR-433. The authors applied qRT-PCR as well as western blot for measuring the miR-433 and SPP1 mRNA expression and SPP1 protein expression, respectively. Dual-luciferase reporter test was applied so as to clarify the direct interaction between SPP1 mRNA and miR-433. It was shown that TBI increased the incident formation of callus and heterotopic ossification in those suffering from fractures; nevertheless, TBI had no effect on the healing time of fracture. In the TBI group, enhanced levels of SPP1 mRNA and protein and also decreased levels of miR-433 compared to the control group were observed. MiR-433 was able to modulate SPP1 mRNA and protein expression through targeting the 3′-untranslated area of SPP1 mRNA. In conclusion, increased levels of protein and SPP1 mRNA were expected in tissues of callus, heterotopic ossification, and plasma of those with the tibial fracture and TBI, which may be because of decreased miR-433 expression [76].
LncRNAs and Brain Trauma
As mentioned earlier, lncRNAs have been introduced as one of the groups of ncRNAs known to be consisted of more than 200 bases [77]. Most of lncRNAs are processed alike to protein-coding messenger RNAs, such as 5′ end capping, splicing, and 3′ end polyadenylation [77, 78]. A growing bulk of evidence is in support of the fact that lncRNAs are involved virtually in all physiological cellular processes, including the expression of gene, activation of enzyme, and epigenetic modulation [79]. Although lncRNAs do not encode proteins, it was revealed that lncRNAs modulate gene expression at transcription, post-transcription, and epigenetic levels mostly through histone modifications, chromatin remodeling, and DNA demethylation [80, 81]. LncRNAs can increase or decrease the levels of target genes via binding to them or the employment of transcription factors [82, 83]. With the tremendous advancements in genome-wide techniques, some lncRNAs with the capacity of neurogenesis in adults have been explored, which confirmed the important functions of lncRNAs in the nervous system [84, 85]. According to several researches, the expression of lncRNAs is altered in brain injury; moreover, they may be participated in neurological consequences arise following brain injury [14, 86,87,88,89,90,91,92,93,94].
TNF-α is considered as one of the major triggers of cytokine secretion and an inducer of cell adhesion molecule expression, hence leading to progression of inflammation. It can modulate some of important cellular signaling pathways like JNK/AP-1 and IKK/NF-κB, thereby moderating the expression of downstream genes [95]. Moreover, TNF-α was shown to be capable of stimulating apoptosis; nonetheless, the exact mechanism is yet to be understood. It has been hypothesized that TNF-α may act with death ligands, including Apo2L/TRAIL and FasL to eliminate cells, which are infected with the pathogens. Furthermore, the other hypothesized mechanism is activating Bcl-2 gene and caspase families via stimulating JNK cascade [96].
Yu et al. [97] explored that lncRNA Gm4419 stimulated the apoptosis of astrocytes via TNF-α overexpression in an in vitro model of TBI. The authors found overexpression of Gm4419 in astrocytes following TBI. Gm4419 also was shown to be able to overexpress TNF-α expression and astrocyte apoptosis following TBI via targeting miR-466 l. Gm4419 served as the sponge for miR-466 l that is able to prohibit expression of TNF. Hence, throughout TBI, Gm4419 is able to overexpress the expression of TNF-α via binding to miR-466 l, contributing to neuroinflammation, neurological dysfunction, and the apoptosis of astrocytes. Altogether, this research provided a better insight of the mechanism by which Gm4419 stimulated neuroinflammation and neurological impairments in TBI [97].
Additionally, myeloid differentiation factor 88 (MYD88) adaptor protein has been known as a major member of the Toll-like receptor (TLR) and was revealed that it is involved in progression of diseases [98]. There is an evidence in support of the fact that MYD88 is highly overexpressed in an in vivo model of TBI, and its downregulation is capable of ameliorating TBI via decreasing microglial activation [99]. Moreover, its downregulation also can prohibit the secretion of inflammatory factors [100, 101]. Recently, great amount of interests has been driven toward roles of ubiquitin–proteasome systems in different diseases [102]. It has been demonstrated that E3 ubiquitin ligase mediates disease progression via modulating the ubiquitination of proteins [103]. Of note, Nrdp1, which is one of the members of E3 ubiquitin ligase family was demonstrated to be able to decrease the expression of MYD88 through regulating MYD88 ubiquitination [93].
Cheng et al. [104] illustrated lncRNA HOTAIR contribution to activation of microglia and inflammation responses in TBI in vivo. Therefore, LPS stimulation and Feeney’s free-fall impact methods were recruited to establish an in vivo mouse model of TBI model and in vitro microglial activation, respectively. They also employed qRT-PCR in order to identify lncRNA HOTAIR expression in activated microglia. Then, they evaluated the microglial activation marker, namely Iba-1, and lncRNA HOTAIR expression levels utilizing qRT-PCR and the levels of inflammatory factors were measured utilizing western blot and ELISA. After that, RNA pull-down assay was employed by investigators to evaluate the association of lncRNA HOTAIR with MYD88 in vitro (Table 2). Additionally, researchers evaluated lncRNA HOTAIR effects on MYD88 stability by immunoprecipitation, ubiquitination, and cycloheximide (CHX) chase tests that determined MYD88 ubiquitination and detected overexpression of lncRNA HOTAIR in activated microglia. Its repression was associated with a significant decrease in microglial activation and secretion of inflammatory factors. It was shown that lncRNA HOTAIR was able to bind to MYD88 protein. Moreover, lncRNA HOTAIR upregulation was associated with increased MYD88 stability and prohibited Nrdp1-mediated ubiquitination of MYD88. Collectively, their findings revealed that lncRNA HOTAIR is upregulated in the activated microglia so that its repression was able to prohibit microglial activation and secretion of inflammatory factors through stimulating Nrdp1-mediated ubiquitination of MYD88 [104].
In addition, Nlrp3 inflammasome has been known as one of the major contributors to progression of inflammation and its presence is mandatory for the release of inflammatory cytokines, which contribute to inflammatory responses [105,106,107]. The activation of NLRP3 inflammasome, which is initiated when they detect misfolded or aggregated amyloid-β, prion protein, α-synuclein, or superoxide dismutase, was shown to be associated with enhanced cleavage and caspase-1 and IL-1β secretion activity [105, 106]. The mentioned process is not restricted to microglia and can be found in other brain cells like astrocytes and neurons [105]. A body of evidence revealed that NLRP3 inflammasome was participated in the process of neurodegenerative disease [105]. Additionally, inhibition of activation and production of NLRP3 inflammasome can be a potential treatment for TBI patients [107]. It has been shown that Nlrp3 serves as one of the miR-7 targets in the primary microglia. Emerging evidence has shown that lncRNAs serve as the competing endogenous RNAs (ceRNAs) to prohibit miRNA activities and regulate the expression of mRNA, and imbalance of ceRNA networks leads to different diseases and progression of the diseases [108].
Meng et al. [109] sought to explore the mechanism Meg3 regulates inflammatory response and microglial activation in the in vitro TBI model. Investigators demonstrated that lipopolysaccharide in combination with ATP overexpressed Meg3, stimulated the activation of microglia and Nlrp3/caspase-1, and notably decreased miR-7a-5p. MiR-7a-5p upregulation was associated with decreased microglial activation stimulated by Meg3; nevertheless, its upregulation did not affect the expression of Meg3. Bioinformatic assays revealed that Meg3 serves as the target of miR-7a-5p that is able to decrease miR-7a-5p expression. Moreover, their findings reveled that Meg3 served as the ceRNA for miR-7a-5p. It also promoted microglial inflammation via modulating the expression of Nlrp3. Collectively, the current study elucidated that Meg3 modulates microglial inflammation via affecting the miR-7a-5p/Nlrp3 signaling pathway [109].
According to the research, NOD-like receptors (NLRs) have been introduced as the intracellular proteins involved in several defensive processes categorized into two groups: regulatory and inflammasome-forming NLRs. The inflammasome-forming NLRs have been known as components that are capable of starting the multiprotein inflammasome complex formation. Inflammasome formation improves the activation and maturation of IL-18 and IL-1β. To date, a number of research have been conducted to assess the inflammasome-forming NLRs in TBI. For instance, a number of clinical and experimental research have demonstrated that NLRP1 as well as NLRP3 have modulatory functions in TBI [110,111,112]. It has been reported that NLRX1 provokes reactive oxygen species formation so as to augment JNK and NF-κB signaling pathways [113]. An in vivo research provided an evidence that NLRX1 inhibits TBI progression via modulating the NF-κB signaling pathway [114]. Recently, a study found out that NLRX1 downregulation is able to modulate neuronal tissue insults following TBI [115].
He et al. [116] made an attempt to assess the role of the lncRNA-transmitted nuclear factor κB-interacting lncRNA (NKILA)-containing astrocyte-derived extracellular vehicles (EVs) in TBI. Therefore, in vivo and in vitro models of TBI were established utilizing mechanical damage to human neurons and CCI, respectively. Researchers conducted gain-of-function and loss-of-function assays to identify NKILA, miR-195, as well as NLRX1 functions in damaged neurons in vitro. EVs were isolated from the NKILA-overexpressing astrocytes and applied for treating damaged neurons. They detected lower levels of NKILA expression in damaged neurons. Moreover, it was shown that NKILA can modulate NLRX1 expression via targeting miR-195. NKILA or NLRX1 upregulation was shown to be able to attenuate neuronal damages via apoptosis prohibition and stimulate neuronal proliferation. Astrocyte-derived EVs transferred NKILA into the neurons and contributed to NLRX1 overexpression, miR-195 downregulation, enhanced cell proliferation, and reduced cell apoptosis. They also revealed that NKILA-containing EVs exerted protective effects on brain injuries following TBI. In summary, astrocyte-derived EVs containing NKILA can ameliorate neural damages in TBI via binding to miR-195 and overexpressing NLRX1 [116].
As reported in a study, enhancer of zeste homolog 2 (EZH2) is recognized as one of the histone methyltransferases, which is able to decrease Krüppel-like factor 4 (KLF4) expression via targeting its promoter [117]. It was shown that KLF4 suppression has the potential to ameliorate neuronal injuries following TBI [118]. A report revealed that ischemic/reperfusion damage can lead to overexpression of EZH2 in microglia, whereas its prohibition modulates brain damage in an in vivo model of the brain injury induced by middle cerebral artery occlusion [119]. Additionally, EZH2 prohibition is able to attenuate neuroinflammation in rats with subarachnoid hemorrhage, emphasizing the importance of EZH2 prohibition in neural damages [120]. EZH2 was shown to be a core sub-unit of the polycomb repressive complex 2 (PRC2) that suppresses the gene transcription by its histone methyltransferase effects on lysine 27 (H3K27me3). Bulk of evidence exists in the support of the fact that metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) employs PRC2 in order to increase H3K27me3 activity, leading to gene transcription suppression, mostly in malignancy and sepsis [121,122,123,124]. Despite that, recent reports revealed that EZH2 also can exert inhibition of gene transcription independent of activation of H3K27me3 [125,126,127].
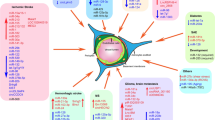
Wu et al. [128] designed an in vivo and in vitro experiment to evaluate the contribution of MALAT1 to TBI. CCI was pertained to established a mouse model of TBI, and thus, researchers utilized oxygen–glucose deprivation as an in vitro model of TBI. Experiment findings demonstrated that MALAT1 suppression was associated with prohibition of endothelial cell viability and tube formation, while its silencing was associated with enhanced migration. They also illustrated that MALAT1 increased endothelial cell proliferation, functional vessel density, and cerebral perfusion in the injured areas following TBI. Bioinformatic tests showed that EZH2 serves as a downstream effector of MALAT1 in endothelial cells. Additionally, angiogenesis activities of MALAT1 were reversed by the activation of Jagged 1, the Notch homolog 1 (NOTCH1). Altogether, their findings clarified that MALAT1 maintains a mandatory role in angiogenesis following TBI via regulating the EZH2/NOTCH1 signaling pathway [128]. Figure 2 displays the various functions of lncRNAs in the pathophysiological of TBI.
The mechanisms involved in the effects of lncRNAs on TBI. Noteworthy, researchers identified several lncRNAs to maintain various fundamental contributions to the pathophysiology of TBI. GAS5 knockdown is associated with ameliorated RGC oxidative stress injury through overexpressing miR-124. GAS5 is able to prohibit microglial M2 polarization via blocking TRF4 transcription. LincRNA-p21 can induce the activation of microglia via a p53-dependent transcriptional pathway. Additionally, lncRNA MALAT1 is involved in astrocyte apoptosis and neurodegeneration through alternative splicing of PKC δII and CREB signaling pathway. Moreover, there was a relationship between astrocyte apoptosis and the nuclear enriched abundant transcript 1 (NEAT1) and Gm4419 in which Gm4419 may act as a sponge for miR-466 l to target TNF-α. In addition, H19 has the capability to ameliorate hypoxia-induced damage via downregulation of miR-28 expression. Finally, SNHG1 can prevent hypoxia and the secondary cerebral ischemia through sponging miR-338. This figure was adapted from Li et al. [129]
Circular RNAs and Brain Trauma
Considering some studies in the field, circular RNAs (circRNAs) have been introduced as one of the novel groups of endogenous ncRNAs generated during splicing process [130, 131]. They were shown to be conserved and highly abundant in the brain [132, 133]. It has been shown that they participated in several biological processes such as translation and transcription regulation and served as miRNA sponges [10, 134,135,136,137]. Abnormal values of circRNAs have been observed in several diseases like heart diseases, neurodegenerative diseases, as well as malignancy [138, 139]. The expression of circRNAs was shown to be changed in those with TBI, indicating their potential for TBI management [140,141,142,143].
CXCR2, a G protein–coupled receptor, is shown to be involved in leukocyte recruitment from the circulation into locations of infection [144, 145]. Additionally, throughout neuroinflammation, CXCR2 participates in the activation of cerebral endothelial cells and, as a result, recruitment of leukocytes [146]. It also was shown to have a pivotal contribution to numerous inflammatory illnesses, including pancreatic inflammation [147], lung inflammation [148, 149], kidney inflammation [150], and skin inflammation [151].
Chen et al. [152] addressed whether circRNA expression near damage location in the mouse model of TBI is altered or not. A mouse model of TBI was established using a CCI model to cause brain trauma. The investigators extracted RNA from the cortex near the injury location so as to conduct RNA sequencing. In addition, 8036 circRNAs with differential expression have been detected of which 16 were significantly changed. Significantly enhanced levels of the circRNA chr8_87,859,283–87,904,548 were found in the cerebral cortex near the damage location following TBI. The mentioned circRNAs facilitated neuroinflammation via enhancing the expression of CXCR2 through the sponging mmu-let-7a-5p (Table 3). Therefore, these five circRNAs have detrimental impact on the neurological function improvement in mouse model of TBI [152].
Researchers found the generation of TNF-α and IL-1β at the site of inflammation primarily by monocytes and macrophages is capable of inducing neurite growth by glial cell line–derived neurotrophic factor [153]. Moreover, IL-1β was revealed to have dual opposite effects on neurons [154,155,156]. The findings of an in vitro study suggested that IL-1β exerts its neuroprotective effects through prohibiting N-type Ca2+ channel protein expression, leading to deactivation of N-type Ca2+ channels [157]. Intraparenchymal injection of IL-1β into the medial forebrain bundle largely reduced the number of dopaminergic neurons in striatum [158]. Conversely, IL-1β was shown to have the potential to promote the apoptosis of neurons via altering the expression activity of the p38 mitogen–activated protein kinase and caspase-3 in rats following spinal cord injury [159]. An in vitro investigation illustrated that IL-1β is important for promoting neural precursor cell apoptosis and cell cycle arrest [156]. Additionally, emerging evidence demonstrated that JAK2/STAT3 signaling pathway activation and IL-1β generation are dependent on the inhibitor of the cytokine signaling 1 (SOCS-1) downregulation and STAT3 phosphorylation, respectively [160, 161].
As stated in a study, oxygen–glucose deprivation (GOD)-activated microglia promote apoptosis by secreting several cytokines and several miRNAs may regulate this process. Wang et al. [162] designed a study to evaluate how circPTK2 and miR-29b expression can affect the OGD-activated microglia-induced neuronal apoptosis in vitro. The authors demonstrated enhanced IL-1β and TNF-α as well as lower levels of miR-29b in OGD-activated microglia. In addition, miR-29b prohibited OGD-activated microglia-induced neuronal apoptosis. Moreover, miR-29b could augment and reduce SOCS-1 expression and JAK2/STAT3 signaling, respectively. Furthermore, JAK2/STAT3 prohibition was associated with lower levels of IL-1β, whereas overexpression of miR-29b or SOCS-1 was associated with lower levels of IL-1β. In fact, IL-1β has been demonstrated to be involved in the hippocampal neuron apoptosis. Additionally, both SOCS-1 overexpression and prohibition of JAK2/STAT3 signaling pathway can inhibit OGD-activated microglia-induced neuronal apoptosis, indicating miR-29b prohibits OGD-activated microglia-induced neuronal apoptosis through stimulating SOCS-1 expression, prohibiting JNK2/STAT3 signaling pathway and IL-1β expression. They also showed that circPTK2 was capable of modulating microglia-induced neuronal apoptosis via serving as an inhibitor of miR-29b [162].
As stated in the research, DNA damage–regulated autophagy modulator protein 2 (DRAM2) encodes 6 transmembrane domains and a protein with 266 amino acids [163]. This protein has been identified as a lysosomal protein involved in autophagy activation [164]. HOTAIRM1 maintains a vital contribution to modulation of autophagy pathways via PML-RARA degradation. HOTAIRM1 serves as the miRNA sponge in the pathway containing miR-20a, miR-106b, and miR-125b-5p as well as the respective targets DRAM2, E2F1, and ULK1 [165]. The gene can act as an autophagy modulator via acting as a potential target of miR-125b-1 [166]. It was also revealed that human miR144* through targeting DRAM2 is able to prohibit immune responses to Mycobacterium tuberculosis [167]. Moreover, Bai et al. [168] observed miR-125b-5p, which is overexpressed in retinoblastoma, via suppressing DRAM2 which enhances retinoblastoma cell invasion and proliferation.
Treatment with dexmedetomidine (DEX) was associated with favorable outcomes in those with TBI, which was mediated by prohibiting NLRP3/caspase-1. Li et al. [169] designed an in vivo study to evaluate the role of circLrp1b in DEX-mediated TBI of brain and underlying mechanism in those with TBI. A rat TBI model was developed via CCI to induce brain trauma. Thereafter, rats were given intracerebroventricular injections of lentiviral vector and then intraperitoneal injection of DEX. Therefore, DEX treatment was shown to be able to improve TBI-induced autophagy, inflammation, and neurological deficits in vivo, associated with overexpression of Dram2 and circLrp1b and miR-27a-3p downregulation. It was demonstrated that these effects were augmented by combined treatment with circLrp1b, which were reversed by miR-27a-3p prohibition or Dram2 upregulation. CircLrp1b can enhance the expression of Dram2 through acting as a sponge for miR-27a-3p in order to stimulate TBI-induced autophagy that is attenuated by administration of DEX. Altogether, this research declared that DEX is able to prohibit TBI-induced autophagy and inflammatory responses via inhibiting the circLrp1b/miR-27a-3p/Dram2 signaling pathway [169].
Ferroptosis is a non-apoptotic type of regulated cell death which is highly dependent on iron and has drawn a quite amount of attention due to its role in TBI. Its role in TBI has been demonstrated; however, the exact mechanism is yet to be clear [170]. Besides, it is widely accepted that melatonin has a neuroprotective property [171]. Nevertheless, researchers did not clarify the antiferroptotic impacts of melatonin on TBI. Very recently, Wu et al. [172] dealt with the evaluation of ferroptosis expression following TBI, and melatonin administration can be used as a protective agent against endoplasmic reticulum (ER) stress and ferroptosis following TBI. The authors reached out that the levels of lipid peroxidation increased following TBI, whereas melatonin treatment led to the improvement of the TBI-induced neurological deficits and sleep disorders in TBI mice. Furthermore, melatonin treatment was able to decrease lipid peroxidation and iron accumulation, leading to ameliorated ER stress and ferroptosis. Moreover, authors found upregulation and downregulation of 905 and 921 circRNAs in brain tissues collected from TBI mice treated with melatonin. They found that melatonin treatment is associated with lower values of circPtpn14, which was shown to be positively associated with ferroptosis-related 5-lipoxygenase (5-LOX) expression. Furthermore, circPtpn14 upregulation eliminated prohibitory impacts of melatonin on ferroptosis. Altogether, the investigators suggested that melatonin exhibits its anti-ER stress and antiferroptotic impacts on TBI via ameliorating lipid peroxidation by the circPtpn14/miR-351-5p/5-LOX signaling pathway (Fig. 3) [172].
The mechanism behind antiferroptotic and ER stress properties of melatonin in TBI. Intracranial hematoma following TBI contributes to the entrance of free-iron ions to the cell via TfR1. As mentioned earlier, the intracellular divalent iron ions are capable of catalyzing the production of lipid peroxides by reaction of Fenton. Therefore, lipid peroxide accumulation results in ferroptosis and ER stress. Melatonin is able to pass the cell membranes and BBB easily. It can immediately chelate the free-iron ions to inhibit the lipid peroxidation. Moreover, melatonin can indirectly decrease the expression of 5-LOX via suppressing circPtpn14 expression, leading to accumulation of reducing lipid peroxide and prohibition of ferroptosis and ER stress. This figure was adapted from Wu et al. [172]
Exosomal miRNAs and Brain Trauma
Exosomes are small EVs that can be released by lots of various types of cells and are categorized into three major groups based on their size. They can communicate with the remaining cells on the surface of the cell through cell receptors or release their contents including protein, lipids, as well as nucleic acids into the cell cytoplasm through endocytosis. Since exosomes release protein, lipids, and nucleic acids into recipient cells, they essentially contribute to many physiological and pathophysiological cellular processes [173, 174]. However, there has been growing interest toward the roles of exosomes miRNA in TBI [175]. Figure 4 exhibits the neuroprotective and neuroinflammatory impacts of EVs on TBI [176].
Rab GTPases, which are encoded by a group of more than 60 human genes, are known as important regulators of intracellular trafficking processes [177]. Rab11 is a member of Rab GTPase family. It was shown that Rab11 participated in the early as well as late phase of autophagy and has a fundamental role in autophagosome maturation [178,179,180]. Rab11 was shown to participate in interactions of multivesicular bodies with autophagic pathways in K652 cells [181]. Recycling endosomes (REs) serve as a provider of membrane for autophagosome biogenesis, and Rab11 may be involved in transporting vesicles to the location of autophagosome formation [182, 183]. A research revealed that Rab11 probably simplify binding the autophagosomes and endosomes via eliminating hook from mature late endosomes [184]. Recent evidence claimed that Rab11a, which is a subgroup of Rab11, maintains a mandatory role in autophagy. In 2018, a study demonstrated that Rab11a-positive membrane is an essential component for autophagosomes to evolve and its loss disrupts the assembly and recruitment of autophagic machinery. Figure 4 was adapted from Puri et al. [185].
Li et al. [186] designed an experimental research to evaluate the potential and underlying mechanism of exosomal miR-21-5p treatment in TBI. Brain extracts were isolated from a repetitive TBI (rTBI) mouse model and were applied to cultured HT-22 neurons to mimic the microenvironment of TBI in vitro. Exosomes were purified using ultracentrifugation, and their characteristics were determined, utilizing transmission electron microscopy and NanoSight technology. The authors developed an in vitro model of TBI to evaluate the impacts of the miR-21-5p administration on neuron autophagy. Furthermore, miR-21-5p expression is enhanced in exosomal neurons following rTBI mouse brain extract treatment. Higher levels of autophagy were detected in an in vitro model of TBI (Table 4). Treatment with exosomal miR-21-5p had the potential to prohibit autophagy via enhancing the miR-21-5p level in damaged neurons. MiR-21-5p by targeting the translational site of Rab11a suppressed Rab11a expression, thereby inhibiting Rab11a-induced autophagy. Altogether, higher values of miR-21-5p can be detected in chronic TBI compared to acute TBI. Exosomal miR-21-5p exerts its protective effects via prohibiting autophagy by targeting Rab11a in rats with TBI [186].
According to the studies, brain-derived neurotrophic factor (BDNF) has been known as one of the neurotrophic factors that maintain a mandatory contribution to neuron survival, axonal sprouting, and regeneration in response to TBI [187]. As a result, several experimental and clinical researches assessed BDNF impacts on TBI recovery [188, 189]. BDNF was introduced as the most broadly distributed neurotrophin in the CNS, participating in plenty of facets of brain function, including neuron survival, differentiation, and synaptic plasticity [187, 190, 191]. It is able to stimulate the neuron growth and decrease neuron death, provoking differentiation of the neural stem cells and MSCs into the neurons [192,193,194]. It exerts its impacts via tyrosine kinase receptor B (TrkB) [195]. It has been shown that TBI and spinal cord injury lead to upregulation of TrkB mRNA at TBI as well as spinal cord injury [196, 197].
It was unveiled that injecting exosomes extracted from the mesenchymal stromal cells (MSCs-Exo) is associated with better neurological function in those with TBI. Xu et al. [198] made a hypothesis that treatment with BDNF-stimulated MSCs-Exo may improve neurological function and neuron regeneration in a rat model of TBI. Then, researchers isolated MSCs from the bone marrow of rats and added them to BDNF, the supernatant was gathered, and exosomes were isolated and purified by hypercentrifugation. The properties of exosomes were determined using western blot, transmission electron microscopy, and particle size analysis. The authors demonstrated that a BDNF-stimulated MSCs-Exo group was significantly better than a MSCs-Exo group in terms of spatial learning capability and regaining of sensorimotor function. Treatment with BDNF-stimulated MSCs-Exo prohibited the inflammation and provoked neurogenesis in rats and in vitro. The investigators also found out that miR-216a-5p was expressed in significantly higher levels in the BDNF-stimulated MSCs-Exo group relative to the MSCs-Exo group, and the function of miR-216a-5p is alike to BDNF-stimulated MSCs-Exo. Briefly, treatment with BDNF-stimulated MSCs-Exo has the potential to improve neural regeneration and inhibit neural apoptosis, which is thought to be owing to miR-216a-5p expression [198].
Conclusion
The incidence of TBI, known as a deleterious condition, is increasing globally. The mechanisms involved in secondary brain damage are profoundly complex, and the mechanisms involved in TBI are not yet fully clear. NcRNAs are known as a group of diverse RNA, which are classified into 2 main groups including regulatory and housekeeping ncRNAs. It has been shown that they are expressed in the brain at high levels and may be pivotal for some of brain functions and neurodevelopment. Lots of experimental and clinical research has demonstrated that expressing different ncRNAs was modified after TBI. Additionally, it has been unveiled that they may be involved in the pathophysiology of TBI and its related complications. Moreover, several studies have evaluated the diagnostic and prognostic significances of various ncRNAs and found that they could be utilized as one of the biomarkers for TBI discrimination and diagnosis in the future, given that it is not possible to completely clarify the function of non-coding transcripts out of a physiological context, especially because they are poorly conserved between species, making the in vivo experiments not easily translatable for applications in humans, and because, if compared to coding genes, they are more difficult to be explored. A lot of novel ncRNAs are completely uncharacterized by making the understanding of their role more complex. Besides, since they have a crucial role in TBI development and pathophysiology, they may be considered as a possible target for TBI treatment.
Data Availability
The primary data for this study is available from the authors on request.
References
Timofeev I, Santarius T, Kolias AG, Hutchinson PJ (2012) Decompressive craniectomy - operative technique and perioperative care. Adv Tech Stand Neurosurg 38:115–136
Faul M, Wald MM, Xu L, Coronado VG (2010) Traumatic brain injury in the United States. Emergency department visits, hospitalizations, and deaths 2002–2006
Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A et al (2017) Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. The Lancet Neurology 16(12):987–1048
National Institute for Health and Care Excellence (NICE) (2017) Head injury: assessment and early management. Clinical guideline [CG176]. London, NICE
Huber A, Dorn A, Witzmann A, Cervós-Navarro J (1993) Microthrombi formation after severe head trauma. Int J Legal Med 106(3):152–155
Mahjoubin-Tehran M, Rezaei S, Jesmani A, Birang N, Morshedi K, Khanbabaei H et al (2021) New epigenetic players in stroke pathogenesis: from non-coding RNAs to exosomal non-coding RNAs. Biomed Pharmacother 140:111753
ENCODE Project Consortium, Stamatoyannopoulos JA, Dutta A, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z et al (2007) Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447:799–816
Wade JT, Grainger DC (2014) Pervasive transcription: illuminating the dark matter of bacterial transcriptomes. Nat Rev Microbiol 12(9):647–653
Kitagawa M, Kitagawa K, Kotake Y, Niida H, Ohhata T (2013) Cell cycle regulation by long non-coding RNAs. Cell Mol Life Sci 70(24):4785–4794
Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A et al (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495(7441):333–338
Wang WX, Huang Q, Hu Y, Stromberg AJ, Nelson PT (2011) Patterns of microRNA expression in normal and early Alzheimer’s disease human temporal cortex: white matter versus gray matter. Acta Neuropathol 121(2):193–205
Thome AD, Harms AS (2016) microRNA-155 regulates alpha-synuclein-induced inflammatory responses in models of Parkinson disease. 36(8):2383–90
Nelson PT, Wang WX (2010) MiR-107 is reduced in Alzheimer’s disease brain neocortex: validation study. J Alzheimer Dis: JAD 21(1):75–79
Dharap A, Nakka VP, Vemuganti R (2012) Effect of focal ischemia on long noncoding RNAs. Stroke 43(10):2800–2802
Dharap A, Nakka VP, Vemuganti R (2011) Altered expression of PIWI RNA in the rat brain after transient focal ischemia. Stroke 42(4):1105–1109
Dharap A, Vemuganti R (2010) Ischemic pre-conditioning alters cerebral microRNAs that are upstream to neuroprotective signaling pathways. J Neurochem 113(6):1685–1691
Dharap A, Bowen K, Place R, Li LC, Vemuganti R (2009) Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab 29(4):675–687
Pan Y-B, Sun Z-L, Feng D-F (2017) The role of microRNA in traumatic brain injury. Neuroscience 367:189–199
Barry G (2014) Integrating the roles of long and small non-coding RNA in brain function and disease. Mol Psychiatry 19(4):410–416
Redell JB, Moore AN, Ward NH III, Hergenroeder GW, Dash PK (2010) Human traumatic brain injury alters plasma microRNA levels. J Neurotrauma 27(12):2147–2156
Miao W, Bao T, Han J, Yin M, Yan Y, Wang W et al (2015) Voluntary exercise prior to traumatic brain injury alters miRNA expression in the injured mouse cerebral cortex. Braz J Med Biol Res 48(5):433–439
Quinlan S, Kenny A, Medina M, Engel T, Jimenez-Mateos EM (2017) MicroRNAs in neurodegenerative diseases. Int Rev Cell Mol Biol 334:309–343
Danborg PB, Simonsen AH, Waldemar G, Heegaard NH (2014) The potential of microRNAs as biofluid markers of neurodegenerative diseases–a systematic review. Biomarkers 19(4):259–268
Pereira P, Queiroz JA, Figueiras A, Sousa F (2017) Current progress on microRNAs-based therapeutics in neurodegenerative diseases. Wiley Interdisciplinary Reviews: RNA 8(3):e1409
Theus MH, Brickler T, Meza AL, Coutermarsh-Ott S, Hazy A, Gris D et al (2017) Loss of NLRX1 exacerbates neural tissue damage and NF-κB signaling following brain injury. J Immunol 199(10):3547–3558
Tao L, Li D, Liu H, Jiang F, Xu Y, Cao Y et al (2018) Neuroprotective effects of metformin on traumatic brain injury in rats associated with NF-κB and MAPK signaling pathway. Brain Res Bull 140:154–161
Ferreira APO, Rodrigues FS, Della-Pace ID, Mota BC, Oliveira SM, Gewehr CdCV et al (2013) The effect of NADPH-oxidase inhibitor apocynin on cognitive impairment induced by moderate lateral fluid percussion injury: role of inflammatory and oxidative brain damage. Neurochem Int 63(6):583–93
Bhowmick S, D’Mello V, Caruso D, Abdul-Muneer P (2019) Traumatic brain injury-induced downregulation of Nrf2 activates inflammatory response and apoptotic cell death. J Mol Med 97(12):1627–1641
Zhang L, Zhao L, Zhu W, Ding Y, Chen H, Chi N (2020) miR-146a mimics ameliorates traumatic brain injury involving JNK and NF-κB signaling pathway. NeuroMol Med 22(4):484–492
Hsueh YP, Yang FC, Kharazia V, Naisbitt S, Cohen AR, Weinberg RJ et al (1998) Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J Cell Biol 142(1):139–151
Hata Y, Butz S, Südhof TC (1996) CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci: Off J Soc Neurosci 16(8):2488–2494
Hsueh YP, Sheng M (1999) Regulated expression and subcellular localization of syndecan heparan sulfate proteoglycans and the syndecan-binding protein CASK/LIN-2 during rat brain development. J Neurosci: Off J Soc Neurosci 19(17):7415–7425
Carasatorre M, Ramírez-Amaya V (2013) Network, cellular, and molecular mechanisms underlying long-term memory formation. Curr Top Behav Neurosci 15:73–115
Nisticò R, Pignatelli M, Piccinin S, Mercuri NB, Collingridge G (2012) Targeting synaptic dysfunction in Alzheimer’s disease therapy. Mol Neurobiol 46(3):572–587
Doxakis E (2010) Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J Biol Chem 285(17):12726–12734
Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL et al (2002) Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci: Off J Soc Neurosci 22(20):8797–8807
Clayton DF, George JM (1999) Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res 58(1):120–129
Hsu LJ, Mallory M, Xia Y, Veinbergs I, Hashimoto M, Yoshimoto M et al (1998) Expression pattern of synucleins (non-Abeta component of Alzheimer’s disease amyloid precursor protein/alpha-synuclein) during murine brain development. J Neurochem 71(1):338–344
Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S et al (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18(2):106–108
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A et al (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science (New York, NY) 276(5321):2045–2047
Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH (2005) Neural activity controls the synaptic accumulation of alpha-synuclein. J Neurosci: Off J Soc Neurosci 25(47):10913–10921
Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK et al (2010) Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65(1):66–79
Su E, Bell MJ, Wisniewski SR, Adelson PD, Janesko-Feldman KL, Salonia R et al (2010) α-Synuclein levels are elevated in cerebrospinal fluid following traumatic brain injury in infants and children: the effect of therapeutic hypothermia. Dev Neurosci 32(5–6):385–395
Mondello S, Buki A, Italiano D, Jeromin A (2013) α-Synuclein in CSF of patients with severe traumatic brain injury. Neurology 80(18):1662–1668
Uryu K, Giasson B, Longhi L, Martinez D, Murray I, Conte V et al (2003) Age-dependent synuclein pathology following traumatic brain injury in mice. Exp Neurol 184(1):214–224
Liu L, Sun T, Liu Z, Chen X, Zhao L, Qu G et al (2014) Traumatic brain injury dysregulates microRNAs to modulate cell signaling in rat hippocampus. PLoS ONE 9(8):e103948
Wang J, Zhou J-Y, Kho D, Reiners JJ, Wu GS (2016) Role for DUSP1 (dual-specificity protein phosphatase 1) in the regulation of autophagy. Autophagy 12(10):1791–1803
Shah S, King EM, Chandrasekhar A, Newton R (2014) Roles for the mitogen-activated protein kinase (MAPK) phosphatase, DUSP1, in feedback control of inflammatory gene expression and repression by dexamethasone*. J Biol Chem 289(19):13667–13679
Shen J, Zhang Y, Yu H, Shen B, Liang Y, Jin R et al (2016) Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy. Cancer Med 5(8):2061–2068
Lin X, Wang M, Zhang J, Xu R (2014) p38 MAPK: a potential target of chronic pain. Curr Med Chem 21(38):4405–4418
Clark AR, Dean JL (2016) The control of inflammation via the phosphorylation and dephosphorylation of tristetraprolin: a tale of two phosphatases. Biochem Soc Trans 44(5):1321–1337
Lopes LJS, Tesser-Gamba F, Petrilli AS, de Seixas Alves MT, Garcia-Filho RJ, Toledo SRC (2017) MAPK pathways regulation by DUSP1 in the development of osteosarcoma: potential markers and therapeutic targets. Mol Carcinog 56(6):1630–1641
Qi R, Wang X (2020) Inhibition of miR-429 improves neurological recovery of traumatic brain injury mice and attenuates microglial neuroinflammation. Int Immunopharmacol 79:106091
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9(1):47–59
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM et al (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397(6718):441–446
Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86(1):147–157
Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J et al (2003) Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4(4):321–328
Engel T, Plesnila N, Prehn JH, Henshall DC (2011) In vivo contributions of BH3-only proteins to neuronal death following seizures, ischemia, and traumatic brain injury. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab 31(5):1196–1210
Yakovlev AG, Di Giovanni S, Wang G, Liu W, Stoica B, Faden AI (2004) BOK and NOXA are essential mediators of p53-dependent apoptosis. J Biol Chem 279(27):28367–28374
Zareen N, Biswas SC, Greene LA (2013) A feed-forward loop involving Trib3, Akt and FoxO mediates death of NGF-deprived neurons. Cell Death Differ 20(12):1719–1730
Akhter R, Sanphui P, Biswas SC (2014) The essential role of p53-up-regulated modulator of apoptosis (Puma) and its regulation by FoxO3a transcription factor in β-amyloid-induced neuron death. J Biol Chem 289(15):10812–10822
Ambacher KK, Pitzul KB, Karajgikar M, Hamilton A, Ferguson SS, Cregan SP (2012) The JNK- and AKT/GSK3β- signaling pathways converge to regulate Puma induction and neuronal apoptosis induced by trophic factor deprivation. PLoS ONE 7(10):e46885
Gilley J, Coffer PJ, Ham J (2003) FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol 162(4):613–622
You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M et al (2006) FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med 203(7):1657–1663
Sabirzhanov B, Stoica BA, Zhao Z, Loane DJ, Wu J, Dorsey SG et al (2016) miR-711 upregulation induces neuronal cell death after traumatic brain injury. Cell Death Differ 23(4):654–668
Yan J, Bu X, Li Z, Wu J, Wang C, Li D et al (2019) Screening the expression of several miRNAs from TaqMan low density array in traumatic brain injury: miR‐219a‐5p regulates neuronal apoptosis by modulating CCNA2 and CACUL1 150(2):202–217
Pang X, Gong K, Zhang X, Wu S, Cui Y, Qian BZ (2019) Osteopontin as a multifaceted driver of bone metastasis and drug resistance. Pharmacol Res 144:235–244
Chen G, Zhang X, Li R, Fang L, Niu X, Zheng Y et al (2010) Role of osteopontin in synovial Th17 differentiation in rheumatoid arthritis. Arthritis Rheum 62(10):2900–2908
Gao SG, Li KH, Zeng KB, Tu M, Xu M, Lei GH (2010) Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthr Cartil 18(1):82–87
Gattorno M, Gregorio A, Ferlito F, Gerloni V, Parafioriti A, Felici E et al (2004) Synovial expression of osteopontin correlates with angiogenesis in juvenile idiopathic arthritis. Rheumatology (Oxford) 43(9):1091–1096
Jiang K, Teng GD, Chen YQ (2020) MicroRNA-23 suppresses osteogenic differentiation of human bone marrow mesenchymal stem cells by targeting the MEF2C-mediated MAPK signaling pathway. J Gene Med 22(10):e3216
Tao L, Bei Y, Chen P, Lei Z, Fu S, Zhang H et al (2016) Crucial role of miR-433 in regulating cardiac fibrosis. Theranostics 6(12):2068–2083
Zhang T, Jiang K, Zhu X, Zhao G, Wu H, Deng G et al (2018) miR-433 inhibits breast cancer cell growth via the MAPK signaling pathway by targeting Rap1a. Int J Biol Sci 14(6):622–632
Shi Q, Wang Y, Mu Y, Wang X, Fan Q (2018) MiR-433-3p inhibits proliferation and invasion of esophageal squamous cell carcinoma by targeting GRB2. Cell Physiol Biochem: Int J Exp Cell Physiol, Biochem, Pharmacol 46(5):2187–2196
Sun S, Wang X, Xu X, Di H, Du J, Xu B et al (2017) MiR-433-3p suppresses cell growth and enhances chemosensitivity by targeting CREB in human glioma. Oncotarget 8(3):5057–5068
Han Z, Shi F, Chen Y, Dong X, Zhang B, Li M (2021) Relationship between miRNA-433 and SPP1 in the presence of fracture and traumatic brain injury. Exp Ther Med 22(3):928
Jaé N, Heumüller AW, Fouani Y, Dimmeler S (2019) Long non-coding RNAs in vascular biology and disease. Vascul Pharmacol 114:13–22
Wilusz JE (2016) Long noncoding RNAs: re-writing dogmas of RNA processing and stability. Biochim et Biophys Acta (BBA)-Gene Regul Mech 1859(1):128–38
Yao D, Zhan X, Zhan X, Kwoh CK, Li P, Wang J (2020) A random forest based computational model for predicting novel lncRNA-disease associations. BMC Bioinformatics 21(1):1–18
Batista PJ, Chang HY (2013) Long noncoding RNAs: cellular address codes in development and disease. Cell 152(6):1298–1307
Dahariya S, Paddibhatla I, Kumar S, Raghuwanshi S, Pallepati A, Gutti RK (2019) Long non-coding RNA: classification, biogenesis and functions in blood cells. Mol Immunol 112:82–92
Zhang L, Wang H (2019) Long non-coding RNA in CNS injuries: a new target for therapeutic intervention. Mol Ther-Nucleic Acids 17:754–766
Li J, Kuang Y, Chen L, Wang J (2018) LncRNA ZNF667-AS1 inhibits inflammatory response and promotes recovery of spinal cord injury via suppressing JAK-STAT pathway. Eur Rev Med Pharmacol Sci 22(22):7614–7620
Hong SH, Kwon JT, Kim J, Jeong J, Kim J, Lee S et al (2018) Profiling of testis-specific long noncoding RNAs in mice. BMC Genomics 19(1):1–12
Ramos AD, Diaz A, Nellore A, Delgado RN, Park K-Y, Gonzales-Roybal G et al (2013) Integration of genome-wide approaches identifies lncRNAs of adult neural stem cells and their progeny in vivo. Cell Stem Cell 12(5):616–628
Das S, Shah R, Dimmeler S, Freedman JE, Holley C, Lee JM et al (2020) Noncoding RNAs in cardiovascular disease: current knowledge, tools and technologies for investigation, and future directions: a scientific statement from the American Heart Association. Circ Genomic Precis Med 13(4):e000062
Qureshi IA, Mehler MF (2012) Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat Rev Neurosci 13(8):528–541
Riva P, Ratti A, Venturin M (2016) The long non-coding RNAs in neurodegenerative diseases: novel mechanisms of pathogenesis. Curr Alzheimer Res 13(11):1219–1231
Schmitt AM, Chang HY (2016) Long noncoding RNAs in cancer pathways. Cancer Cell 29(4):452–463
Yin K-J, Hamblin M, Chen YE (2014) Non-coding RNAs in cerebral endothelial pathophysiology: emerging roles in stroke. Neurochem Int 77:9–16
Zhang X, Hamblin MH, Yin K-J (2017) The long noncoding RNA Malat1: its physiological and pathophysiological functions. RNA Biol 14(12):1705–1714
Zhang X, Hamblin MH, Yin KJ (2019) Noncoding RNAs and stroke. 25(1):22-6
Wang C, Chen T, Zhang J, Yang M, Li N, Xu X et al (2009) The E3 ubiquitin ligase Nrdp1‘preferentially’ promotes TLR-mediated production of type I interferon. Nat Immunol 10(7):744–752
Zhang X, Tang X, Liu K, Hamblin MH, Yin KJ (2017) Long noncoding RNA Malat1 regulates cerebrovascular pathologies in ischemic stroke. 37(7):1797–806
Wajant H, Pfizenmaier K, Scheurich P (2003) Tumor necrosis factor signaling. Cell Death Differ 10(1):45–65
Varfolomeev EE, Ashkenazi A (2004) Tumor necrosis factor: an apoptosis JuNKie? Cell 116(4):491–497
Yu Y, Cao F, Ran Q, Wang F (2017) Long non-coding RNA Gm4419 promotes trauma-induced astrocyte apoptosis by targeting tumor necrosis factor α. Biochem Biophys Res Commun 491(2):478–485
Leite FRM, de Aquino SG, Guimarães MR, Cirelli JA, Zamboni DS, Silva JS et al (2015) Relevance of the myeloid differentiation factor 88 (MyD88) on RANKL, OPG, and nod expressions induced by TLR and IL-1R signaling in bone marrow stromal cells. Inflammation 38(1):1–8
Zhu H-t, Bian C, Yuan J-c, Chu W-h, Xiang X, Chen F et al (2014) Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway in experimental traumatic brain injury. J Neuroinflammation 11(1):1–17
Kim SY, Jin CY, Kim CH, Yoo YH, Choi SH, Kim GY et al (2019) Isorhamnetin alleviates lipopolysaccharide-induced inflammatory responses in BV2 microglia by inactivating NF-κB, blocking the TLR4 pathway and reducing ROS generation. Int J Mol Med 43(2):682–692
Ding F, Li Y, Hou X, Zhang R, Hu S, Wang Y (2016) Oxymatrine inhibits microglia activation via HSP60-TLR4 signaling. Biomedical Reports 5(5):623–628
Pallante P, Malapelle U, Berlingieri MT, Bellevicine C, Sepe R, Federico A et al (2013) UbcH10 overexpression in human lung carcinomas and its correlation with EGFR and p53 mutational status. Eur J Cancer 49(5):1117–1126
Zheng N, Shabek N (2017) Ubiquitin ligases: structure, function, and regulation. Annu Rev Biochem 86:129–157
Cheng S, Zhang Y, Chen S, Zhou Y (2021) LncRNA HOTAIR participates in microglia activation and inflammatory factor release by regulating the ubiquitination of MYD88 in traumatic brain injury. 71(1):169–77
Heneka MT, McManus RM, Latz E (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19(10):610–621
Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A et al (2019) NLRP3 inflammasome activation drives tau pathology. Nature 575(7784):669–673
Kuwar R, Rolfe A, Di L, Xu H, He L, Jiang Y et al (2019) A novel small molecular NLRP3 inflammasome inhibitor alleviates neuroinflammatory response following traumatic brain injury 16(1):81
Sen R, Ghosal S, Das S, Balti S, Chakrabarti J (2014) Competing endogenous RNA: the key to posttranscriptional regulation. Sci World J 2014
Meng J, Ding T, Chen Y, Long T, Xu Q, Lian W et al (2021) LncRNA-Meg3 promotes Nlrp3-mediated microglial inflammation by targeting miR-7a-5p. Int Immunopharmacol 90:107141
Brickler T, Gresham K, Meza A, Coutermarsh-Ott S, Williams TM, Rothschild DE et al (2016) Nonessential role for the NLRP1 inflammasome complex in a murine model of traumatic brain injury. Mediators Inflamm 2016:6373506
Ma J, Xiao W, Wang J, Wu J, Ren J, Hou J et al (2016) Propofol inhibits NLRP3 inflammasome and attenuates Blast-induced traumatic brain injury in rats. Inflammation 39(6):2094–2103
Liu HD, Li W, Chen ZR, Hu YC, Zhang DD, Shen W et al (2013) Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res 38(10):2072–2083
Tattoli I, Carneiro LA, Jéhanno M, Magalhaes JG, Shu Y, Philpott DJ et al (2008) NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species production. EMBO Rep 9(3):293–300
Allen IC, Meza A, Brickler T, Coutermarsh-Ott S, Ives A, Bertke A, et al (2016) NLRX1 attenuates damage following traumatic brain injury through negatively regulating NF-κB signaling. Am Assoc Immnol
Theus MH, Brickler T (2017) Loss of NLRX1 exacerbates neural tissue damage and NF-κB signaling following brain injury. 199(10):3547–58
He B, Chen W, Zeng J, Tong W, Zheng P (2021) Long noncoding RNA NKILA transferred by astrocyte-derived extracellular vesicles protects against neuronal injury by upregulating NLRX1 through binding to mir-195 in traumatic brain injury. Aging 13(6):8127–8145
Shao H, Dong D, Shao F (2019) Long non-coding RNA TUG1-mediated down-regulation of KLF4 contributes to metastasis and the epithelial-to-mesenchymal transition of colorectal cancer by miR-153-1. Cancer Manag Res 11:8699–8710
Cui DM, Zeng T, Ren J, Wang K, Jin Y, Zhou L et al (2017) KLF4 knockdown attenuates TBI-induced neuronal damage through p53 and JAK-STAT3 signaling. CNS Neurosci Ther 23(2):106–118
Chen J, Zhang M, Zhang X, Fan L, Liu P, Yu L et al (2019) EZH2 inhibitor DZNep modulates microglial activation and protects against ischaemic brain injury after experimental stroke. Eur J Pharmacol 857:172452
Luo Y, Fang Y, Kang R, Lenahan C, Gamdzyk M, Zhang Z et al (2020) Inhibition of EZH2 (enhancer of zeste homolog 2) attenuates neuroinflammation via H3k27me3/SOCS3/TRAF6/NF-κB (trimethylation of histone 3 lysine 27/suppressor of cytokine signaling 3/tumor necrosis factor receptor family 6/nuclear factor-κB) in a rat model of subarachnoid hemorrhage. Stroke 51(11):3320–3331
Wang D, Ding L, Wang L, Zhao Y, Sun Z, Karnes RJ et al (2015) LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget 6(38):41045
Huo Y, Li Q, Wang X, Jiao X, Zheng J, Li Z et al (2017) MALAT1 predicts poor survival in osteosarcoma patients and promotes cell metastasis through associating with EZH2. Oncotarget 8(29):46993
Lin N, Yao Z, Xu M, Chen J, Lu Y, Yuan L et al (2019) Long noncoding RNA MALAT1 potentiates growth and inhibits senescence by antagonizing ABI3BP in gallbladder cancer cells. J Exp Clin Cancer Res 38(1):1–15
Yong H, Wu G, Chen J, Liu X, Bai Y, Tang N et al (2020) lncRNA MALAT1 accelerates skeletal muscle cell apoptosis and inflammatory response in sepsis by decreasing BRCA1 expression by recruiting EZH2. Mol Ther-Nucleic Acids 19:97–108
Lee ST, Li Z, Wu Z, Aau M, Guan P, Karuturi RM et al (2011) Context-specific regulation of NF-κB target gene expression by EZH2 in breast cancers. Mol Cell 43(5):798–810
Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT et al (2012) EZH2 oncogenic activity in castration-resistant prostate cancer cells is polycomb-independent. Science (New York, NY) 338(6113):1465–1469
Asangani IA, Ateeq B, Cao Q, Dodson L, Pandhi M, Kunju LP et al (2013) Characterization of the EZH2-MMSET histone methyltransferase regulatory axis in cancer. Mol Cell 49(1):80–93
Wu N, Cheng CJ, Zhong JJ, He JC, Zhang ZS, Wang ZG et al (2022) Essential role of MALAT1 in reducing traumatic brain injury. Neural Regen Res 17(8):1776–1784
Li Z, Han K, Zhang D, Chen J, Xu Z, Hou L (2019) The role of long noncoding RNA in traumatic brain injury. Neuropsychiatr Dis Treat 15:1671–1677
Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M et al (2014) circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 56(1):55–66
Qu S, Yang X, Li X, Wang J, Gao Y, Shang R et al (2015) Circular RNA: a new star of noncoding RNAs. Cancer Lett 365(2):141–148
Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S et al (2015) Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell 58(5):870–885
Lasda E, Parker R (2014) Circular RNAs: diversity of form and function. RNA 20(12):1829–1842
Li X, Yang L, Chen L-L (2018) The biogenesis, functions, and challenges of circular RNAs. Mol Cell 71(3):428–442
Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B et al (2016) Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun 7(1):1–13
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK et al (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495(7441):384–388
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P et al (2017) CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer 16(1):1–8
Hansen TB, Kjems J, Damgaard CK (2013) Circular RNA and miR-7 in cancer. Can Res 73(18):5609–5612
Mehta SL, Pandi G, Vemuganti R (2017) Circular RNA expression profiles alter significantly in mouse brain after transient focal ischemia. Stroke 48(9):2541–2548
Jiang YJ, Cao SQ, Gao LB, Wang YY, Zhou B, Hu X et al (2019) Circular ribonucleic acid expression profile in mouse cortex after traumatic brain injury. J Neurotrauma 36(7):1018–1028
Zhao RT, Zhou J, Dong XL, Bi CW, Jiang RC, Dong JF et al (2018) Circular ribonucleic acid expression alteration in exosomes from the brain extracellular space after traumatic brain injury in mice. J Neurotrauma 35(17):2056–2066
Xie BS, Wang YQ, Lin Y, Zhao CC, Mao Q, Feng JF et al (2018) Circular RNA expression profiles alter significantly after traumatic brain injury in rats. J Neurotrauma 35(14):1659–1666
Qu X, Li Z, Chen J, Hou L (2020) The emerging roles of circular RNAs in CNS injuries. 98(7):1485-97
Marchelletta RR, Gareau MG, Okamoto S, Guiney DG, Barrett KE, Fierer J (2015) Salmonella-induced diarrhea occurs in the absence of IL-8 receptor (CXCR2)-dependent neutrophilic inflammation. J Infect Dis 212(1):128–136
Bajrami B, Zhu H, Kwak H-J, Mondal S, Hou Q, Geng G et al (2016) G-CSF maintains controlled neutrophil mobilization during acute inflammation by negatively regulating CXCR2 signaling. J Exp Med 213(10):1999–2018
Wu F, Zhao Y, Jiao T, Shi D, Zhu X, Zhang M et al (2015) CXCR2 is essential for cerebral endothelial activation and leukocyte recruitment during neuroinflammation. J Neuroinflammation 12(1):1–15
Steele CW, Karim SA, Foth M, Rishi L, Leach JD, Porter RJ et al (2015) CXCR2 inhibition suppresses acute and chronic pancreatic inflammation. J Pathol 237(1):85–97
Tiwari N, Marudamuthu AS, Tsukasaki Y, Ikebe M, Fu J, Shetty S (2016) p53-and PAI-1-mediated induction of CXC chemokines and CXCR2: importance in pulmonary inflammation due to cigarette smoke exposure. Am J Physiol-Lung Cell Mol Physiol 310(6):L496–L506
Lerner CA, Lei W, Sundar IK, Rahman I (2016) Genetic ablation of CXCR2 protects against cigarette smoke-induced lung inflammation and injury. Front Pharmacol 7:391
Ranganathan P, Jayakumar C, Manicassamy S, Ramesh G (2013) CXCR2 knockout mice are protected against DSS-colitis-induced acute kidney injury and inflammation. Am J Physiol-Renal Physiol 305(10):F1422–F1427
Hsieh C-Y, Chen C-L, Lin Y-S, Yeh T-M, Tsai T-T, Hong M-Y et al (2014) Macrophage migration inhibitory factor triggers chemotaxis of CD74+CXCR2+ NKT cells in chemically induced IFN-γ–mediated skin inflammation. J Immunol 193(7):3693–3703
Chen Z, Wang H, Zhong J, Yang J, Darwazeh R, Tian X et al (2019) Significant changes in circular RNA in the mouse cerebral cortex around an injury site after traumatic brain injury. Exp Neurol 313:37–48
Gougeon P-Y, Lourenssen S, Han TY, Nair DG, Ropeleski MJ, Blennerhassett MG (2013) The pro-inflammatory cytokines IL-1β and TNFα are neurotrophic for enteric neurons. J Neurosci 33(8):3339–3351
Clausen F, Hånell A, Israelsson C, Hedin J, Ebendal T, Mir AK et al (2011) Neutralization of interleukin-1β reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur J Neurosci 34(1):110–123
Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K (1995) Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke 26(4):676–681
Guadagno J, Swan P, Shaikh R, Cregan S (2015) Microglia-derived IL-1β triggers p53-mediated cell cycle arrest and apoptosis in neural precursor cells. Cell Death Dis 6(6):e1779-e
Zhou C, Ye H-H, Wang S-Q, Chai Z (2006) Interleukin-1beta regulation of N-type Ca2+ channels in cortical neurons. Neurosci Lett 403(1–2):181–185
Carvey P, Chen E-Y, Lipton J, Tong C, Chang Q, Ling Z (2005) Intra-parenchymal injection of tumor necrosis factor-α and interleukin 1-β produces dopamine neuron loss in the rat. J Neural Transm 112(5):601–612
Wang X-j, Kong K-m, Qi W-l, Ye W-l, Song P-s (2005) Interleukin-1 beta induction of neuron apoptosis depends on p38 mitogen-activated protein kinase activity after spinal cord injury. Acta Pharmacol Sin 26(8):934–942
Zhang Y, Zhou B, Zhang F, Wu J, Hu Y, Liu Y et al (2012) Amyloid-β induces hepatic insulin resistance by activating JAK2/STAT3/SOCS-1 signaling pathway. Diabetes 61(6):1434–1443
Samavati L, Rastogi R, Du W, Hüttemann M, Fite A, Franchi L (2009) STAT3 tyrosine phosphorylation is critical for interleukin 1 beta and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol Immunol 46(8–9):1867–1877
Wang H, Li Z, Gao J, Liao Q (2019) Circular RNA circPTK2 regulates oxygen-glucose deprivation-activated microglia-induced hippocampal neuronal apoptosis via miR-29b-SOCS-1-JAK2/STAT3-IL-1β signaling. Int J Biol Macromol 129:488–496
El-Asrag ME, Sergouniotis PI, McKibbin M, Plagnol V, Sheridan E, Waseem N et al (2015) Biallelic mutations in the autophagy regulator DRAM2 cause retinal dystrophy with early macular involvement. Am J Hum Genet 96(6):948–954
Yoon JH, Her S, Kim M, Jang IS, Park J (2012) The expression of damage-regulated autophagy modulator 2 (DRAM2) contributes to autophagy induction. Mol Biol Rep 39(2):1087–1093
Chen ZH, Wang WT, Huang W, Fang K, Sun YM, Liu SR et al (2017) The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death Differ 24(2):212–224
Zeng CW, Chen ZH, Zhang XJ, Han BW, Lin KY, Li XJ et al (2014) MIR125B1 represses the degradation of the PML-RARA oncoprotein by an autophagy-lysosomal pathway in acute promyelocytic leukemia. Autophagy 10(10):1726–1737
Kim JK, Lee HM, Park KS, Shin DM, Kim TS, Kim YS et al (2017) MIR144* inhibits antimicrobial responses against Mycobacterium tuberculosis in human monocytes and macrophages by targeting the autophagy protein DRAM2. Autophagy 13(2):423–441
Bai S, Tian B, Li A, Yao Q, Zhang G, Li F (2016) MicroRNA-125b promotes tumor growth and suppresses apoptosis by targeting DRAM2 in retinoblastoma. Eye (Lond) 30(12):1630–1638
Li H, Lu C, Yao W, Xu L, Zhou J, Zheng B (2020) Dexmedetomidine inhibits inflammatory response and autophagy through the circLrp1b/miR-27a-3p/Dram2 pathway in a rat model of traumatic brain injury. Aging 12(21):21687–21705
Geng Z, Guo Z, Guo R, Ye R, Zhu W, Yan BJBRB (2021) Ferroptosis and traumatic brain injury. 172:212-9
Naseem M, Parvez SJTswj (2014) Role of melatonin in traumatic brain injury and spinal cord injury. 2014
Wu C, Du M, Yu R, Cheng Y, Wu B, Fu J, et al (2022) A novel mechanism linking ferroptosis and endoplasmic reticulum stress via the circPtpn14/miR-351–5p/5-LOX signaling in melatonin-mediated treatment of traumatic brain injury. 178:271–94
Thompson AG, Gray E, Heman-Ackah SM, Mäger I, Talbot K, Andaloussi SE et al (2016) Extracellular vesicles in neurodegenerative disease - pathogenesis to biomarkers. Nat Rev Neurol 12(6):346–357
Shi M, Sheng L, Stewart T, Zabetian CP, Zhang J (2019) New windows into the brain: central nervous system-derived extracellular vesicles in blood. Prog Neurobiol 175:96–106
Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y et al (2018) Exosomes from MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem: Int J Exp Cell Physiol, Biochem, Pharmacol 47(2):864–878
Panaro MA, Benameur T, Porro CJB (2020) Extracellular vesicles miRNA cargo for microglia polarization in traumatic brain injury. 10(6):901
Rivero-Ríos P, Gómez-Suaga P, Fernández B, Madero-Pérez J, Schwab AJ, Ebert AD et al (2015) Alterations in late endocytic trafficking related to the pathobiology of LRRK2-linked Parkinson’s disease. Biochem Soc Trans 43(3):390–395
Lamb CA, Longatti A, Tooze SA (2016) Rabs and GAPs in starvation-induced autophagy. Small GTPases 7(4):265–269
Longatti A, Lamb CA, Razi M, Yoshimura S, Barr FA, Tooze SA (2012) TBC1D14 regulates autophagosome formation via Rab11- and ULK1-positive recycling endosomes. J Cell Biol 197(5):659–675
Richards P, Didszun C, Campesan S, Simpson A, Horley B, Young KW et al (2011) Dendritic spine loss and neurodegeneration is rescued by Rab11 in models of Huntington’s disease. Cell Death Differ 18(2):191–200
Fader CM, Sánchez D, Furlán M, Colombo MI (2008) Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic (Copenhagen, Denmark) 9(2):230–250
Knævelsrud H, Søreng K, Raiborg C, Håberg K, Rasmuson F, Brech A et al (2013) Membrane remodeling by the PX-BAR protein SNX18 promotes autophagosome formation. J Cell Biol 202(2):331–349
Puri C, Renna M, Bento CF, Moreau K, Rubinsztein DC (2013) Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell 154(6):1285–1299
Szatmári Z, Kis V, Lippai M, Hegedus K, Faragó T, Lorincz P et al (2014) Rab11 facilitates cross-talk between autophagy and endosomal pathway through regulation of Hook localization. Mol Biol Cell 25(4):522–531
Puri C, Vicinanza M, Ashkenazi A, Gratian MJ, Zhang Q, Bento CF et al (2018) The RAB11A-positive compartment is a primary platform for autophagosome assembly mediated by WIPI2 recognition of PI3P-RAB11A. Dev Cell 45(1):114–31.e8
Li D, Huang S, Zhu J, Hu T, Han Z, Zhang S et al (2019) Exosomes from MiR-21-5p-increased neurons play a role in neuroprotection by suppressing Rab11a-mediated neuronal autophagy in vitro after traumatic brain injury. Med Sci Monit: Int Med J Exp Clin Res 25:1871–1885
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736
Zeiler FA, McFadyen C, Newcombe VFJ, Synnot A, Donoghue EL, Ripatti S et al (2021) Genetic influences on patient-oriented outcomes in traumatic brain injury: a living systematic review of non-apolipoprotein E single-nucleotide polymorphisms. J Neurotrauma 38(8):1107–1123
Lipsky RH, Marini AM (2007) Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci 1122:130–143
Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P et al (1989) Molecular cloning and expression of brain-derived neurotrophic factor. Nature 341(6238):149–152
Poo MM (2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci 2(1):24–32
Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J (2018) BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol 38(3):579–593
Singh M, Kakkar A, Sharma R, Kharbanda OP, Monga N, Kumar M et al (2017) Synergistic effect of BDNF and FGF2 in efficient generation of functional dopaminergic neurons from human mesenchymal stem cells. Sci Rep 7(1):10378
Ortiz JB, Mathewson CM, Hoffman AN, Hanavan PD, Terwilliger EF, Conrad CD (2014) Hippocampal brain-derived neurotrophic factor mediates recovery from chronic stress-induced spatial reference memory deficits. Eur J Neurosci 40(9):3351–3362
Klein R, Jing SQ, Nanduri V, O’Rourke E, Barbacid M (1991) The trk proto-oncogene encodes a receptor for nerve growth factor. Cell 65(1):189–197
Frisén J, Verge VM, Fried K, Risling M, Persson H, Trotter J et al (1993) Characterization of glial trkB receptors: differential response to injury in the central and peripheral nervous systems. Proc Natl Acad Sci USA 90(11):4971–4975
Rostami E, Krueger F, Plantman S, Davidsson J, Agoston D, Grafman J et al (2014) Alteration in BDNF and its receptors, full-length and truncated TrkB and p75(NTR) following penetrating traumatic brain injury. Brain Res 1542:195–205
Xu H, Jia Z, Ma K, Zhang J, Dai C, Yao Z et al (2020) Protective effect of BMSCs-derived exosomes mediated by BDNF on TBI via miR-216a-5p. Med Sci Monit: Int Med J Exp Clin Res 26:e920855
Xiao X, Jiang Y, Liang W, Wang Y, Cao S, Yan H et al (2019) miR-212-5p attenuates ferroptotic neuronal death after traumatic brain injury by targeting Ptgs2. Mol Brain 12(1):78
Kumar A, Stoica BA, Loane DJ, Yang M, Abulwerdi G, Khan N et al (2017) Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation 14(1):47
Bai W, Zhang X, Su X, Kong C, Yang Y, Ye Y et al (2020) Activation of miR-124–3p/Notch pathway promotes proliferation and differentiation of rat neural stem cells after traumatic brain injury. Xi bao yu fen zi mian yi xue za zhi = Chin J Cell Mol Immunol 36(1):49–55
Henry RJ, Doran SJ, Barrett JP, Meadows VE, Sabirzhanov B, Stoica BA, et al. Inhibition of miR-155 limits neuroinflammation and improves functional recovery after experimental traumatic brain injury in mice. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2019;16(1):216–30.
Yang T, Song J, Bu X, Wang C, Wu J, Cai J, et al (2016) Elevated serum miR-93, miR-191, and miR-499 are noninvasive biomarkers for the presence and progression of traumatic brain injury. 137(1):122–9
Ge X, Li W, Huang S, Yin Z, Yang M, Han Z et al (2019) Increased miR-21-3p in injured brain microvascular endothelial cells after traumatic brain injury aggravates blood-brain barrier damage by promoting cellular apoptosis and inflammation through targeting MAT2B. J Neurotrauma 36(8):1291–1305
Sabirzhanov B, Zhao Z, Stoica BA, Loane DJ, Wu J, Borroto C et al (2014) Downregulation of miR-23a and miR-27a following experimental traumatic brain injury induces neuronal cell death through activation of proapoptotic Bcl-2 proteins. J Neurosci: Off J Soc Neurosci 34(30):10055–10071
Das Gupta S, Ciszek R, Heiskanen M (2021) Plasma miR-9–3p and miR-136–3p as potential novel diagnostic biomarkers for experimental and human mild traumatic brain injury. 22(4)
Li Z, Wang Y, Zeng G, Zheng X, Wang W, Ling Y et al (2017) Increased miR-155 and heme oxygenase-1 expression is involved in the protective effects of formononetin in traumatic brain injury in rats. Am J Transl Res 9(12):5653–5661
Ge X, Huang S, Gao H, Han Z, Chen F, Zhang S et al (2016) miR-21-5p alleviates leakage of injured brain microvascular endothelial barrier in vitro through suppressing inflammation and apoptosis. Brain Res 1650:31–40
Yan J, Bu X, Li Z, Wu J, Wang C, Li D, et al (2019) Screening the expression of several miRNAs from TaqMan low density array in traumatic brain injury: miR-219a-5p regulates neuronal apoptosis by modulating CCNA2 and CACUL1. 150(2):202–17
Wang WX, Wilfred BR, Madathil SK, Tang G, Hu Y, Dimayuga J et al (2010) miR-107 regulates granulin/progranulin with implications for traumatic brain injury and neurodegenerative disease. Am J Pathol 177(1):334–345
Cui C, Zhang D, Sun K, Li H, Xu L, Lin G, et al (2021) Propofol maintains Th17/Treg cell balance and reduces inflammation in rats with traumatic brain injury via the miR‑145‑3p/NFATc2/NF‑κB axis. Int J Mol Med 48(1)
Vuokila N, Das Gupta S, Huusko R, Tohka J, Puhakka N, Pitkänen A (2020) Elevated acute plasma miR-124-3p level relates to evolution of larger cortical lesion area after traumatic brain injury. Neuroscience 433:21–35
Si L, Wang H, Wang L (2020) Suppression of miR-193a alleviates neuroinflammation and improves neurological function recovery after traumatic brain injury (TBI) in mice. Biochem Biophys Res Commun 523(2):527–534
Vuokila N, Aronica E, Korotkov A, van Vliet EA (2020) Chronic regulation of miR-124–3p in the perilesional cortex after experimental and human TBI. 21(7)
Zhu J, Chen Z, Tian J, Meng Z, Ju M, Wu G et al (2017) miR-34b attenuates trauma-induced anxiety-like behavior by targeting CRHR1. Int J Mol Med 40(1):90–100
Chen W, Zhao L, Zhang J, Wang B, Xu G, Lin C et al (2019) Elevated expression of miR-302 cluster improves traumatic brain injury by inhibiting phosphorylation of connexin43 via ERK signaling. J Chem Neuroanat 99:1–8
Pinchi E, Frati A, Cipolloni L, Aromatario M, Gatto V, La Russa R et al (2018) Clinical-pathological study on β-APP, IL-1β, GFAP, NFL, spectrin II, 8OHdG, TUNEL, miR-21, miR-16, miR-92 expressions to verify DAI-diagnosis, grade and prognosis. Sci Rep 8(1):2387
Jia Q, Yan S, Huang J, Xu S (2020) Restored microRNA-133a-3p or depleted PSAT1 restrains endothelial cell damage-induced intracranial aneurysm via suppressing the GSK3β/β-catenin pathway. Nanoscale Res Lett 15(1):177
Burek M, König A, Lang M, Fiedler J, Oerter S, Roewer N et al (2019) Hypoxia-induced microRNA-212/132 alter blood-brain barrier integrity through inhibition of tight junction-associated proteins in human and mouse brain microvascular endothelial cells. Transl Stroke Res 10(6):672–683
Vuokila N, Lukasiuk K, Bot AM, van Vliet EA, Aronica E, Pitkänen A et al (2018) miR-124-3p is a chronic regulator of gene expression after brain injury. Cell Mol Life Sci: CMLS 75(24):4557–4581
Ge XT, Lei P, Wang HC, Zhang AL, Han ZL, Chen X et al (2014) miR-21 improves the neurological outcome after traumatic brain injury in rats. Sci Rep 4:6718
Wang L, Zhao C, Wu S, Xiao G, Zhuge X, Lei P et al (2018) Hydrogen gas treatment improves the neurological outcome after traumatic brain injury via increasing miR-21 expression. Shock (Augusta, GA) 50(3):308–315
Yang M, Wang X, Fan Y, Chen Y, Sun D, Xu X et al (2019) Semaphorin 3A contributes to secondary blood-brain barrier damage after traumatic brain injury. Front Cell Neurosci 13:117
Qin X, Li L, Lv Q, Shu Q, Zhang Y, Wang Y (2018) Expression profile of plasma microRNAs and their roles in diagnosis of mild to severe traumatic brain injury. 13(9):e0204051
Truettner JS, Motti D, Dietrich WD (2013) MicroRNA overexpression increases cortical neuronal vulnerability to injury. Brain Res 1533:122–130
Guo S, Zhen Y, Zhu Z, Zhou G, Zheng X (2019) Cinnamic acid rescues behavioral deficits in a mouse model of traumatic brain injury by targeting miR-455-3p/HDAC2. Life Sci 235:116819
Zhao L, Zhang L, Zhu W, Chen H, Ding Y, Cui G (2021) Inhibition of microRNA-203 protects against traumatic brain injury induced neural damages via suppressing neuronal apoptosis and dementia-related molecues. Physiol Behav 228:113190
Sandhir R, Gregory E, Berman NE (2014) Differential response of miRNA-21 and its targets after traumatic brain injury in aging mice. Neurochem Int 78:117–121
Zhang J, Knight R, Wang Y, Sawyer TW, Martyniuk CJ, Langlois VS (2019) Hair follicle miRNAs: a novel biomarker for primary blast induced-mild traumatic brain injury. 24(2):166–79
Li Q, Cheng K, Wang AY, Xu QG, Fu ZF, He SY et al (2019) microRNA-126 inhibits tube formation of HUVECs by interacting with EGFL7 and down-regulating PI3K/AKT signaling pathway. Biomed Pharmacother = Biomed Pharmacother 116:109007
Miao W, Bao TH, Han JH, Yin M, Yan Y, Wang WW et al (2015) Voluntary exercise prior to traumatic brain injury alters miRNA expression in the injured mouse cerebral cortex. Braz J Med Biol Res = Rev Bras de Pesquisas Medicas e Biologicas. 48(5):433–9
Johnson D, Cartagena CM, Tortella FC, Dave JR, Schmid KE, Boutté AM (2017) Acute and subacute microRNA dysregulation is associated with cytokine responses in the rodent model of penetrating ballistic-like brain injury. J Trauma Acute Care Surg 83(1 Suppl 1):S145–S149
Osei J, Kelly W, Toffolo K, Donahue K, Levy B, Bard J et al (2018) Thymosin beta 4 induces significant changes in the plasma miRNA profile following severe traumatic brain injury in the rat lateral fluid percussion injury model. Expert Opin Biol Ther 18(sup1):159–164
Redell JB, Zhao J, Dash PK (2011) Altered expression of miRNA-21 and its targets in the hippocampus after traumatic brain injury. J Neurosci Res 89(2):212–221
Wang WX, Visavadiya NP, Pandya JD, Nelson PT, Sullivan PG, Springer JE (2015) Mitochondria-associated microRNAs in rat hippocampus following traumatic brain injury. Exp Neurol 265:84–93
Wang Y, Guo F, Pan C, Lou Y, Zhang P, Guo S et al (2012) Effects of low temperatures on proliferation-related signaling pathways in the hippocampus after traumatic brain injury. Exp Biol Med (Maywood) 237(12):1424–1432
Sun TY, Chen XR, Liu ZL, Zhao LL, Jiang YX, Qu GQ et al (2014) Expression profiling of microRNAs in hippocampus of rats following traumatic brain injury. J Huazhong Univ Sci Technol Med Sci = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban 34(4):548–53
Han Z, Chen F, Ge X, Tan J, Lei P, Zhang J (2014) miR-21 alleviated apoptosis of cortical neurons through promoting PTEN-Akt signaling pathway in vitro after experimental traumatic brain injury. Brain Res 1582:12–20
Puhakka N, Bot AM, Vuokila N, Debski KJ, Lukasiuk K, Pitkänen A (2017) Chronically dysregulated NOTCH1 interactome in the dentate gyrus after traumatic brain injury. PLoS ONE 12(3):e0172521
Lei P, Li Y, Chen X, Yang S, Zhang J (2009) Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Res 1284:191–201
Dai X, Yi M, Wang D, Chen Y, Xu X (2019) Changqin NO. 1 inhibits neuronal apoptosis via suppressing GAS5 expression in a traumatic brain injury mice model. Biol Chem 400(6):753–63
Nan A, Chen L, Zhang N, Liu Z, Yang T, Wang Z et al (2017) A novel regulatory network among LncRpa, CircRar1, MiR-671 and apoptotic genes promotes lead-induced neuronal cell apoptosis. Arch Toxicol 91(4):1671–1684
Yang LX, Yang LK, Zhu J, Chen JH, Wang YH, Xiong K (2019) Expression signatures of long non-coding RNA and mRNA in human traumatic brain injury. Neural Regen Res 14(4):632–641
Wang CF, Zhao CC, Weng WJ, Lei J, Lin Y, Mao Q et al (2017) Alteration in long non-coding RNA expression after traumatic brain injury in rats. J Neurotrauma 34(13):2100–2108
Feng J, Zhou Z, Feng R, Zeng C, Wei M, Hong T (2021) Silencing long non-coding RNA zinc finger antisense 1 restricts secondary cerebral edema and neuron injuries after traumatic brain injury. Neurosci Lett 756:135958
Zhong J, Jiang L, Huang Z, Zhang H, Cheng C, Liu H et al (2017) The long non-coding RNA Neat1 is an important mediator of the therapeutic effect of bexarotene on traumatic brain injury in mice. Brain Behav Immun 65:183–194
Yang X, Chen Y, Li J, Chen L, Ren H, Liu Y et al (2019) Hypertonic saline maintains coagulofibrinolytic homeostasis following moderate-to-severe traumatic brain injury by regulating monocyte phenotype via expression of lncRNAs. Mol Med Rep 19(2):1083–1091
Yi M, Dai X, Li Q, Xu X, Chen Y, Wang D (2019) Downregulated lncRNA CRNDE contributes to the enhancement of nerve repair after traumatic brain injury in rats. 18(18):2332-43
Shao HF, Li ZZ, Zheng XF, Wang HJ, Wang YG, Ma ZL et al (2019) Research on the correlation of changes in plasma lncRNA MEG3 with change in inflammatory factors and prognosis in patients with traumatic brain injury. Eur Rev Med Pharmacol Sci 23(10):4341–4347
Liu N, Sun H, Li X, Cao W, Peng A, Dong S et al (2021) Downregulation of lncRNA KCNQ1OT1 relieves traumatic brain injury induced neurological deficits via promoting “M2” microglia polarization. Brain Res Bull 171:91–102
Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y et al (2018) Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J: Off Publ Fed Am Soc Exp Biol 32(1):512–528
Yang Y, Ye Y, Kong C, Su X, Zhang X, Bai W et al (2019) MiR-124 enriched exosomes promoted the M2 polarization of microglia and enhanced hippocampus neurogenesis after traumatic brain injury by inhibiting TLR4 pathway. Neurochem Res 44(4):811–828
Ge X, Guo M, Hu T, Li W, Huang S, Yin Z et al (2020) Increased microglial exosomal miR-124-3p alleviates neurodegeneration and improves cognitive outcome after rmTBI. Mol Ther: J Am Soc Gene Ther 28(2):503–522
Long X, Yao X, Jiang Q, Yang Y, He X, Tian W et al (2020) Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J Neuroinflammation 17(1):89
Yin Z, Han Z, Hu T, Zhang S, Ge X, Huang S et al (2020) Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain Behav Immun 83:270–282
Pusic KM, Pusic AD, Kraig RP (2016) Environmental enrichment stimulates immune cell secretion of exosomes that promote CNS myelination and may regulate inflammation. Cell Mol Neurobiol 36(3):313–325
Author information
Authors and Affiliations
Contributions
H. M. was involved in the conception, design, statistical analysis, and drafting of the manuscript. O. M., M. H., F. M., A. J. Y., A. O., A. S., S. S. T. Z., G. O. S., S. A. T. Z., M. R. H., and A. H. contributed in the data collection and manuscript drafting. All authors approved the final version for submission.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohamadzadeh, O., Hajinouri, M., Moammer, F. et al. Non-coding RNAs and Exosomal Non-coding RNAs in Traumatic Brain Injury: the Small Player with Big Actions. Mol Neurobiol 60, 4064–4083 (2023). https://doi.org/10.1007/s12035-023-03321-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03321-y