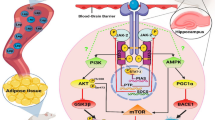
Abstract
Alzheimer’s disease (AD) is closely related to type 2 diabetes (T2D). This study investigated the impact of high-intensity interval training (HIIT) on diabetes-induced disturbances in AD-related factors (including AMP-activated protein kinase (AMPK), glycogen synthase kinase-3β (GSK3β), and tau protein) in the hippocampus, with the main focus on adiponectin signaling.
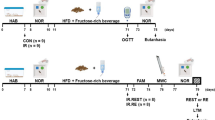
In total, 28 male Wistar rats at the age of 8 weeks were randomly assigned to four groups (n = 7 in each group): control (Con), type 2 diabetes (T2D), HIIT (Ex), and type 2 diabetes + HIIT (T2D + Ex). T2D was induced by a high-fat diet plus a single dose of streptozotocin (STZ). Rats in Ex and T2D + Ex groups performed 8 weeks of HIIT (running at 8–95% of Vmax, 4–10 intervals). Insulin and adiponectin levels in serum and hippocampus were measured along with hippocampal expression of insulin and adiponectin receptors, phosphorylated AMPK, dephosphorylated GSK3β, and phosphorylated tau. Homeostasis model assessment for insulin resistance (HOMA-IR), homeostasis model assessment for insulin resistance beta (HOMA-β), and quantitative insulin sensitivity check index (QUICKI) were calculated to assess insulin resistance and sensitivity. T2D decreased insulin and adiponectin levels in serum and hippocampus, as well as the hippocampal levels of insulin and adiponectin receptors and AMPK, but increased GSK3β and tau in the hippocampus. HIIT reversed diabetes-induced impairments and consequently decreased tau accumulation in the hippocampus of diabetic rats. HOMA-IR, HOMA-β, and QUICKI were improved in Ex and T2D + Ex groups. Overall, our results confirmed that T2D has undesirable effects on the levels of some Alzheimer’s-related factors in the hippocampus, and HIIT could ameliorate these impairments in the hippocampus.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sedentary lifestyles and an increased tendency toward high-calorie and high-fat foods contribute to obesity [1], a risk factor for many diseases, including diabetes and cardiovascular disease. Diabetes is a metabolic disease that disrupts fat metabolism, increases adipose tissue (especially visceral), and causes glucose intolerance [2]. Usually associated with obesity, type 2 diabetes (T2D) is a disease in which the body does not respond to insulin normally. This insulin resistance (IR) is the main cause of T2D [1]. In addition to IR, diabetes can disrupt adipokine production and secretion [3]. Adipokines are cytokines secreted by adipose tissue acting at autocrine/paracrine and endocrine levels [4]. Adipokines have a role in regulating glucose and lipid metabolism, energy homeostasis, healthy behavior, insulin sensitivity, inflammation, immune system function, fat accumulation, vascular function, coagulation, and cognitive function [5]. Evidence suggests that obesity and T2D can lead to cognitive disorders by disrupting adipokines’ functions [4]. Today, the number of people with cognitive disorders is estimated to be around 55 million worldwide and will reach more than 131 million by 2050 [6, 7]. In total, 98% of people with cognitive disorders such as Alzheimer’s disease (AD) suffer from one or more common diseases such as obesity and diabetes. Type 2 diabetes increases the risk of cognitive disorders by about 1.6 times [8, 9].
Adiponectin is an adipokine that mediates the cross-talk between adipose tissue and the central nervous system and is considered the leading player in diabetes-induced cognitive disorders. Two adiponectin receptors, AdipoR1 and AdipoR2, have been identified in the brain [10]. Based on the available evidence, adiponectin crosses the blood–brain barrier. It binds to its receptors to activate cellular pathways, including insulin metabolism, mitochondrial biogenesis, and oxidative stress through AMP-activated protein kinase (AMPK) [11]. On the other hand, phosphorylation of p38-mitogen-activated protein kinases (p38-MAPK) stimulates neurogenesis through glycogen synthase kinase-3β (GSK3β). In addition, there is a cross-talk between the AMPK and GSK-3β signaling pathways in which GSK-3β suppresses AMPK [11].
Adiponectin function in the central nervous system and its relation to cognitive disorders has not yet been fully understood. However, because IR is commonly seen in early AD (hence called type 3 diabetes [12]), adiponectin might be a vital player in AD [13]. Adiponectin levels have been reported to increase [14], decrease [15], and in some cases, remain unchanged in AD [16]. To compensate for the increase in IR in the central nervous system, plasma adiponectin levels increase to stimulate the decreased activity of insulin receptors (i.e., a compensatory response) [17]. However, after crossing the blood–brain barrier and activating AMPK and p53-MAPK cascades, adiponectin increases the phosphorylated and toxic form of tau protein, which leads to neuronal and synaptic cell death. Therefore, both positive and negative role is imaginable for adiponectin in cognitive disorders [17, 18].
Exercise effectively counteracts various metabolic problems, age-related loss of function, and physiological issues. Various studies have shown that different modalities of exercise, such as treadmills [19,20,21], voluntary exercise [22,23,24], and swimming [25, 26], are effective in AD and T2D diseases. Among all interventions, exercise could be used as a preventive strategy, does not have side effects, and has many positive physiological and psychological effects [27]. Regular exercise training could control dysglycemia and hyperinsulinemia, increase insulin sensitivity, decrease body fat, and decrease blood pressure [28,29,30,31]. Exercise effects on metabolic disorders could be mediated through adiponectin [32]. Research has also shown that adiponectin’s role in T2D and AD is remarkable [17] and can be considered a link between the two diseases. Thus, exercise could be a preferred choice for preventing T2D-induced cognitive disorders. However, the therapeutic effect of exercise shows a dose–response pattern, meaning that training variables should be manipulated carefully to reach the desirable adaption. It has been shown that adiponectin response to exercise is intensity dependent, with high-intensity interval training (HIIT) as the most effective. It has been demonstrated that 12 weeks of HIIT increased adiponectin levels and insulin sensitivity more than the same amount of moderate intensity in obese individuals [33]. In addition, Martinez et al. [34],while a high-fat diet disrupted heart levels of adiponectin, exercise training could ameliorate this change. They suggested that the effect of exercise might be type-dependent. This study investigated the effect of 8-week HIIT on adiponectin signaling and phosphorylated AMPK, dephosphorylated GSK3β, and phosphorylated tau protein in hippocampus of male rats with T2D.
Material and Methods
Animal Care
In the present study, we purchased 28 8-week-old male Wistar rats with an average weight of 200 g from the animal farm of Kerman University of Medical Sciences (KUMS) and kept them at 23 ± 2 °C and a 12:12 dark–light cycle in special polycarbonate cages. All animals had free access to water and food. The ethics committee of KUMS approved the study protocol prior to any experiments being carried out (ethics approval code: IR.KMU.REC.1399.503).
After being habituated to the laboratory environment, the animals were randomly assigned to four groups (n = 7 in each group): control (Con), type 2 diabetes (T2D), exercise (Ex), and type 2 diabetes + exercise (T2D + Ex). The Ex and T2D + Ex groups performed 8 weeks of HIIT.
Induction of Diabetes
T2D + Ex and T2D groups were fed a high-fat diet (HFD) for 2 months (Table 1). After 2 months, the animals fasted for 12 h, and a single dose of 35 mg/kg streptozotocin (STZ) was injected intraperitoneally. Animal blood glucose was measured three days after STZ injection using a glucometer. Animals with fasting blood glucose (FBG) above 300 mg/dl were considered diabetic and included in the study [35]. Animals’ FBG were measured before starting the intervention (month 0), after diabetes induction (2 months of high-fat diet and STZ injection), and 48 h after the training period using a glucometer (Accu-Chek, USA).
Exercise Protocol
All animals were familiarized with the motorized treadmill. They ran on the treadmill at 8 m/min with an incline of 0% and 10 min/day for 5 consecutive days prior to the experiments. The Ex and T2D + Ex groups performed an incremental running test to determine their maximum speed (Vmax). They ran for 2 min at a 6 m/min speed, and every 2 min, 2 m/min was added to the speed until they became exhausted. The last min tolerated speed was considered Vmax. Finally, the HIIT protocol was carried out five times a week for 8 weeks by rats in Ex and T2D + Ex groups [30]. Rats’ Vmax was measured every 2 weeks, and the new Vmax was used to calculate relative speed in the next 2 weeks. This protocol was designed in our lab, and we named it the K1 protocol (Table 2).
Serum and Tissue Sampling
A total of 48 h after the last training session, animals were anesthetized by intraperitoneal injection of ketamine(80 mg/kg) and xylazine(10 mg/kg) and blood samples were taken from the animal’s heart after 12 h of fasting, and hippocampal tissues were harvested. Blood samples were then placed at room temperature for 30 min and then centrifuged at 1000 g for 20 min at 4 °C, and serum samples were stored at a temperature of – 80 °C. The hippocampus was washed in PBS (1.37 M NaCl, 27 mM KCl, 100 mM Na2HPO4, 18 mM KH2PO4, and PH: 7.4) solution. An ultrasonic homogenizer performed homogenization in Ripa buffer (150 mM NaCl, 1.0% IGEPAL® CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, and PH: 8.0) solution with protease inhibitor on ice. The homogenate was centrifuged at 4 °C at 17,982 g for 20 min, and the supernatant was kept at – 80 °C.
Western Blot
Western blotting was used to measure the amount of phosphorylated AMPK, dephosphorylated (Santa Cruz Biotechnology Inc., sc-33524), GSK3-β (Santa Cruz Biotechnology Inc., sc-81462), AdipoR1 (Santa Cruz Biotechnology Inc., sc-518030), AdipoR2 (Santa Cruz Biotechnology Inc., sc-514045), and the phosphorylated form of tau protein (Santa Cruz Biotechnology Inc., sc-21796) and insulin receptor beta subunit (InsRB) (Santa Cruz Biotechnology Inc., sc-57342). The total protein concentration in the hippocampal samples was measured by the Lowry method, while bovine serum albumin was used as standard. After matching the concentrations, 40 μg of protein from each sample was mixed with a buffer sample. Then electrophorese was performed for 75 min using 11% SDS-PAGE gel. After that, the proteins separated in the gel were transferred to PVDF paper. The membrane was then incubated in a 2% block solution overnight (at 4 °C). In the next step, the membrane was quenched four times each, washed with TBST (tris-buffered saline with tween 20) solution as a detergent for 5 min, and incubated for 3 h with the initial antibody (concentration 1:200) for each of the mentioned proteins. Then, the membrane was exposed to a secondary antibody (with a concentration of 1:1000) for 1 h. In the next step, immune detection was recorded using Chemi Doc XRS + imaging system (Bio-Rad Company, USA) and analyzed by ImageJ software [36]. β-actin was used as a control, and the final data was corrected by β-actin expression.
ELISA
The adiponectin and insulin levels were assessed using ELISA in the serum. According to the manufacturer’s instructions, serum and tissue adiponectin and insulin were assayed using relevant kits. Adiponectin (Rat ELISA Kit, Eastbiopharm) and insulin (Rat ELISA Kit, Eastbiopharm) [36,37,38].
Calculation of Insulin Resistance/Sensitivity Indices
The homeostasis model assessment (HOMA) was used to assess insulin resistance (HOMA-IR) and homeostasis model assessment of β-cell function (HOMA-β). Specifically, HOMA-IR and HOMA-β scores were calculated using the following formula: HOMA-IR = [(fasting glucose (mmol/l) × fasting insulin (μU/ml))/22.5]. HOMA β-cell = [(20 × fasting insulin (μU/ml))/(fasting glucose (mmol/l) – 3.5) [39]. The quantitative insulin sensitivity check index (QUICKI) was also used as it has a better linear correlation with glucose clamp determinations of insulin sensitivity than minimal-model estimates. QUICKI was calculated using Katz et al. formula [40] (i.e., 1/[log (fasting insulin in μU/ml) + log (fasting glucose in mg/dl)]).
Statistical Analysis
The data are reported as mean ± standard deviation (SD). Statistical analysis was performed using SPSS version 21. Normality and homogeneity of variances were assessed using Shapiro–Wilk and Leven tests, respectively. Two-way ANOVA followed by Tukey’s post hoc was used to analyze the data. P-values less than 0.05 were considered statistically significance.
Results
Animal Weight and Blood Glucose
We assessed FBG to confirm our diabetes induction method. Our results showed that blood glucose was significantly increased after diabetes induction (2 months of high-fat diet and STZ injection) (month 2) compared with before baseline (month 0) in T2D and T2D + Ex group (P = 0.000), with no significant difference between these groups. In addition, HIIT reduced blood glucose significantly (P = 0.000) (Fig. 1A). Animals’ weight showed a significant increase in T2D and T2D + Ex groups after diabetes induction (2 months of high-fat diet and STZ injection) (P = 0.000). In addition, the weight was decreased in T2D and T2D + Ex groups (P = 0.000), with more decrease in the T2D group (P = 0.02) (Fig. 1B).
Fasting blood glucose (A) and body weight (B) before starting the intervention (month 0), after diabetes induction (2 months of high-fat diet and STZ injection) (month 2), and 48 h after the last training session (month 4) in all groups (mean ± SD). FBG: Fasting blood glucose, Con: control, T2D: type 2 diabetic (STZ injected), Ex: exercise only, and T2D + Ex: type 2 diabetic + exercise. * shows a significant difference between T2D and T2D + Ex with other groups
Insulin Resistance/Sensitivity Indexes
To assess if T2D and Ex could improve insulin sensitivity, we evaluated insulin sensitivity indices (i.e., HOMA-IR, HOMAβ, and QUICKI). Our result showed that T2D and Ex increased and decreased HOMA-IR, respectively (P < 0.05). In addition, a significant interaction was seen between T2D and Ex (P < 0.05) (Fig. 2A). Also, HOMAβ decreased by T2D and increased by Ex (P < 0.05). T2D and Ex showed significant interaction (P < 0.05) (Fig. 2B). Furthermore, a significant decrease was seen in QUICKI after T2D induction decreased but it increased after Ex. T2D and Ex interaction was also significant (P < 0.05) (Fig. 2C). All in all, these results suggested that T2D increased IR but Ex decreased it.
Insulin and Adiponectin Levels in Serum
Our results showed that serum insulin levels were significantly decreased by T2D and increased by Ex (P < 0.05) (Fig. 3A). In addition, T2D and Ex showed significant interaction (P < 0.05). Our results showed that serum adiponectin levels were significantly decreased by T2D and increased by Ex (P < 0.05). In addition, T2D and Ex showed significant interaction (P < 0.05). (Fig. 3B).
InsRB, APNR1, and APNR2 Expression in Hippocampus
Our results showed that InsRB levels in hippocampus were significantly decreased by T2D and increased by Ex (P < 0.05). In addition, T2D and Ex showed significant interaction (P < 0.05) (Fig. 4A). APNR1 and APNR2 levels showed a significant decrease and increase by T2D and Ex, respectively (P < 0.05). Furthermore, our results showed significant interaction for T2D and Ex (P < 0.05) (Fig. 4B and C).
Phosphorylated AMPK and Dephosphorylated GSK3β Expression in the Hippocampus
Phosphorylated AMPK levels decreased by T2D and increased by Ex (P < 0.05). In addition, a significant interaction was seen for T2D and Ex (P < 0.05) (Fig. 5A). Dephosphorylated GSK3β levels increased by T2D and decreased by Ex (P < 0.05). In addition, a significant interaction was seen for T2D and Ex (P < 0.05) (Fig. 5B).
Phosphorylated AMPK (A) and dephosphorylated GSK3β (B) expression (mean ± SD) in the hippocampus. P-AMPK, phosphorylated AMP-activated protein kinase; Co, control; T2D, type 2 diabetic; Ex, exercise; Ex + T2D, type 2 diabetic + exercise. The western bond order is as follows: Con, T2D, Ex, and T2D + Ex
Phosphorylated Tau Expression in the Hippocampus
Phosphorylated tau levels increased by T2D and decreased by Ex (P < 0.05). In addition, a significant interaction was seen for T2D and Ex (P < 0.05) (Fig. 6).
Discussion
This study was designed to investigate the effect of 8-week HIIT on adiponectin signaling and AD risk factors in the hippocampus of male rats with T2D. Our results showed that diabetes reduced both insulin and adiponectin levels in serum. Diabetes also reduced the expression of adiponectin and insulin receptors and AMPK and increased dephosphorylated GSK3-β and tau in the hippocampus. HIIT recovered these impairments fully or partially. HOMA-IR, HOMA-β, and QUICKI (i.e., indices of insulin resistance, β cell function, and insulin sensitivity, respectively) were also improved by HIIT. In line with our results, Ghiasi et al. [41] reported that while HOMA-IR increased and QUICKI and HOMA-β decreased in diabetes, HIIT returned these indices to the normal ranges.
It has been suggested that diabetes-induced peripheral IR could finally lead to central IR. This can explain the decrease in insulin receptor expression in the T2D group, consistent with Biessels et al. results [42].
One study suggested that hypoadiponectinemia is associated with a decrease in hippocampus volume in patients with T2D [34], and adiponectin levels are critical for brain function. Pousti et al. [43] revealed that adiponectin modulates synaptic plasticity in the hippocampal dentate gyrus. Furthermore, Weisz et al. [44] demonstrated that adiponectin signaling could regulate hippocampal synaptic transmission. The recovery of adiponectin receptors in the hippocampus of diabetic rats through exercise, reported in the present study, shows that HIIT may be used as a non-pharmacological strategy for the prevention and treatment of hippocampus function impairments induced by diabetes/AD. At the behavioral level, diabetes can adversely affect cognitive-related functions, such as the results of the Morris water maze [8], and adiponectin improved animal performance in this test [45, 46]. Our results also showed a negative effect of diabetes on adiponectin receptors in the hippocampus, which are in agreement with other studies [47,48,49].
In line with our results, many studies have shown that exercise can improve HOMA indices and IR [50, 51]. In addition, it has been suggested that HIIT can improve insulin sensitivity and increase insulin secretion [52]. Our observations also confirmed the positive effects of HIIT on insulin sensitivity and insulin secretion. Hemmatinafar et al. [53] reported that HIIT is a time-efficient method for increasing adiponectin levels and reducing body fat, considering the point that exercise intensity is the vital variable in affecting adiponectin. It means that the more exercise intensity the more increase in adiponectin. Added to this, the interval nature of HIIT allows to repeat several bouts of high-intensity exercises, reinforcing the HIIT associate advantages [54].
In addition, we saw increased p-tau levels in the hippocampus in T2D, which is in line with Hobday et al. [55] results. P-AMPK/DeP-GSK-3β pathway dysfunction is considered the main reason for increased tau accumulation because of increased DeP-GSK-3β activity, which finally leads to its hyper-phosphorylation [56]. Adiponectin could also stimulate p-AMPK in the hypothalamus, and increases food intake [57]. This may justify the weight loss we saw in diabetic animals.
Our results also showed that the hippocampus level of adiponectin receptors and p-AMPK are reduced in diabetic rats, but HIIT seems to recover these changes. In peripheral tissue, adiponectin activates p-AMPK through AdipoR1. Furthermore, p-AMPK promotes Akt phosphorylation and GSK-3β inhibition, both of which increase insulin sensitivity. Activating this signaling pathway also suppresses apoptosis, oxidative stress, and neuroinflammation, and it could reduce neurodegeneration. It has been shown that adiponectin could improve memory and synaptic plasticity in a rat model of dementia, and p-AMPK is essential for the memory-improving effect of adiponectin [58]. Barone et al. [59] showed that the interplay between oxidative stress, brain IR, and p-AMPK dysfunction contributes to neurodegeneration in T2D and AD. They reported a decrease in p-AMPK in the hippocampus of diabetic patients and consider decreased adiponectin, increased oxidative stress and inflammation, and increased DeP-GSK3B activity as the main players in this scenario [59]. Many studies [60, 61] have shown that HIIT can increase p-AMPK levels and expression in the skeletal muscle. Exercise has long been known to activate AMPK/Sirtuin1 (AMPK/SIRT1) pathway and enhance brain-derived neurotrophic factor (BDNF) production. The activation of AMPK/SIRT1 and BDNF plays an important role in the exercise-related mitigation of dementia pathology. AMPK/SIRT1 and BDNF can directly affect intracellular Aβ production, tau phosphorylation, and neurogenesis via regulating α-, β-, and γ-secretases, and GSK3 [62]. Activated GSK-3 phosphorylates and thereby inactivates glycogen synthase, an enzyme that converts glucose to glycogen for storage [63].
Insulin activates AKT/protein kinase B through a well-defined mechanism mediated by the IRS1/PI3K pathway. This leads to the phosphorylation of GSK3B at serin9 residual, resulting in its inactivation [64]. Our results showed increased expression of GDeP-GSK3β in the hippocampus following T2D. Adiponectin can modulate the GDeP-GSK3β signaling pathway [63], and evidence supports GDeP-GSK3β’s role in producing some of AD’s characteristic hallmarks, such as extracellular accumulation of amyloid-β protein (Aβ) and intraneuronal neurofibrillary tangles composed of hyper-phosphorylated tau and inflammatory markers. These effects contribute to synaptic and neuronal loss and memory decline [65]. GSK3β was recognized as a primary kinase involved in tau phosphorylation, as was apparent from the first study termed tau protein kinase-I. Thus, GDeP-GSK3β has been identified as one of the major enzymes mediating tau hyper-phosphorylation at the residues implicated in neurodegenerative tauopathies, including AD [66]. Our results showed its increased expression in the hippocampus following T2D. Thota et al. [67] results are consistent with our data. The recovery of adiponectin levels and reduction of DeP-GSK3β expression toward normal by HIIT in diabetic rats imply that this type of exercise would benefit patients with diabetes to prevent the progression of their memory loss during the course of the disease.
Conclusion
Our results confirmed the destructive effects of T2D on the levels of specific Alzheimer’s-related markers in the hippocampus through adiponectin signaling. T2D decreased insulin and adiponectin levels in serum as well as the hippocampal levels of insulin and adiponectin receptors and p-AMKP but increased DeP-GSK3β and P-tau in the hippocampus, and HIIT as a non-pharmacological intervention could recover these impartments.
Limitations
Due to financial limitations, we could not perform an immunochemistry analysis. Using immunochemistry could show us the exact reign of molecular changes, which is important because, for example, CA1 is closely related to cognitive impairments. In addition, to reduce the number of animals (as suggested by the ethical committee), we had to use the whole hippocampus, not the special reign/s for western blotting.
Data Availability
All data and material is available at https://kmu.ac.ir/en by request.
Materials Availability
All data and material is available at https://kmu.ac.ir/en by request.
References
Araujo MYC et al (2020) Obesity increases costs with productivity loss due to disability retirements, independent of physical activity: a cohort study. J Occup Environ Med 62(5):325–330
Alford F et al (2018) Glucose effectiveness is a critical pathogenic factor leading to glucose intolerance and type 2 diabetes: an ignored hypothesis. Diabetes Metab Res Rev 34(4):e2989
Mather KJ et al (2008) Adiponectin, change in adiponectin, and progression to diabetes in the Diabetes Prevention Program. Diabetes 57(4):980–986
Maccio A, Madeddu C, Mantovani G (2009) Adipose tissue as target organ in the treatment of hormone-dependent breast cancer: new therapeutic perspectives. Obes Rev 10(6):660–670
Maury E, Brichard S (2010) Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol 314(1):1–16
Picone P, Di Carlo M, Nuzzo D (2020) Obesity and Alzheimer’s disease: molecular bases. Eur J Neurosci 52(8):3944–3950
Khaleghi M et al (2021) Estrogen attenuates physical and psychological stress-induced cognitive impairments in ovariectomized rats. Brain and Behavior 11(5):e02139
Zare D et al (2021) Inhibition of protease-activated receptor 1 (PAR1) ameliorates cognitive performance and synaptic plasticity impairments in animal model of Alzheimer’s diseases. Psychopharmacology 238(6):1645–1656
Diaz-Kenney RV et al (2010) A historical overview of the United States-Mexico border diabetes prevention and control project. Rev Panam Salud Publica 28(3):143–150
Kadowaki T, Yamauchi T (2005) Adiponectin and adiponectin receptors. Endocr Rev 26(3):439–451
Fang H, Judd RL (2011) Adiponectin regulation and function. Compr Physiol 8(3):1031–1063
Michailidis M et al (2022) Alzheimer’s disease as type 3 diabetes: common pathophysiological mechanisms between Alzheimer’s disease and type 2 diabetes. Int J Mol Sci 23(5):2687
Nguyen TT et al (2020) Type 3 diabetes and its role implications in Alzheimer’s disease. Int J Mol Sci 21(9):3165
Ma J et al (2016) Peripheral blood adipokines and insulin levels in patients with Alzheimer’s disease: a replication study and meta-analysis. Curr Alzheimer Res 13(3):223–233
Teixeira AL et al (2013) Decreased levels of circulating adiponectin in mild cognitive impairment and Alzheimer’s disease. NeuroMol Med 15(1):115–121
Dukic L et al (2016) The role of human kallikrein 6, clusterin and adiponectin as potential blood biomarkers of dementia. Clin Biochem 49(3):213–218
Benavente KS, Raymond P, Royall DR (2018) Serum adiponectin is related to dementia. Am J Geriatr Psychiatry 26(3):S94
Rajizadeh MA et al (2021) Does caffeine therapy improve cognitive impairments in valproic acid rat model of autism? Toxin Reviews 40(4):654–664
Bejeshk M-A et al (2018) Combinatorial effect of lower extremity blood flow restriction and low intensity endurance exercise on aorta of old male rats: histomorphological and molecular approach. Artery Research 24:22–31
Rajizadeh MA et al (2019) The effects of high intensity exercise on learning and memory impairments followed by combination of sleep deprivation and demyelination induced by etidium bromide. Int J Neurosci 129(12):1166–1178
Raji-Amirhasani A et al (2018) Mild exercise along with limb blood-flow restriction modulates the electrocardiogram, angiotensin, and apelin receptors of the heart in aging rats. Iran J Basic Med Sci 21(6):558
Rajizadeh MA et al (2020) Voluntary exercise modulates learning & memory and synaptic plasticity impairments in sleep deprived female rats. Brain Res 1729:146598
Rajizadeh MA et al (2018) Voluntary exercise impact on cognitive impairments in sleep-deprived intact female rats. Physiol Behav 188:58–66
Rajizadeh MA et al (2020) Cognitive impairments of sleep-deprived ovariectomized (OVX) female rats by voluntary exercise. Basic and Clinical Neuroscience 11(5):573
Amirazodi F et al (2020) The combination effects of resveratrol and swimming HIIT exercise on novel object recognition and open-field tasks in aged rats. Exp Aging Res 46(4):336–358
Amirazodi M et al (2022) The effects of combined resveratrol and high intensity interval training on the hippocampus in aged male rats: an investigation into some signaling pathways related to mitochondria. Iran J Basic Med Sci 25(2):254
Kadoglou NP et al (2007) The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Prev Cardiol 14(6):837–843
Wewege MA et al (2021) The effect of resistance training in healthy adults on body fat percentage, fat mass and visceral fat: a systematic review and meta-analysis. Sports Med 52(2):287–300
Rajabi A et al (2022) Effect of 8 weeks aerobic training and saffron supplementation on inflammation and metabolism in middle-aged obese women with type 2 diabetes mellitus. Sports 10(11):167
Orumiyehei A et al (2022) High-intensity interval training-induced hippocampal molecular changes associated with improvement in anxiety-like behavior but not cognitive function in rats with type 2 diabetes. Brain Sci 12(10):1280
Khajehlandi M et al (2021) Endurance training regulates expression of some angiogenesis-related genes in cardiac tissue of experimentally induced diabetic rats. Biomolecules 11(4):498
Vardar SA et al (2018) High-intensity interval training acutely alters plasma adipokine levels in young overweight/obese women. Arch Physiol Biochem 124(2):149–155
Racil G et al (2013) Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur J Appl Physiol 113(10):2531–2540
Martínez-Huenchullán SF et al (2020) Constant-moderate versus high-intensity interval training on heart adiponectin levels in high-fat fed mice: a preventive and treatment approach. Arch Physiol Biochem 1–5
Eliza J et al (2009) Antidiabetic and antilipidemic effect of eremanthin from Costus speciosus (Koen.) Sm., STZ-induced diabetic rats. Chemico-Biol Interact 182(1):67–72
Frankenberg ADv, Reis AF, Gerchman F (2017) Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: a literature review. Arch Endocrinol Metab 61:614–622
Parastesh M et al (2019) The effect of aerobic training on serum levels of adiponectin, hypothalamic-pituitary-gonadal axis and sperm quality in diabetic rats. Urol J 16(06):592–597
Rajizadeh MA et al (2019) Anti-inflammatory and anti-oxidative effects of myrtenol in the rats with allergic asthma. Iran J Pharm Res: IJPR 18(3):1488
Sims-Robinson C et al (2010) How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol 6(10):551–559
Katz A et al (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85(7):2402–2410
Ghiasi R et al (2015) Swim training improves HOMA-IR in type 2 diabetes induced by high fat diet and low dose of streptozotocin in male rats. Adv Pharm Bull 5(3):379
Biessels GJ et al (2020) Understanding multifactorial brain changes in type 2 diabetes: a biomarker perspective. Lancet Neurol 19(8):699–710
Pousti F et al (2018) Adiponectin modulates synaptic plasticity in hippocampal dentate gyrus. Neurosci Lett 662:227–232
Weisz F et al (2017) The role of adiponectin receptors in the regulation of synaptic transmission in the hippocampus. Synapse 71(5):e21964
Hahm JR et al (2020) Metabolic stress alters antioxidant systems, suppresses the adiponectin receptor 1 and induces Alzheimer’s like pathology in mice brain. Cells 9(1):249
Zhao C-J et al (2019) Effect of moxibustion at “Shenque”(CV8) and “Zusanli”(ST36) on expression of adiponectin and adiponectin receptor in Alzheimer’s disease rats. Zhen ci yan jiu = Acupuncture Res 44(6):419–423
Aleidi S et al (2015) Adiponectin serum levels correlate with insulin resistance in type 2 diabetic patients. Saudi Pharm J 23(3):250–256
Wang Y et al (2018) Plasma adiponectin levels and type 2 diabetes risk: a nested case-control study in a Chinese population and an updated meta-analysis. Sci Rep 8(1):1–13
Khoramipour K et al (2021) Metabolomics in exercise and sports: a systematic review. Sports Med 1–37
Ha CH et al (2015) Effects of combined exercise on HOMA-IR, HOMA β-cell and atherogenic index in Korean obese female. Sport Sci Health 11(1):49–55
Shaban N, Kenno K, Milne K (2014) The effects of a 2 week modified high intensity interval training program on the homeostatic model of insulin resistance (HOMA-IR) in adults with type 2 diabetes. J Sports Med Phys Fitness 54(2):203–209
Eizadi M et al (2017) Relationship between TCF7L2 relative expression in pancreas tissue with changes in insulin by high intensity interval training (HIIT) in type 2 diabetes rats. SSU_Journals 24(12):981–993
Hemmatinafar M et al (2013) The effect of high intensity interval training (HIIT) on plasma adiponectin levels, insulin sensitivity and resistance in sedentary young men. J Adv Med Biomed Res 21(84):1–12
de Mello MB et al (2022) Effect of high-intensity interval training protocols on VO2max and HbA1c level in people with type 2 diabetes: a systematic review and meta-analysis. Ann Phys Rehabil Med 65(5):101586
Hobday AL, Parmar MS (2021) The link between diabetes mellitus and tau hyperphosphorylation: implications for risk of Alzheimer’s disease. Cureus 13(9)
Zhang Y et al (2018) Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav Brain Res 339:57–65
Kubota N et al (2007) Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6(1):55–68
Rashtiani S et al (2021) Adenosine monophosphate activated protein kinase (AMPK) is essential for the memory improving effect of adiponectin. Neurosci Lett 749:135721
Barone E et al (2021) The interplay among oxidative stress, brain insulin resistance and AMPK dysfunction contribute to neurodegeneration in type 2 diabetes and Alzheimer disease. Free Radical Biol Med 176:16–33
Apró W et al (2015) Resistance exercise-induced S6K1 kinase activity is not inhibited in human skeletal muscle despite prior activation of AMPK by high-intensity interval cycling. Am J Physiol-Endocrinol Metab 308(6):E470–E481
Bartlett JD et al (2012) Matched work high-intensity interval and continuous running induce similar increases in PGC-1α mRNA, AMPK, p38, and p53 phosphorylation in human skeletal muscle. J Appl Physiol 112(7):1135–1143
Nigam SM et al (2017) Exercise and BDNF reduce Aβ production by enhancing α-secretase processing of APP. J Neurochem 142(2):286–296
Yong J et al (2021) Adiponectin as well as compressive forces regulate in vitro β-catenin expression on cementoblasts via mitogen-activated protein kinase signaling activation. Front Cell Dev Biol 9:874
Leng S et al (2010) Glycogen synthase kinase 3β mediates high glucose-induced ubiquitination and proteasome degradation of insulin receptor substrate 1. J Endocrinol 206(2):171
Salcedo-Tello P, Ortiz-Matamoros A, Arias C (2011) GSK3 function in the brain during development, neuronal plasticity, and neurodegeneration.Int J Alzheimer’s disease 2011:189728. https://doi.org/10.4061/2011/189728
Pei J-J et al (1997) Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol 56(1):70–78
Thota RN et al (2020) Dietary supplementation with curcumin reduce circulating levels of glycogen synthase kinase-3β and islet amyloid polypeptide in adults with high risk of type 2 diabetes and Alzheimer’s disease. Nutrients 12(4):1032
Acknowledgements
We would like to thank the Physiology Research Center and Neuroscience Research Center of Kerman University of Medical sciences for their financial help.
Funding
This study was funded by Physiology Research Center and Neuroscience Research Center of Kerman University of Medical Sciences (grants No. 99000443 and 99–42).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Kayvan Khoramipour, Mohammad Abbas Bejeshk, and Mohammad Amin Rajizadeh. The first draft of the manuscript was written by Kayvan Khoramipour, Mohammad Abbas Bejeshk, and Mohammad Amin Rajizadeh. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the ethics committee of KUMS (ethics approval code: IR.KMU.REC.1399.503).
Research involving Human Participants and/or Animals
Yes.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khoramipour, K., Bejeshk, M.A., Rajizadeh, M.A. et al. High-Intensity Interval Training Ameliorates Molecular Changes in the Hippocampus of Male Rats with the Diabetic Brain: the Role of Adiponectin. Mol Neurobiol 60, 3486–3495 (2023). https://doi.org/10.1007/s12035-023-03285-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03285-z