Abstract
Schizophrenia (SCZ), bipolar disorder (BD), and major depressive disorder (MDD) are common neuropsychiatric disorders that lead to neuroinflammation in the pathogenesis. It is possible to further explore the connection between inflammation in the brain and SCZ, BD, and MDD. Therefore, we systematically reviewed PubMed and Web of Science on brain inflammatory markers measured in SCZ, BD, and MDD postmortem brains. Out of 2166 studies yielded by the search, 46 studies met the inclusion criteria in SCZ, BD, and MDD postmortem brains. The results were variable across inflammatory markers. For example, 26 studies were included to measure the differential expression between SCZ and control subjects. Similarly, seven of the included studies measured the differential expression of inflammatory markers in patients with BD. The heterogeneity from the included studies is not clear at present, which may be caused by several factors, including the measured brain region, disease stage, brain source, medication, and other factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia (SCZ), bipolar disorder (BD), and major depressive disorder (MDD) are three types of common neuropsychiatric disorders that are characterized by severity and recurrence and are among the leading causes of serious self-harm or even suicidal behavior in young people [1,2,3]. In the early stages of the disease, they are usually difficult to distinguish by clinical diagnosis with symptoms overlapping across diagnoses, and shared phenotypes [4]. Although the etiology of these psychiatric disorders is still unknown and there are no effective drugs for treatment, several neuropathology [5], oligodendrocyte abnormalities [6], and metabolic disturbances [7] have been proposed. Among these underlying biological factors, several studies support the role of neuroinflammation in the pathogenesis of these mental disorders [8,9,10].
Neuroinflammation is an inflammatory response within the central nervous system (CNS) characterized by the proliferation and activation of glial cells (e.g., microglia and astrocytes) [11, 12]. Microglia are macrophages in the central nervous system that mediate innate and adaptive immune responses in the brain [13]. Under abnormal conditions such as brain infection, injury, or disease, microglia change from ramified (“resting”) state to an “activated” state, releasing proinflammatory cytokines such as interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, or several chemokines [14]. Proinflammatory cytokines released from microglia can activate astrocytes, which are generally manifested by increased glial fibrillary acidic protein (GFAP) expression [8].
Given the pathology of inflammation in the brain, most studies have attempted to elucidate the link between inflammation and psychiatric disorders during the past 20 years. For example, advances in molecular biology and genetics have shown that genes involved in regulating the immune system are highly associated with the risk of SCZ, BD, and MDD [15]. Inflammatory biomarkers derived from peripheral blood of major psychiatric diseases have been investigated by several studies, which is due to the easy accessibility of the “blood and periphery as a window to the brain” hypothesis [16]. Elevated serum and plasma levels of proinflammatory cytokines, such as IL-1β and TNF-α, have been found in SCZ and BD [17, 18]. In addition, a recent meta-analysis focused on cerebrospinal fluid (CSF) cytokines in patients with SCZ, BD, and MDD and found that CSF levels of IL-6 and IL-8 were similarly elevated in these patients [19].
Although the above studies have suggested overall similarities in the pattern of blood cytokine alterations in patients with SCZ, BD, and MDD and raise the possibility that inflammation is involved in a potential brain pathologic pathway for these mental disorders, peripheral inflammatory markers are not representative of cerebral inflammatory markers since the CNS is the ultimate site of disease. However, in the literature related to major psychiatric disorders such as SCZ, BD, and MDD, there have been a large number of reports evaluating markers related to inflammation in the brain. This makes it possible to further explore the connection between inflammation in the brain and disease. Therefore, we systematically reviewed the literature on brain inflammatory markers measured in SCZ, BD, and MDD postmortem brains to identify more elevated inflammatory markers in the postmortem brain of these patients, and to provide a preliminary conclusion on the inflammatory pathways by which postmortem brain samples of these diseases are affected.
Methods
We performed this systematic review as stated in a prospective protocol following guidelines that are recommended by the PRISMA Statement (Preferred Reporting Items for Systematic reviews) [20].
Literature Search Strategy
We performed a literature search for records indexed within PubMed (1974 to 8th May 2022) and Web of Science (1985 to 8th May 2022) using the following search terms: “(schizophrenia or bipolar disorder or major depressive disorder or depression) and (inflammation or cytokine or chemokine or interleukin or interferon or tumor necrosis factor or colony-stimulating factor) and (postmortem or brain sample).”
Eligibility Criteria
Studies were screened for relevance based on their title and abstract by two researchers independently. The full text of potentially relevant articles was retrieved and screened against the following inclusion criteria: (1) studies that focused on postmortem brain samples, including SCZ, BD, and MDD; (2) measurement of inflammation-related markers, including any kind of inflammatory cytokine/chemokines, and other related markers were considered if the authors mentioned their role in neuroinflammation; and (3) matched healthy controls were included. Duplicates and articles that did not meet the above criteria were excluded. In addition, review articles, in vitro studies, and animal studies were excluded. Finally, conference abstracts and non-English papers were also excluded.
Data Extraction
Eligible studies were assessed, the data were extracted into an Excel spreadsheet by the researcher, and any disagreements were resolved by discussion. For the eligible studies, the first author’s name, publication year, brain bank, sample size, sex, age, and death from suicide were extracted as background information. In addition to inflammatory markers measured, measuring techniques and in which brain regions the measurements were made were all extracted, along with comparative results between the patient subjects and the healthy controls. Graphical data were extracted using a Web plotter (https://apps.automeris.io/wpd/).
Given the differences in the brain regions measured, we decided that if studies measured the same parameters in the cortical (or subcortical) layer and at least two sets of data were available, we performed a systematic review of these studies. The effective size (ES) was used except where stated. ES was produced by sample size, mean concentration, and standard deviation (SD), or by sample size and P value if the data of mean concentration were not available [21]. This excluded several studies (e.g., which used medians and interquartile ranges).
Results
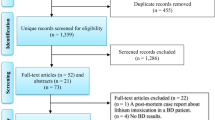
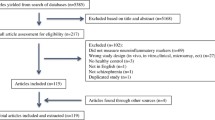
Our search strategy resulted in the identification of 2166 unique studies from the initial search. After screening the titles and abstracts for relevance, 77 articles were full-text screened against the inclusion criteria. Out of the 77 articles, 31 articles were excluded because they did not measure inflammation-related markers (12 studies); data was not separable from other diagnostic groups (8 studies); postmortem brain from the dataset (2 studies); and incorrect measurement methods, such as cDNA microarray experiments, and gene network analysis (9 studies). Thus, a total of 46 studies were ultimately included in this review (Fig. 1).
Characteristics of the Included Studies
Table 1 summarizes the basic demographics of these included studies. The incorporated studies contained relatively small numbers of subjects with BD, which may be due to the BD postmortem brain samples being scarce. Most studies involving donor subjects were defined according to the Diagnostic and Statistical Manual of Mental Disorders (DSM)-III or DSM-IV, but the remaining studies used other criteria or were not specified and were therefore not included in the statistics. Although several studies included one or more diagnostic groups, our results were still discussed by disease classification and summarized in Tables 2, 3 and 4, highlighting the main results the authors reported as being statistically significant in their study.
Studied Brain Areas
The regions of the brain studied in the included studies mainly included the anterior cingulate cortex (ACC; Brodmann area (BA)24), dorsolateral prefrontal cortex (DLPFC; BA46), frontal cortex (FC), hippocampus, orbitofrontal cortex (OFC; BA11), prefrontal cortex (PFC; BA9), medial frontal gyrus (MFG), superior frontal gyrus (SFG), and superior temporal gyrus (STG).
Immune/Inflammation Response, Cell Regulatory Proteins, Glia/Macrophage Proliferation, Metabolic Pathway, and Chemokines in Postmortem Studies of SCZ
A total of 39 studies were included to measure the difference between SCZ and control subjects. In detail, immune/inflammation response (Arion et al. [22], Dean et al. [23], Durrenberger et al. [24], Fillman et al. [25], Fillman et al. [26], Foster et al. [27], Harris et al. [28], Hoseth et al. [29], Hwang et al. [30], Iwamoto et al. [31], Izumi et al. [32], Kim et al. [33], Kindler et al. [34], Lanz et al. [35], López-González et al. [36], Maida et al. [37], Murphy et al. [38], Murphy et al. [39], Pandey et al. [40], Saetre et al. [41], Schmitt et al. [42], Toyooka et al. [43], Volk et al. [44], Volk et al. [45], Yokota et al. [46], Zhang et al. [47]), cell regulatory proteins (Abdolmaleky et al. [48], Catts et al. [49], Gibbons et al. [50]), glia/macrophage proliferation (Busse et al. [51], Purves-Tyson et al. [52], Sneeboer et al. [53], Zhang et al. [54], Zhang et al. [55]), metabolic pathway (Afia et al. [56], Tang et al. [57]), and chemokines (Hill et al. [58], Nakatani et al. [59], Volk et al. [60]) were measured in postmortem brains of SCZ. The characteristics of included studies are summarized in Table 2.
As shown in Table 2, the main regions of the postmortem brains of patients with SCZ consisted of the DLPFC, PFC, CB, HPC, temporal lobe, cortical gray, STG, etc. The different regions of brain were analyzed using polymerase chain reaction (PCR) , Western blot (WB), enzyme linked immunosorbent assay (ELISA), and immunohistochemical (IHC) methods. Therefore, the analysis of different regions of the brain showed differential expression of inflammatory factors. For example, 8 studies reported the expression of TNF-α protein levels; 3 studies on DLPFC and one for cortical gay and STG respectively found no difference in expression; one study on PFC, STG, and FC respectively found an increase. Furthermore, other inflammatory factors including IL-1β, IL-4, and IL-10 had similar results.
Immune/Inflammation Response, Cell Regulatory Proteins, Glia/Macrophage Proliferation, and Chemokines in Postmortem Studies of BD
Regarding BD, 18 studies were included. The immune/inflammation response (Bezchlibnyk et al. [61], Dean et al. [23], Fillman et al. [26], Foster et al. [27], Hoseth et al. [29], Iwamoto et al. [31], Kim et al. [62], Kim et al. [63], Kim et al. [33], Lanz et al. [35], Maida et al. [37], Nascimento et al. [64], Rao et al. [65]), cell regulatory proteins (Abdolmaleky et al. [48], Catts et al. [49]), glia/macrophage proliferation (Zhang et al. [55]), and chemokines (Hill et al. [58], Nakatani et al. [59]) were measured in postmortem brains of BD (Table 3).
Table 3 shows that the main regions of the postmortem brains of patients with BD consisted of DLPFC, FC, OFC, ACC, MFG, CB, etc. The different regions of brain were analyzed using PCR, WB, ELISA, and IHC methods. Few studies were included in the analyses of postmortem brains of BD. Therefore, the analysis of inflammatory factors was less than that of SCZ. Unlike SCZ, the postmortem brains of BD showed different expressions in the same regions of the brain in different studies. For example, two studies analyzed the expression of TNF-α protein levels in DLPFC, one study an increase, and one study no difference. This may be due to the number of deaths from suicide, technique, and/or other factors affecting the heterogeneity of the study.
Immune/Inflammation Response, Cell Regulatory Proteins, Glia/Macrophage Proliferation, and Metabolic Pathways in Postmortem Studies of MDD
As shown in Table 4, there were 21 studies included for MDD, immune/inflammation response (Böttcher et al. [66], Dean et al. [67], Dean et al. [23], Foster et al. [27], Khundakar et al. [68], Lanz et al. [35], Maida et al. [37], Martín-Hernández et al. [69], Morrison et al. [70], Pandey et al. [71], Pandey et al. [72], Pandey et al. [73], Pantazatos et al. [74], Thomas et al. [75], Torres-Platas et al. [76], Wang et al. [77], Zhao et al. [78]), cell regulatory proteins (Tanti et al. [79]), glia/macrophage proliferation (Zhang et al. [55]), and metabolic pathway (Clark et al. [80]) were measured in postmortem brains of MDD.
The main regions of the postmortem brains of patients with MDD consisted of frontal the lobe, temporal lobe, thalamus, subventricular zone, DLPFC, BA, and OC. The methodological approaches mainly used CyTOF measurements, GC/MS, PCR, WB, and IHC. Similar to SCZ, the analysis of different regions of the brain showed different expressions of inflammatory factors. For example, 10 studies reported the expression of TNF-α protein levels; four studies on the DLPFC and one for the frontal lobe, ACC, and PC respectively found no difference in expression; one study on the ACC, VLPFC, and PFC respectively found an increase, and one study for the VLPFC found a decrease. Moreover, other inflammatory factors included HLA-DR, TNFR1, and NF-κB, but only one study reported it, and therefore, it cannot be summarized effectively.
Discussion
SCZ, BD, and MDD have been linked to neuroinflammation and metabolic disorders [81], which have been shown to have aberrant blood cytokines in blood [15, 82]. This study systematically reviewed the literature reporting brain inflammatory markers in the postmortem brains of SCZ, BD, and MDD patients.
Multiple studies have evaluated neuroinflammation markers, chemokines, and microglial activation in postmortem brain samples of SCZ [8, 83, 84]. However, it is impossible to determine whether there are the abovementioned facts in postmortem brain samples of SCZ due to a large number of null studies. For example, 39 studies were included to measure the differential expression between SCZ and control subjects. Out of 26 studies that evaluated inflammatory markers, 12 examined IL-1β. Therefore, whereas eight studies found no differences, three found a decrease, and one had elevated IL-1β expression. Similarly, six studies evaluated the anti-inflammatory cytokine IL-10, three found no effect of SCZ, two studies found a decrease, and one study found an increase. Previous studies have implicated proinflammatory profiles in psychiatric disorders, where the most consistent findings were alterations in TNF-α and related pathways [85,86,87], which have been reported in peripheral blood. Thus, the researchers set out to examine TNF pathway-related molecules at the protein and mRNA levels in the postmortem brain of SCZ patients in search of a larger association. TNF-α protein levels or mRNA expression were determined in eight studies, six had no effect, and two studies found an increase. Cytokine modulators (Toll-like receptors, colony-stimulating factors, and members of the complement system) have been evaluated in several studies. Three studies reported TLR4 in the postmortem brains of SCZ patients; one study found an increase and two studies found a decrease. The analysis of different regions of the brain is one of the heterogeneous variables in studies. For example, studies evaluating IL-1β expression have analyzed nine brain regions, including the PFC, DLPFC, cortical gray, STG, FC, HPC, STC, and CFC. Therefore, eight of them with no differences included the PFC, DLPFC, cortical gray, STG, and HPC. For the PFC analysis, four studies found no differences and one found a decrease. Nevertheless, although more studies have indicated no difference in IL-1β expression in the PFC, not all studies.
SCZ is a common mental illness associated with suicide [88]. A previous study found that there was a trend in microglial density and elevated proinflammatory cytokines in SCZ [89, 90]. In this study, fewer included studies analyzed the differential expression of inflammatory factors between suicide and nonsuicide. However, there is evidence that there is a difference in the expression of inflammatory factors between suicide and nonsuicide SZ patients [40]. Previous studies have shown an effect of SCZ on neurokinin receptors compared to suicide victims, which may confound the results [91]. Although some studies have considered the impact of suicide on the measurements of inflammatory factors, many studies have not reported these data or included it in statistical analysis, which makes it a limitation. Treatment for SCZ might reduce proinflammatory markers [92]. These findings may be associated with potential effects on neuroinflammatory markers in SCZ in postmortem brains. This is noteworthy because not all studies measured antipsychotic levels at death or corrected for this potential confounding factor. Furthermore, the separation of antipsychotics was not considered in the statistical analysis. In addition, subjects in the control group were not exposed to antipsychotics, which may cause confusion between the HC group and the SCZ group.
The same effect was also observed for BD and MDD, and it is impossible to determine whether inflammatory factors were significantly expressed in postmortem brain samples of BD and MDD. For example, seven of the included studies that measured the differential expression of inflammatory markers between the BD and control groups examined TNF-α. Therefore, six studies found no differences, and one had elevated TNF-α expression. Similar to BD, the MDD results found that six studies found no effect, one decreased, and three studies found an increase. Previous studies have indicated that inflammation is documented extensively in BD and MDD [93, 94]. The heterogeneity of our systematic review may be explained by these results. One of the heterogeneous variables may be the brain region. Significant functional and structural alterations in the neural circuits of emotion or reward processing may explain the heterogeneity. During emotional, reward, and/or cognitive related tasks, different activation patterns occur in the neural network including the amygdala, ACC, PFC, and striatum [95]. In addition, different stages of BD and MDD have distinct neurobiological changes in the related brain regions [96, 97]. Similar to SCZ, the treatments for inflammation also influence the expression of neuroinflammation markers. Aspirin has beneficial effects in clinical trials of mood disorders; it inhibits the inflammatory response and reduces the levels of inflammatory biomarkers, including C-reactive protein, TNF-α, and IL-6 [98]. These missing treatment statistics may influence the effects on neuroinflammatory markers in BD and MDD in postmortem brains. Another variable may be the differences in the methodological approaches. Although most techniques of the included studies were PCR and WB, other detection methods such as CyTOF measurements may contribute to the heterogeneous results. A previous study showed that the kynurenine pathway in the CNS of suicide attempts is chronically dysregulated, and an increase in inflammatory cytokines is associated with more severe symptoms [99]. In addition, a similar study also reported on BD, which is related to baseline biomarkers of suicide attempts with clinical outcomes [100]. Therefore, it is important to consider elevated proinflammatory cytokines in postmortem brains of suicide victims, which may confound the results. Several other confounding factors, such as age, lifestyle choices, and brain banks (diagnostic methods, storage, inclusion and exclusion criteria, and many other variables), may also need consideration.
These findings indicate that inflammatory markers appear to have different expression patterns in each psychiatric disease, which is of great significance for us to realize the pathophysiology of inflammatory markers in major psychiatric diseases and provide new directions for therapy. However, because the samples all came from postmortem brains, there was no record of the use of antipsychotic drugs before death. It is a limitation of this paper that the influence of antipsychotic drugs on the expression of the protein and molecules in the samples cannot be taken into account in statistics.
In conclusion, although numerous included studies have noted a lack of changes in neuroinflammatory markers in postmortem brain samples of SCZ, BD, and MDD, there are still multiple studies that have indicated an increase or decrease in neuroinflammatory markers. The heterogeneity is not clear at present, and may be caused by several factors, including the measured brain region, disease stage, brain source, medication, and other factors. The expression of neuroinflammatory markers was different, which means that inflammation was accompanied by the occurrence of neuropsychiatric disorders. Whether the inflammation in the brain is the pathogeny of schizophrenia, bipolar disorder, and major depressive disorder or the pathological manifestations of these diseases. According to some preclinical studies [101, 102], after some anti-inflammatory treatment, the neuropsychiatric disorder symptoms have improved significantly. This finding revealed that inflammation in the brain may be the pathogenesis of schizophrenia, bipolar disorder, and major depressive disorder.
In the future, these potential sources of heterogeneity should be considered to measure neuroinflammatory markers in postmortem brain samples for patients with SCZ, BD, and MDD, which will contribute to the successful construction of a similar study.
Data availability
All data and materials are contained within the manuscript.
References
Carvalho AF, Firth J, Vieta E (2020) Bipolar disorder. N Engl J Med 383:58–66
Kahn RS et al (2015) Schizophrenia. Nat Rev Dis Primers 1:15067
Otte C et al (2016) Major depressive disorder. Nat Rev Dis Primers 2:16065
Meyer JH et al (2020) Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry 7:1064–1074
Harrison PJ, Colbourne L, Harrison CH (2020) The neuropathology of bipolar disorder: systematic review and meta-analysis. Mol Psychiatry 25:1787–1808
Karoutzou G, Emrich HM, Dietrich DE (2008) The myelin-pathogenesis puzzle in schizophrenia: a literature review. Mol Psychiatry 13:245–260
MacDonald K et al (2019) Biomarkers for major depressive and bipolar disorders using metabolomics: a systematic review. Am J Med Genet B Neuropsychiatr Genet 180:122–137
Trépanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP (2016) Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Molec Psychiatry 21:1009–1026
Giridharan VV et al (2020) Postmortem evidence of brain inflammatory markers in bipolar disorder: a systematic review. Mol Psychiatry 25:94–113
Enache D, Pariante CM, Mondelli V (2019) Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun 81:24–40
Narayanaswami V et al (2018) Emerging PET radiotracers and targets for imaging of neuroinflammation in neurodegenerative diseases: outlook beyond TSPO. Mol Imaging 17:1536012118792317
Carriba P, Comella JX (2015) Neurodegeneration and neuroinflammation: two processes, one target. Neural Regen Res 10:1581–1583
Wong M (2019) The role of glia in epilepsy, intellectual disability, and other neurodevelopmental disorders in tuberous sclerosis complex. J Neurodev Disord 11:30
Cherry JD, Olschowka JA, O’Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98
Goldsmith DR, Rapaport MH, Miller BJ (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21:1696–1709
Liu SH, Shi XJ, Fan FC, Cheng Y (2021) Peripheral blood neurotrophic factor levels in children with autism spectrum disorder: a meta-analysis. Sci Rep 11:15
Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B (2011) Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70:663–671
Sayana P et al (2017) A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J Psychiatr Res 92:160–182
Wang AK, Miller BJ (2018) Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull 44:75–83
Liberati A et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1-34
Cohen J (1992) A power primer. Psychol Bull 112:155–159
Arion D, Unger T, Lewis DA, Levitt P, Mirnics K (2007) Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry 62:711–721
Dean B et al (2013) Different changes in cortical tumor necrosis factor-α-related pathways in schizophrenia and mood disorders. Mol Psychiatry 18:767–773
Durrenberger PF et al (2015) Common mechanisms in neurodegeneration and neuroinflammation: a BrainNet Europe gene expression microarray study. J Neural Transmiss (Vienna, Austria : 1996) 122:1055–1068
Fillman SG et al (2013) Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 18:206–214
Fillman SG, Sinclair D, Fung SJ, Webster MJ, Shannon Weickert C (2014) Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Translat Psychiatry 4:e365
Foster R et al (2006) Calprotectin in microglia from frontal cortex is up-regulated in schizophrenia: evidence for an inflammatory process? Eur J Neurosci 24:3561–3566
Harris LW et al (2012) Comparison of peripheral and central schizophrenia biomarker profiles. PloS one 7:e46368
Hoseth EZ et al (2017) A study of TNF pathway activation in schizophrenia and bipolar disorder in plasma and brain tissue. Schizophrenia Bull 43:881–890
Hwang Y et al (2013) Gene expression profiling by mRNA sequencing reveals increased expression of immune/inflammation-related genes in the hippocampus of individuals with schizophrenia. Translat Psychiatry 3:e321
Iwamoto K, Kakiuchi C, Bundo M, Ikeda K, Kato T (2004) Molecular characterization of bipolar disorder by comparing gene expression profiles of postmortem brains of major mental disorders. Mol Psychiatry 9:406–416
Izumi R et al (2021) Detailed postmortem profiling of inflammatory mediators expression revealed post-inflammatory alternation in the superior temporal gyrus of schizophrenia. Front Psychiatry 12:653821
Kim HK, Andreazza AC, Elmi N, Chen W, Young LT (2016) Nod-like receptor pyrin containing 3 (NLRP3) in the post-mortem frontal cortex from patients with bipolar disorder: a potential mediator between mitochondria and immune-activation. J Psychiatric Res 72:43–50
Kindler J et al (2020) Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry 25:2860–2872
Lanz TA et al (2019) Postmortem transcriptional profiling reveals widespread increase in inflammation in schizophrenia: a comparison of prefrontal cortex, striatum, and hippocampus among matched tetrads of controls with subjects diagnosed with schizophrenia, bipolar or major depressive disorder. Translat Psychiatry 9:151
López-González I et al (2019) Neuroinflammation in the dorsolateral prefrontal cortex in elderly chronic schizophrenia. Eur Neuropsychopharmacol 29:384–396
Maida ME, Hurley SD, Daeschner JA, Moore AH, O’Banion MK (2006) Cytosolic prostaglandin E2 synthase (cPGES) expression is decreased in discrete cortical regions in psychiatric disease. Brain Res 1103:164–172
Murphy CE et al (2020) Nuclear factor kappa B activation appears weaker in schizophrenia patients with high brain cytokines than in non-schizophrenic controls with high brain cytokines. J Neuroinflamm 17:215
Murphy CE et al (2020) Regional, cellular and species difference of two key neuroinflammatory genes implicated in schizophrenia. Brain, Behav Immun 88:826–839
Pandey GN, Rizavi HS, Zhang H, Ren X (2018) Abnormal gene and protein expression of inflammatory cytokines in the postmortem brain of schizophrenia patients. Schizophrenia Res 192:247–254
Saetre P et al (2007) Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry 7:46
Schmitt A et al (2011) Regulation of immune-modulatory genes in left superior temporal cortex of schizophrenia patients: a genome-wide microarray study. World J Biol Psychiatry 12:201–215
Toyooka K et al (2003) A decrease in interleukin-1 receptor antagonist expression in the prefrontal cortex of schizophrenic patients. Neurosci Res 46:299–307
Volk DW et al (2015) Molecular mechanisms and timing of cortical immune activation in schizophrenia. Am J Psychiatry 172:1112–1121
Volk DW, Moroco AE, Roman KM, Edelson JR, Lewis DA (2019) The role of the nuclear factor-κB transcriptional complex in cortical immune activation in schizophrenia. Biol Psychiatry 85:25–34
Yokota O et al (2004) Neuronal expression of cyclooxygenase-2, a pro-inflammatory protein, in the hippocampus of patients with schizophrenia. Progr Neuro-Psychopharmacol Biol Psychiatry 28:715–721
Zhang Y et al (2016) Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Translat Psychiatry 6:e982
Abdolmaleky HM et al (2019) Aberrant transcriptomes and DNA methylomes define pathways that drive pathogenesis and loss of brain laterality/asymmetry in schizophrenia and bipolar disorder. Am J Med Genet B, Neuropsychiatric 180:138–149
Catts VS, Weickert CS (2012) Gene expression analysis implicates a death receptor pathway in schizophrenia pathology. PloS one 7:e35511
Gibbons AS, Hoyer D, Dean B (2021) SMAD4 protein is decreased in the dorsolateral prefrontal and anterior cingulate cortices in schizophrenia. World J Biol Psychiatry 22:70–77
Busse S et al (2012) Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain, Behav Immun 26:1273–1279
Purves-Tyson TD et al (2020) Increased macrophages and C1qA, C3, C4 transcripts in the midbrain of people with schizophrenia. Front Immunol 11:2002
Sneeboer MAM et al (2020) Microglial activation in schizophrenia: is translocator 18 kDa protein (TSPO) the right marker? Schizophrenia Res 215:167–172
Zhang L, Verwer RWH, Lucassen PJ, Huitinga I, Swaab DF (2020) Prefrontal cortex alterations in glia gene expression in schizophrenia with and without suicide. J Psychiatric Res 121:31–38
Zhang J, Chang L, Pu Y, Hashimoto K (2020) Abnormal expression of colony stimulating factor 1 receptor (CSF1R) and transcription factor PU.1 (SPI1) in the spleen from patients with major psychiatric disorders: a role of brain-spleen axis. J Affect Disorders 272:110–115
Afia AB et al (2021) Kynurenine pathway in post-mortem prefrontal cortex and cerebellum in schizophrenia: relationship with monoamines and symptomatology. J Neuroinflamm 18:198
Tang B, Capitao C, Dean B, Thomas EA (2012) Differential age- and disease-related effects on the expression of genes related to the arachidonic acid signaling pathway in schizophrenia. Psychiatry Res 196:201–206
Hill SL, Shao L, Beasley CL (2021) Diminished levels of the chemokine fractalkine in post-mortem prefrontal cortex in schizophrenia but not bipolar disorder. World J Biol Psychiatry 22:94–103
Nakatani N et al (2006) Genome-wide expression analysis detects eight genes with robust alterations specific to bipolar I disorder: relevance to neuronal network perturbation. Human Molec Genet 15:1949–1962
Volk DW, Chitrapu A, Edelson JR, Lewis DA (2015) Chemokine receptors and cortical interneuron dysfunction in schizophrenia. Schizophrenia Res 167:12–17
Bezchlibnyk YB, Wang JF, McQueen GM, Young LT (2001) Gene expression differences in bipolar disorder revealed by cDNA array analysis of post-mortem frontal cortex. J Neurochem 79:826–834
Kim HW, Rapoport SI, Rao JS (2010) Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis 37:596–603
Kim HW, Rapoport SI, Rao JS (2011) Altered arachidonic acid cascade enzymes in postmortem brain from bipolar disorder patients. Molec Psychiatry 16:419–428
Nascimento C et al (2020) Differential levels of inflammatory and neuroendocrine markers in the hippocampus and anterior cingulate cortex of bipolar disorder subjects: a post-mortem study. Brain, Behav Immun 90:286–293
Rao JS, Harry GJ, Rapoport SI, Kim HW (2010) Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Molec Psychiatry 15:384–392
Böttcher C et al (2020) Single-cell mass cytometry of microglia in major depressive disorder reveals a non-inflammatory phenotype with increased homeostatic marker expression. Translat Psychiatry 10:310
Dean B, Tawadros N, Scarr E, Gibbons AS (2010) Regionally-specific changes in levels of tumour necrosis factor in the dorsolateral prefrontal cortex obtained postmortem from subjects with major depressive disorder. J Affect Disorders 120:245–248
Khundakar A, Morris C, Slade J, Thomas AJ (2011) Examination of glucose transporter-1, transforming growth factor-β and neuroglobin immunoreactivity in the orbitofrontal cortex in late-life depression. Psychiatry Clin Neurosci 65:158–164
Martín-Hernández D et al (2018) Intracellular inflammatory and antioxidant pathways in postmortem frontal cortex of subjects with major depression: effect of antidepressants. J Neuroinflamm 15:251
Morrison FG et al (2019) Reduced interleukin 1A gene expression in the dorsolateral prefrontal cortex of individuals with PTSD and depression. Neurosci Lett 692:204–209
Pandey GN, Rizavi HS, Ren X, Bhaumik R, Dwivedi Y (2014) Toll-like receptors in the depressed and suicide brain. J Psychiatric Res 53:62–68
Pandey GN, Rizavi HS, Bhaumik R, Ren X (2019) Innate immunity in the postmortem brain of depressed and suicide subjects: role of Toll-like receptors. Brain, Behav Immun 75:101–111
Pandey GN, Zhang H, Sharma A, Ren X (2021) Innate immunity receptors in depression and suicide: upregulated NOD-like receptors containing pyrin (NLRPs) and hyperactive inflammasomes in the postmortem brains of people who were depressed and died by suicide. J Psychiatry Neurosci 46:E538-e547
Pantazatos SP et al (2017) Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: evidence for altered glial, endothelial and ATPase activity. Molec Psychiatry 22:760–773
Thomas AJ et al (2000) Elevation in late-life depression of intercellular adhesion molecule-1 expression in the dorsolateral prefrontal cortex. Am J Psychiatry 157:1682–1684
Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N (2014) Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain, Behav Immun 42:50–59
Wang Q, Roy B, Turecki G, Shelton RC, Dwivedi Y (2018) Role of complex epigenetic switching in tumor necrosis factor-α upregulation in the prefrontal cortex of suicide subjects. Am J Psychiatry 175:262–274
Zhao J et al (2015) Different stress-related gene expression in depression and suicide. J Psychiatric Res 68:176–185
Tanti A et al (2019) Evidence of decreased gap junction coupling between astrocytes and oligodendrocytes in the anterior cingulate cortex of depressed suicides. Neuropsychopharmacology 44:2099–2111
Clark SM et al (2016) Reduced kynurenine pathway metabolism and cytokine expression in the prefrontal cortex of depressed individuals. J Psychiatry Neurosci 41:386–394
Hughes HK, Ashwood P (2020) Overlapping evidence of innate immune dysfunction in psychotic and affective disorders. Brain, Behav Immun - Health 2:100038
Chen L et al (2021) Altered peripheral immune profiles in first-episode, drug-free patients with schizophrenia: response to antipsychotic medications. Front Med 8:757655
Shinko Y et al (2020) Chemokine alterations in the postmortem brains of suicide completers. J Psychiatric Res 120:29–33
Gober R et al (2022) Microglia activation in postmortem brains with schizophrenia demonstrates distinct morphological changes between brain regions. Brain Pathol (Zurich, Switzerland) 32:e13003
Munkholm K, Brauner JV, Kessing LV, Vinberg M (2013) Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res 47:1119–1133
Dean B et al (2013) Different changes in cortical tumor necrosis factor-alpha-related pathways in schizophrenia and mood disorders. Mol Psychiatry 18:767–773
Hoseth EZ et al (2017) A study of TNF pathway activation in schizophrenia and bipolar disorder in plasma and brain tissue. Schizophr Bull 43:881–890
Sher L, Kahn RS (2019) Suicide in schizophrenia: an educational overview. Medicina-lithuania 55(7)
Steiner J et al (2008) Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatric Res 42:151–157
Pandey GN et al (2012) Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatric Res 46:57–63
Tooney PA, Crawter VC, Chahl LA (2001) Increased tachykinin NK(1) receptor immunoreactivity in the prefrontal cortex in schizophrenia. Biol Psychiatry 49:523–527
Zhang L et al (2019) The effect of minocycline on amelioration of cognitive deficits and pro-inflammatory cytokines levels in patients with schizophrenia. Schizophrenia Res 212:92–98
van den Ameele S et al (2018) Markers of inflammation and monoamine metabolism indicate accelerated aging in bipolar disorder. Front Psychiatry 9:250
Martinuzzi E et al (2021) Blood cytokines differentiate bipolar disorder and major depressive disorder during a major depressive episode: initial discovery and independent sample replication. Brain, Behav Immun - Health 13:100232
Han KM, De Berardis D, Fornaro M, Kim YK (2019) Differentiating between bipolar and unipolar depression in functional and structural MRI studies. Progr Neuro-Psychopharmacol Biol Psychiatry 91:20–27
Keramatian K, Su W, Saraf G, Chakrabarty T, Yatham LN (2021) Preservation of gray matter volume in early stage of bipolar disorder: a case for early intervention: Préservation du volume de matière grise au stade précoce du trouble bipolaire: un cas pour intervention précoce. Canadian journal of psychiatry. Revue Canadienne de Psychiatrie 66:139–146
Sawamura D et al (2020) Microstructural alterations in bipolar and major depressive disorders: a diffusion kurtosis imaging study. J Magnet Reson Imaging : JMRI 52:1187–1196
Berk M et al (2013) Aspirin: a review of its neurobiological properties and therapeutic potential for mental illness. BMC Med 11:74
Bay-Richter C et al (2015) A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain, Behav Immun 43:110–117
Isgren A et al (2017) Markers of neuroinflammation and neuronal injury in bipolar disorder: relation to prospective clinical outcomes. Brain, Behav Immun 65:195–201
Leng L, Zhuang K, Liu Z et al (2018) Menin deficiency leads to depressive-like behaviors in mice by modulating astrocyte-mediated neuroinflammation. Neuron. 100(3):551–563
Guo L, Xiao P, Zhang X et al (2021) Inulin ameliorates schizophrenia via modulation of the gut microbiota and anti-inflammation in mice. Food Funct. 12(3):1156–1175
Acknowledgements
We acknowledge the support of the National Science Foundation of China (82071676, 81703492) to Professor Yong Cheng.
Funding
This study was supported by the National Science Foundation of China (82071676, 81703492).
Author information
Authors and Affiliations
Contributions
Yong Cheng and Qingshan Liu designed this study; Yangwen Ai and Shu-Han Liu searched the database and identified eligible studies; Yang Du and Lei Chen extracted the data; Yangwen Ai drafted the manuscript. All authors have read and agree with the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ai, Yw., Du, Y., Chen, L. et al. Brain Inflammatory Marker Abnormalities in Major Psychiatric Diseases: a Systematic Review of Postmortem Brain Studies. Mol Neurobiol 60, 2116–2134 (2023). https://doi.org/10.1007/s12035-022-03199-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03199-2