Abstract
There have been a large number of reports about glial cell dysfunction being related to major psychiatric diseases such as schizophrenia (SCZ), bipolar disorder (BD), and major depressive disorder (MDD). In this review, we provide an overview of postmortem studies analyzing the structural changes of glial cells in these three major psychiatric diseases, including the density, number and size of glial cells, and the expression of related markers. Up to May 1, 2021, 108 articles that met the inclusion criteria were identified by searching PubMed and Web of Science. Although most studies evaluating total glial cells did not show abnormalities in the brains of postmortem patients, astrocytes, microglial cells, and oligodendrocytes seem to have specific patterns of changes in each disease. For example, out of 20 studies that evaluated astrocyte markers in MDD, 11 studies found decreased astrocyte marker expression in MDD patients. Similarly, out of 25 studies evaluating oligodendrocyte markers in SCZ, 15 studies showed decreased expression of oligodendrocyte markers in different brain regions of SCZ patients. In addition, activated microglial cells were observed in patients with SCZ, BD, and MDD, but suicide may be a confounding factor for the observed effects. Although the data from the included studies were heterogeneous and this cannot be fully explained at present, it is likely that there are a variety of contributing factors, including the measured brain regions, methods of measurement, gender, age at time of death, and medications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
George Somjen was prescient in his comments about glial cells in 1988 [1]. Indeed, the crucial role of glia in psychiatric diseases has been neglected over the past two decades [2, 3]. Glial cells are involved in every major aspect of brain development, function, and disease by communicating with neurons and releasing neurotransmitters and other signals [4]. In the central nervous system (CNS), astrocytes, oligodendrocytes, and microglia are the three main types of glial cells, which play an important role in synaptic function, neuronal metabolism and migration [3]. Over the years, there has been a shift in our understanding of the relative number of glial cells, and detailed studies suggest that the proportions vary greatly by brain region [5]. Schizophrenia (SCZ), bipolar disorder (BD), and major depressive disorder (MDD) are three major psychiatric diseases, with some similarities in their occurrence and symptoms, at least in the early stages [6,7,8]. A growing body of research is attempting to clarify the pathogenesis associated with these mental disorders. Genetic studies have shown a high correlation between multiple genes involved in the regulation of the immune system and SCZ, BD, and MDD [9]. Postmortem evidence supports the role of cerebral inflammation in the etiological pathways of these mental disorders [10,11,12]. Nevertheless, the neurobiological mechanisms underlying these diseases are still not fully understood.
In the literature related to major psychiatric diseases such as SCZ, BD, and MDD, there have been a large number of reports on glial cell dysfunction, which undoubtedly provides evidence of a relationship between psychiatric disease and glial pathology [13,14,15]. However, there are problems in these studies, such as the diversity of results due to differences in the studied brain regions and research methods. It is difficult to draw straightforward conclusions based on these results. To help improve our understanding of the pathogenesis and pathophysiology of psychiatric diseases, we systematically reviewed postmortem studies on the structure of brain cells in patients with SCZ, BD, and MDD. The density, number, and size of the astrocytes, microglia, and oligodendrocytes, as well as the expression of cell type-specific markers in postmortem brain samples, were evaluated. By emphasizing the importance of glial cells in these mental diseases, we have provided a preliminary conclusion on the changes of glial cells in the postmortem brain and a guideline for future studies to improve our understanding of the mechanism of mental diseases and to identify new therapeutic targets.
Methods
We performed a literature search for records indexed within PubMed and Web of Science up to May 1, 2021. The search strategy was ‘(glia or microglia or astrocytes or oligodendrocytes) and (schizophrenia or bipolar disorder or major depressive disorder or depression) and (postmortem or brain sample)’.
Studies were screened for relevance based on their title and abstract by two researchers independently. The full text of potentially relevant articles was retrieved and screened against the following inclusion criteria: (1) studies that focused on postmortem brain samples in SCZ, BD, or MDD; (2) measured glial cells, including microglia, astrocytes, or oligodendrocytes, as well as several related markers; and (3) matched psychiatric-disease-free controls. Studies with matched samples (by age, sex, race, brain pH or postmortem interval, etc.) [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109] or studies that statistically adjusted for these specific variables (age and sex primarily) [110,111,112,113,114,115,116] were included. Duplicates and studies that did not meet the above criteria were excluded. In addition, review articles, in vitro studies, and animal studies were excluded.
Eligible studies were assessed and the data were extracted into an Excel spreadsheet by the researcher, and any disagreements were resolved by discussion. For the eligible studies, the first author’s name, publication year, brain bank, sample size, sex, age, and death from suicide were extracted as background information. In addition to glial cell markers measured, measuring techniques and in which brain regions the measurements were made were all extracted, along with comparative results between the patient subjects and the healthy controls.
Results
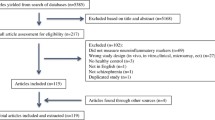
Our search strategy resulted in the identification of 1070 unique studies from the initial search. After screening of the titles and abstracts for relevance, 175 articles were full-text screened against the inclusion criteria. Out of the 175 articles, 69 articles were excluded because they did not measure glial cells (50 studies); data not separable from other diagnostic groups (9 studies); did not include any psychopaths (6 studies) and healthy controls (2 studies); and incorrect measurement methods, such as single-cell sequencing (2 studies). Two more articles were found in the reference section of papers identified in the database search. Thus, a total of 108 studies were ultimately included in this review, including 31 studies on total glial cells that did not differentiate between glial cell types, 41 studies on astrocytes, 24 studies on microglia, and 30 studies on oligodendrocytes (Fig. 1).
The regions of the brain studied among our reviewed papers mainly included the anterior cingulate cortex (ACC), anterior cingulate gyrus (ACG), anterior midcingulate cortex (AMC), anteroventral nucleus (AVN), corpus callosum (CC), caudate nucleus (CN), dorsolateral prefrontal cortex (DLPFC), dorsal raphe nucleus (DRN), entorhinal cortex (EC), frontal cortex (FC), hippocampus, locus coeruleus (LC), medial frontal gyrus (MFG), occipital cortex (OC), orbitofrontal cortex (OFC), prefrontal cortex (PFC), superior frontal gyrus (SFG), superior temporal gyrus (STG), temporal cortex (TC), amygdala, cerebellum, putamen, subiculum, and thalamus (Table 1; Figs. 2 and 3).
Total Glia
In our review, a total of 31 studies evaluated total glial cells (astrocytes, microglia, oligodendrocytes) in postmortem brain samples without the use of cell type-specific markers.
Several studies looking at total glia found that decreased cell density was associated with SCZ. Cotter et al. [37] published their first study in 2001, with glial cell density measured by cresyl violet staining, and observed a decrease in layer VI of the ACC in SCZ and MDD compared to healthy controls, whereas no change was observed in BD. In their second year of study [35], comparable effects were observed again in layer V of the DLPFC. However, in their two subsequent studies, neither Heschl’s gyrus [36] nor OFC [34] observed evidence for changes in glial cell size or density between the three groups of mental diseases and healthy controls. Beasley et al. [18] published a study on the effects on glial cells in 2009. In the white matter near the temporal plane of 15 patients in each group, they found that only schizophrenic patients, not the BD or MDD patients, had a lower glial cell density than the control subjects. Similar decreases in glial cell density were observed in the CA3 and CA4 regions of the hippocampus [41] and layer III of the primary motor cortex [19] of patients with SCZ.
In fact, changes in glial cells were also observed in postmortem brains from BD and MDD patients. For example, Rajkowska et al. [73] found that the density of glial cells decreased in sublayer IIIc of the DLPFC in patients with BD, coupled with enlargement and changes in the shape of the glial nuclei. Brauch et al. [24] also found that the area occupied by the glial cells was reduced in the TC of bipolar patients compared with that of healthy controls. In a study on the amygdala, Nissl staining showed that the density of glial cells decreased only in the MDD group, while there was no significant difference between the BD and control groups [50]. In contrast, glial cell density in the hippocampus was significantly increased in 19 major depressive patients compared with healthy controls [91].
Twenty-two studies evaluating glial cells did not detect any changes associated with SCZ, BD, and MDD. In a cohort of 18 schizophrenic patients [29], there was no difference in the numbers of glial cells in the PFC and ACC, regardless of whether the patients had superimposed mood disturbances. In another cohort of 18 schizophrenic patients, quantified analysis after hematoxylin and eosin staining showed no difference in glial cell density in the CC compared to healthy controls. However, it is worth mentioning they found gliosis in the CC in patients with late-onset SCZ compared with early-onset SCZ and controls [68]. In a study of postmortem brain samples from the Stanley Foundation Neuropathology Consortium, for SCZ, BD, and MDD, Nissl staining revealed no differences in the glial cell density or size in the amygdala [22] and no changes in glial number in the TC [24]. Similarly, whether from the EC [42], PFC [113], OC [113], dentate gyrus [23], fusiform gyrus [78], FC [82], DLPFC [82], lateral geniculate nucleus [83], and primary auditory cortex [87] of SCZ or the OC [75], basolateral amygdala [78, 114], and auditory cortex [87] of MDD, Nissl staining failed to detect any changes in the density or number of glial cells. Cresyl violet staining revealed no changes in glial cell density in the ACC [20, 30] and PFC [117] from SCZ or BD. Gallocyanin, another staining technique, also did not detect an effect of SCZ on glial cell density in the dorsal ACC [53]. Moreover, in Khundakar’s four studies of the MDD postmortem brain, no changes in glial cell density were obtained in the DLPFC [56], OFC [57], CN [58], and ACG [59] compared with healthy controls.
Astroglia
Our search strategy yielded a total of 41 studies assessing astrocytes in postmortem brain samples from patients with major psychiatric diseases.
Glial fibrillary acidic protein (GFAP) is often used as an astrocyte-specific marker in postmortem studies [118]. Among 41 studies, 34 studies evaluated differences in astrocytes by measuring the expression of GFAP or the distribution of immunoreactivity.
The first study to evaluate GFAP was published in 1993. Karson et al. [54] measured the protein level of GFAP in the brains of 25 patients with SCZ by western blot and found no differences in multiple brain regions, including the FC, STG, OC, cerebellum, thalamus, and pons relative to healthy controls. Fatemi et al. [46] later published a study of lateral cerebellum samples from the Stanley Foundation Brain Collection, in which they found that GFAP protein levels were reduced to different degrees in SCZ, BD and MDD. However, only the MDD value remained statistically significant following correction. This is in agreement with several subsequent studies, where reduced GFAP protein levels were observed in the DLPFC [86], LC [119], and OFC [66] of MDD. However, different from the result observed in MDD, two studies detected increased protein levels of GFAP in the DLPFC [47, 63] in SCZ patients compared to healthy controls. Similar increases were also observed in the DLPFC [47] and FC [77] in BD patients.
Similar to the research on GFAP mentioned above, several studies have reported increased GFAP mRNA expression in the brain from SCZ and BD. For example, Farnsworth et al. [110] found that schizophrenic patients had increased GFAP mRNA levels in the PFC and SFG. Qi et al. [72] investigated the expression of GFAP mRNA in the gray matter isolated from the ACC and DLPFC samples from patients with BD and MDD and found that only BD patients had significantly higher levels of GFAP in the ACC than control subjects. The authors did note, however, a lower area fraction of GFAP immunoreactive astrocytes in the ACC of BD. Although GFAP mRNA, as measured by a riboprobe, was decreased in the white matter of the ACC in SCZ and BD [106], Barley et al. [17] again observed that the levels of GFAP mRNA were increased in several brain regions including AVN, putamen, internal capsule, and mediodorsal thalamic nuclei, in patients with SCZ and MDD. In contrast, most studies evaluating GFAP mRNA expression of various brain regions of MDD, including the LC [21], gray matter of the PFC [67], mediodorsal thalamus [97], and CN [97], detected a reduced GFAP mRNA expression in MDD. Not all studies, however, have detected differences in GFAP mRNA expression. For example, no changes were detected between SCZ patients and healthy controls in several brain regions, including the PFC [93], MFG [115], CC [85], and DLPFC [62]. Similarly, no changes in GFAP mRNA were observed in the DLPFC and ACC in patients with BD compared with healthy controls [109].
Other studies have assessed GFAP expression by immunohistochemical analysis. Rajkowska et al. [74] published a study in 2002 of a cohort of 9 schizophrenic brains and found that GFAP-positive astrocyte density in layer V of the DLPFC increased by 81%, whereas the GFAP labeling area was reduced by 32%. These changes were layer-specific, as no difference was detected between layers III and IV. This is slightly different from what two subsequent studies both observed that there were no differences in the density of GFAP cells in the DLPFC [43, 51] of patients with SCZ, although Hercher et al. [51] did find a decreased GFAP fraction area and increased cell clustering in the DLPFC in their study. Similarly, many quantitative studies found no differences in the density of GFAP cells in multiple other brain regions in patients with SCZ, including the hippocampus [69], EC [39, 69, 120, 121], amygdala [39], ACG [43], subiculum [69, 120], STG [43], primary motor cortex [120], DLPFC [28, 43], and subventricular zone [120]. Although Williams [108] and his colleagues examined the cellular structure of the substantia nigra in the postmortem brain with schizophrenia and MDD and found a decrease in astrocyte density in SCZ patients relative to healthy controls, this effect was not detected in patients with MDD.
However, many quantitative immunohistochemical studies have detected a decrease in the density of cells expressing GFAP in the brains of patients with MDD. For instance, Altshuler et al. [16] found a decrease in the density of GFAP-immunoreactive astrocytes in the amygdala of subjects with MDD compared to SCZ, BD, and healthy control postmortem samples. Similarly, in the study of Cobb et al. [33], the authors found that the density of GFAP-immunoreactive astrocytes in the dentate gyrus of MDD patients who did not take antidepressants was significantly lower than that in controls, whereas no change was found in CA1 or CA2/3. Other studies have also found that MDD patients had a lower GFAP-immunoreactive astrocyte density in the LC [119], DLPFC, dorsal CN, and MTN [70]. Nevertheless, not all immunohistochemical studies have detected this effect in MDD. No changes in GFAP cell density in several brain regions, including the ACC [38], hippocampus [48, 112], DLPFC [65], and substantia nigra [108], were observed in other studies.
Other astrocytic markers have also been measured in postmortem brain specimen from psychosis. With the increase in GFAP, aldehyde dehydrogenase (ALDH)1 mRNA in the SCZ and MDD was increased in several brain regions, including the putamen, AVN, internal capsule, and mediodorsal thalamic nucleus [17]. In contrast, two studies found no change in ALDH1 L1 mRNA in the DLPFC of patients with SCZ and BD [47, 109], whereas one other study detected a decrease in the PFC gray matter of MDD [67].
Hamidi and his colleagues examined the astrocyte marker S100b [50]. They did not observe changes in astrocyte density in the amygdala of patients with BD and MDD. Gos et al. [48], however, did find that the numerical density of S100b-immunopositive astrocytes was significantly decreased in the CA1 pyramidal layer in patients with BD and MDD compared to healthy controls. S100b has been measured by qPCR in a few other studies with mixed results. One study found a decrease in S100b mRNA in the LC of patients with MDD [21], and another found no change in the ACC and DLPFC in either BD or MDD [109].
Astrocytes have also been identified in postmortem brain by microscopic analysis with other staining techniques. In a cohort of 10 depressed suicides from the Quebec Suicide Brain Bank, Golgi-impregnated fibrous astrocytes had significantly larger cell bodies and longer, more ramified processes in depressed suicides [96]. This is consistent with another study, where examining astrocyte morphology by immunohistochemistry showed that astrocytes in both the thalamus and CN displayed larger cell bodies and extended more ramified processes across larger domains than cortical astrocytes [97]. However, two stereological counting studies on Nissl-stained astrocytes showed no differences in the number of hippocampal astrocytes, whether in SCZ, BD or MDD [63, 79, 122].
Microglia
From our search, a total of 24 studies assessed microglial marker in postmortem brain samples. Out of these 24 studies, 11 studies reported increased microglial markers in the postmortem brain, whereas 6 studies reported a decrease, and 7 studies found no change.
Cluster of differentiation (CD) is a microglial marker, and multiple studies have found lower CD gene expression in postmortem brains associated with SCZ. For example, in one study, downregulation of CD68 mRNA levels in the DLPFC was detected by qPCR in SCZ patients compared with healthy controls [62]. This is similar to the results of a recent study [85] in which CD68 mRNA levels were also decreased in the CC of SCZ. Similar decreases in the expression of CD68 and CD11b mRNA were observed in the ACC of BD patients [84], despite the expression of CD11b mRNA and protein in the FC was increased [77]. Moreover, Zhang et al. [109] reported a decrease in CD68 mRNA in the DLPFC of BD patients without suicide compared to BD patients with suicide and controls. This effect, however, was not seen in the ACC. Similarly, no difference in CD68 mRNA was detected in the dorsal ACC in MDD [95].
HLA antigen D-related (DR) is an immunohistochemical marker that specifically reacts with activated microglial cells. Bayer et al. [120] found that 3 of 14 schizophrenic patients had positive HLA-DR staining in the hippocampus and FC. This is in agreement with a study conducted the following year, where HLA-DR was increased in the DLPFC and STG [43], and although a similar increase was observed in the ACG, the results were not significant [43]. This increase in the HLA-DR marker appears to be more pronounced in the hippocampus of patients with paranoid SCZ, as HLA-DR was increased in this group compared with the residual SCZ group, but it was not significant compared to the control group [26].
In a microarray analysis, decreases in HLA-DRA and HLA-DRB4, subunits of HLA-DR, and mRNA expression were observed in the temporal lobe of SCZ patients compared to healthy controls [40]. Similar decreases in HLA-DRA1 and HLA-DPB3 mRNA expression were detected in the superior TC of SCZ [80]. The decreases, however, were not statistically significant when mRNA expression was confirmed by qPCR [80].
Other immunohistochemical studies have assessed microglial activation by measuring ionized calcium-binding adapter molecule (IBA)1. In a cohort of 24 patients with MDD, a significant increase in the density of IBA1-immunoreactive amoeboid-like cells was found in the surrounding blood vessels in depression suicide, accompanied by an increase in IBA1 gene expression, although the total microglial densities in the dorsal ACC did not change [95]. This was confirmed in a subsequent study with a larger cohort, in which amoeboid microglial cells were increased in the ventrolateral PFC in depressed patients compared to healthy controls, but the overall density was very low [32]. However, IBA1 as measured by immunohistochemistry, showed no differences in microglial density in several brain regions of SCZ, such as the DLPFC [51], AMC [71], ACC [84], MFG [115], CC [115], PFC [98], and STG [88], as well as the DLPFC [51, 109], ACC [84, 109], and MFG [116] in BD.
Several anatomical studies have also examined microglial density in postmortem brain samples. In the two studies by Steiner et al. [89, 90], there were no changes in microglial density in various brain regions between the psychiatric group and the control group, but it was noted that suicide was accompanied by higher microgliosis. This effect is similar to a recently published study, where there was no significant difference in microglial density among individuals with SCZ, BD and control subjects. However, there was significantly higher microglial density in suicidal BD individuals than in nonsuicidal BD individuals [71]. Similarly, Brisch et al. [25] evaluated HLA-DR-positive microglial cell density in the DRN and found that nonsuicidal depressed patients revealed significantly lower microglial reactions than controls. In addition, two other studies reported no changes in microglial density in the hippocampus [48] of SCZ and the amygdala [50] of BD and MDD.
Changes in microglial cells have also been investigated by electron microscopy. In a cohort of 21 schizophrenic patients, patients with positive and negative symptoms of SCZ both showed significant microglial activation and dystrophic alterations in layer V of the gray matter in the PFC, although the microglial density did not differ from the control group [101].
Oligodendrocytes
Oligodendrocytes have been measured in postmortem brain samples from patients with major psychiatric diseases in 30 studies. Of the 30 studies, 20 studies reported decreased oligodendrocyte-related markers in the postmortem brain, whereas 2 studies reported an increase, and 8 studies found no change.
Several studies assessed differences in the expression of oligodendrocyte-related or myelin-related genes in postmortem brain samples. Tkachev et al. [93] reported a reduction in key oligodendrocyte- and myelin- related genes, such as oligodendrocyte lineage genes (Olig)2, myelin oligodendrocyte glycoprotein (MOG), and coding region of proteolipid protein (PLP)1, in SCZ and BD, and these gene expression changes in the PFC for both disorders showed a high degree of overlap. Wesseling et al. [107] used a labeled multiplexed selected reaction monitoring assay and found that in the PFC of patients with BD, four oligodendrocyte-specific proteins, 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase), MOG, myelin basic protein (MBP), and myelin proteolipid protein (MYPR), showed a decrease, whereas this decrease was less pronounced in SCZ and MDD. However, in other brain regions, such as the AVN, putamen, internal capsule, and mediodorsal thalamic nuclei, the mRNA expression levels of oligodendrocyte-associated genes, such as CNPase, MOG, galactosyl ceramidase (GALC), and myelin-associated glycoprotein (MAG), were only low in SCZ, whereas no significant difference in BD and MDD [17]. The downregulation of MOG [111] and MBP [63] expression seems to be closely related to SCZ. In a cohort of 10 long-term schizophrenic brains, in the middle layer of the STG and stratum lucidum of CA3 in the hippocampus, the thickness of the MOG-positive fiber-like structures decreased significantly along with reduced MOG expression [111]. In a recent study [94], the thickness of MOG-positive fibrous structures also decreased in the middle layer of the STG, regardless of the presence of 22q11DS in patients with SCZ compared to controls. MBP protein expression was decreased in the DLPFC of patients with SCZ in a proteomic analysis [63]. Furthermore, two studies on the expression of oligodendrocyte-related genes in the brains of patients with MDD have yielded conflicting results [76, 92]. Rajkowska et al. [76] detected oligodendrocyte-related mRNA expression of CNPase, PLP1, MBP, MOG, MOBP, oligodendrocyte transcription factor (OLIG) 1, and OLIG2 in the PFC by qPCR and found that PLP1 mRNA levels were significantly reduced in MDD, whereas there was increased mRNA expression of CNPase, OLIG1, and MOG. No changes in protein expression for CNPase, PLP1, MOG, and MAG were detected in the ventromedial prefrontal white matter of suicidal patients with MDD, but there was decreased Olig2 and MBP protein expression [92].
The majority of studies have reported that changes in the number or density of oligodendrocytes are associated with SCZ. Byne et al. [27] reported a decrease in oligodendrocyte number in the anterior principal thalamic nucleus in men with SCZ; although women had a similar trend, the differences were not significant. In one of his subsequent studies [31], however, there were no changes in oligodendrocyte-specific genes in the thalamus of schizophrenic patients, including MAG, CNPase, and MBP. In fact, CNPase is a marker of oligodendrocyte progenitor cells in addition to differentiating and myelinating oligodendrocytes. Hof et al. [52] reported a decrease in oligodendrocyte total number in layer III of the SFG in 7 schizophrenic brains compared with healthy controls, which was measured by CNPase staining and confirmed by Nissl counts. Another study evaluated oligodendrocyte numbers in the entire hippocampus of 10 schizophrenic patients and found a significant decrease in CA4 in the hippocampus [44]. No effect of SCZ was observed in the dentate gyrus, CA1,2/3, and subiculum [44]. Mauney et al. [64] quantified cells that were immunoreactive for neural/glial antigen (NG)2, a selective marker for oligodendrocyte progenitor cells (OPCs), and those that were immunoreactive for OLIG2, an oligodendrocyte lineage marker of mature oligodendrocytes, and found that there was no significant change in the density of NG2-immunoreactive cells in the PFC of patients with SCZ, but the OLIG2-immunoreactive cell density was decreased significantly. The authors suggest that impaired OPC differentiation and myelin sheath lesions appear to be involved in the pathogenesis of SCZ [64]. A disintegrin and metalloprotease (ADAM)12, a member of the family of multidomain metalloprotease-disintegrins that possess cell-binding, cell-signaling and proteolytic properties [45], might also be involved in the pathophysiology of SCZ since the author found that the density of ADAM12-immunoreactive oligodendrocytes in the white matter of the ACC was significantly decreased in schizophrenic patients [45]. Kolomeets et al. [60] found that the density of oligodendrocytes was reduced in layer V of the PFC in patients with SCZ. In their next study with a large sample size, they also found that the numerical density of oligodendrocytes and oligodendrocyte clusters was reduced in the anterior putamen in SCZ [61]. Although this effect was not observed in BD and MDD, the density of oligodendrocyte clusters was significantly reduced in all male clinical cases compared to male controls [61]. Reductions in oligodendrocyte density were also observed in other brain regions of schizophrenic patients, such as the thalamus and [55] layer VI of the PF [102]. This effect, however, was not seen in the anterior cingulum bundle [81] or substantia nigra [81].
A few studies have also detected differences in the density of oligodendrocytes in the postmortem brains of patients with BD and MDD. For example, Gos et al. [48] reported a decreased density of S100B-immunopositive oligodendrocytes in the left alveus of the hippocampus in a cohort of 6 BD patients compared to the MDD and control groups. In contrast, in another stereological study of the posterior hippocampus [122], Nissl staining revealed that BD patients had significantly more oligodendrocytes in the CA1 region of the hippocampus than healthy controls. Similarly, in patients with MDD, the density of oligodendrocytes was higher in the CA2/3 region, CA4 region, and subiculum of the hippocampus. The authors also noted that antidepressant doses correlated with the density and number of oligodendrocytes in CA2/3 [122]. Although one study reported an increase in oligodendrocyte density in the DLPFC of patients with BD [123], in the other two studies, varying degrees of decrease in oligodendrocyte density were observed in the amygdala [50] and layer VI of the PFC [102] in MDD.
The morphological changes in oligodendrocytes have also been investigated by electron microscopy. Vostrikov et al. [104] reported that SCZ patients had a significantly lower number of pericapillary oligodendrocytes than controls. The author also noted obvious ultrastructural dystrophic and degenerative alterations of pericapillary oligodendrocytes in the PFC of schizophrenic brains. In two studies by Uranova et al. [99, 100], significant ultrastructural changes in oligodendrocytes were observed in the PFC [99, 100] and CN [99] of SCZ and BD, despite no changes in the density of oligodendrocytes. This is consistent with what Vikhreva et al. [103] observed, where the ultrastructure of the oligodendrocytes was changed, which was observed in layer IV of the PFC white matter in SCZ. These pathological changes in oligodendrocytes are aggravated when antipsychotic drugs are administered [105].
Discussion
Although the pathogenesis of SCZ, BD and MDD is largely unclear, accumulating postmortem brain sample studies allow for the study of the pathological mechanism of these psychiatric diseases at the cellular level. Therefore, we systematically reviewed the literature reporting glial cell number, density and cell type-specific markers in the postmortem brains of patients with SCZ, BD and MDD.
We tried to elucidate a trend for each cell type in the different diseases, although most of the study designs and results were heterogeneous. Astrocyte dysfunction appears to be a unique pathology in MDD, as multiple autopsy studies have reported a decrease in the number/density of GFAP-reactive astrocytes in several brain regions of patients with MDD, including the LC [119], OFC [66], amygdala [16], and hippocampus [33]. Similarly, decreases in GFAP gene and protein levels were also observed in other brain regions, including the cerebellum [46], DLPFC [86], PFC [67], thalamus, and CN [97] in patients with MDD. However, not all MDD studies have detected this effect. One plausible explanation is that GFAP immunoreactivity varies with the duration of depression because the GFAP protein levels [86] and the packing density of GFAP-immunoreactive astrocytes [65] are positively correlated with the age of death, which may reflect a compensatory response to neuronal injury in elderly patients with MDD [75]. This may also partly explain why Davis and his colleagues [38] observed an increase in the density of GFAP-immunoreactive astrocytes in MDD brains with an average age of 75. Thus, the pattern of astrocyte pathology in the cerebral cortex in younger patients with MDD appears to be different from that in older patients.
Antidepressants are also most likely a confounding factor for this heterogeneous result, as antidepressants have been found to alter the expression pattern of glia-specific genes, including genes encoding GFAP, vimentin, and aquaporin, and affect glial cell numbers [124]. In this respect, it is important to know that a study using the chronic social defeat stress paradigm as an animal model for depression demonstrated that chronic exposure to antidepressant medications (fluoxetine) can counteract the significant reduction in the number of astrocytes induced by stress, resulting in hippocampal astrocyte numbers in the control range [125]. In our systematic review, supporting evidence from Cobb and his colleagues indicated that depressed people who were taking antidepressants (including fluoxetine) had more GFAP-immunoreactive astrocytes in the dentate gyrus [33]. One possible explanation for such findings is that antidepressant treatment may influence glial cell numbers by affecting glial cell proliferation, although the evidence thus far has shown only that fluoxetine-treated experimental animals have an increase in gliogenesis in the prefrontal cortex [126, 127]. Together, although controversial and somewhat inconsistent, the current evidence from postmortem MDD brain studies tends to support the hypothesis that astrocytic pathology represents a prominent feature of MDD and participates in the pathogenesis of MDD.
Astrogliosis was previously considered to be the basis of the changes in the morphology and number of astrocytes in SCZ. However, consistent evidence for astrocytic pathology in postmortem brains from patients with SCZ has proven elusive, in part due to some conflicting findings in the included studies. Investigations of astrogliosis include studies measuring not only GFAP mRNA and GFAP protein but also glial morphology and size. From our search, multiple studies on GFAP expression of genes/proteins have reported increased levels of GFAP gene and protein in different brain regions of SCZ [47, 63, 74, 110]. However, few immunochemical studies have detected an increased number/density of GFAP astrocytes [39, 43, 48, 51, 66, 120, 121]. Antipsychotic medications appear to be an important factor, which was supported by studies in which increased GFAP mRNA levels [17], GFAP protein levels [28], and GFAP immunoreactivity [128] were significantly correlated with lifetime antipsychotic treatment. Therefore, whether astrogliosis is a pathological feature of SCZ and the influence of antipsychotic treatment on glial pathology of SCZ needs further study.
Similarly, our data show that conflicting findings have also been found in studies evaluating microglia in the brains of SCZ patients postmortem. In support of the fact that most studies have not observed a difference in microglial density, several studies have observed changes in gene expression between controls and SCZ patients. One possible explanation is that microglia are known to be highly reactive and can show changes in a host of genes or proteins, which may or may not reflect changes in their morphology or quantity. Our results show that a reduction in CD68 mRNA levels was found in the DLPFC [62] and CC [85] of SCZ patients, which partly reflects that microglial activation was decreased in these brain regions of SCZ patients, as CD68 is generally thought to be highly expressed in round/activated microglia [129]. In two studies, however, more activated microglia were observed in the cortical areas of postmortem brains of several individuals with SCZ [51, 120]. The confounding factor may be that psychiatric patients include some suicide victims, since studies have shown that the suicidal brain may have high levels of pro-inflammatory cytokines [130]. This is consistent with the findings of two studies by Steiner et al. [89, 90] where patients with any psychiatric condition who committed suicide had the highest number of HLA-DR-positive cells. However, when suicide victims were considered, there were no differences in the same groups between diagnostic groups. The same effect was also observed for BD and MDD, and patients who committed suicide tended to have a higher microglial density [25, 71]. Furthermore, the cohort of healthy controls also included suicide victims, which may potentially confound the results.
In postmortem brain studies of oligodendrocytes, oligodendrocyte density and the expression levels of myelin-related genes, such as MAG, Olig2, and CNPase, were decreased in SCZ. A genetic study has shown that variations in oligodendrocyte-related genes affect the microstructural integrity of white matter bundles and cognitive performance in SCZ [131]. Repeated findings of the downregulation of these genes in the brains of postmortem patients with SCZ provides supporting evidence, although the hypothetical function of oligodendrocyte-related genes and white matter structural integrity is currently unclear. Oligodendrocyte dysfunction leads to changes in synaptic formation and function, which in turn leads to cognitive dysfunction, which is considered to be one of the core symptoms of SCZ [132, 133].
However, not all studies have detected this effect, and a plausible explanation is that there may be sex differences in gene expression, since Byne et al. [31] found higher expression of CNPase and MAG in females compared to males in the thalamic regions of SCZ. In addition, multiple studies have detected an effect of a decreased number/density of oligodendrocytes in several brain regions in SCZ patients by immunohistochemistry and Nissl staining, and it is very likely that this effect is due to the loss of mature oligodendrocytes rather than oligodendrocyte precursor cells. A quantitative study detected a significant loss of Olig2-immunoreactive cells in the PFC of patients with SCZ but not NG2-immunoreactive cells [64]. This suggests that in future studies, the use of stage-specific markers to describe the specific stages of differentiation and maturation of oligodendrocytes in patients with SCZ may be more helpful to understand the pathology of oligodendrocytes related to SCZ.
However, there were still several limitations to this study that merit emphasis. Firstly, considering the relatively small number of BD studies that met the inclusion criteria for this review, we cannot draw a definite conclusion, which limited our ability to explain similar and different trends at the cellular level across diseases. Second, anxiety disorders are highly comorbid with MDD. However, the reviewed studies did not report anything with regard to comorbidities with anxiety; therefore, the presence of an anxiety disorder in MDD patients could be an important confounder for the observed between-study heterogeneities [134]. A third limitation is the high level of heterogeneity across studies derived from the research design, measurement methods, and sample selection. For example, some studies have found layer-specific effects in areas of the brain that evaluate glial cells [20, 35, 37, 73]. However, not all studies have measured the cortical layer of glial cells. Although a few studies have considered the impact of suicide on their measurements, many studies have not included it in their statistical analysis, which makes this another limitation. In addition, postmortem histopathological studies often include psychiatric patients with a history of use of various antidepressants and/or antipsychotic medications, so medication therapy itself may also lead to changes in glial morphology and number. Finally, since we did not consider non-English articles and unpublished data, there is a possibility of publication bias. Thus, our findings should be interpreted with caution.
In conclusion, although most studies evaluating total glial cells in postmortem brain samples from patients with psychiatric disease reported no difference, multiple other studies evaluating glial cell subtypes found evidence of glial cell abnormalities (see Supplementary Table S1). For example, although 19 studies did not find any effect of psychiatric disease on astrocyte markers, 22 studies presented both increased and decreased astrocyte markers. In particular, 11 studies found that astrocyte number/density and GFAP expression were decreased in various brain regions in MDD. Similarly, 15 studies reported a decrease in the density and related gene expression of oligodendrocytes in multiple brain regions of SCZ. These findings suggest that glial subtypes seem to have specific patterns of change in each disease, which is of great significance for us to understand the pathophysiology of glial cells in major psychiatric diseases and to provide new directions for disease treatment.
Data Availability
All data are contained within the manuscript.
References
Somjen G (1988) Nervenkitt: notes on the history of the concept of neuroglia. Glia 1:2–9
Miller, G. Neuroscience. The dark side of glia. Science (New York, N.Y.) 308, 778–781 (2005).
Cotter D, Pariante C, Everall I (2001) Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull 55:585–595
Barres B (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60:430–440
Azevedo FA, Carvalho LR et al (2009) Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513:532–541
Otte C, Gold S et al (2016) Major depressive disorder. Nat Rev Dis Primers 2:16065
Kahn R, Sommer I et al (2015) Schizophrenia. Nat Rev Dis Primers 1:15067
Carvalho A, Firth J, Vieta E (2020) Bipolar disorder. N Engl J Med 383:58–66
Goldsmith D, Rapaport M, Miller B (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21:1696–1709
Enache D, Pariante C, Mondelli V (2019) Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun 81:24–40
Giridharan V, Sayana P et al (2020) Postmortem evidence of brain inflammatory markers in bipolar disorder: a systematic review. Mol Psychiatry 25:94–113
Trépanier M, Hopperton K, Mizrahi R, Mechawar N, Bazinet R (2016) Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry 21:1009–1026
Sild M, Ruthazer E, Booij L (2017) Major depressive disorder and anxiety disorders from the glial perspective: etiological mechanisms, intervention and monitoring. Neurosci Biobehav Rev 83:474–488
Gigase F, Snijders G, Boks M, de Witte L (2019) Neurons and glial cells in bipolar disorder: a systematic review of postmortem brain studies of cell number and size. Neurosci Biobehav Rev 103:150–162
Dietz A, Goldman S, Nedergaard M (2020) Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry 7:272–281
Altshuler L, Abulseoud O et al (2010) Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord 12:541–549
Barley K, Dracheva S, Byne W (2009) Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res 112:54–64
Beasley C, Honavar M, Everall I, Cotter D (2009) Two-dimensional assessment of cytoarchitecture in the superior temporal white matter in schizophrenia, major depressive disorder and bipolar disorder. Schizophr Res 115:156–162
Benes F, Davidson J, Bird E (1986) Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch Gen Psychiatry 43:31–35
Benes F, Vincent S, Todtenkopf M (2001) The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiat 50:395–406
Bernard R, Kerman I et al (2011) Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry 16:634–646
Bezchlibnyk Y, Sun X et al (2007) Neuron somal size is decreased in the lateral amygdalar nucleus of subjects with bipolar disorder. J Psychiatry Neuroscience : JPN 32:203–210
Boldrini M, Galfalvy H et al (2019) Resilience is associated with larger dentate gyrus, while suicide decedents with major depressive disorder have fewer granule neurons. Biol Psychiatry 85:850–862
Brauch, R., Adnan El-Masri, M., Parker, J. and El-Mallakh, R. Glial cell number and neuron/glial cell ratios in postmortem brains of bipolar individuals. Journal of affective disorders 91, 87–90 (2006).
Brisch R, Steiner J et al (2017) Microglia in the dorsal raphe nucleus plays a potential role in both suicide facilitation and prevention in affective disorders. Eur Arch Psychiatry Clin Neurosci 267:403–415
Busse S, Busse M et al (2012) Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun 26:1273–1279
Byne W, Kidkardnee S et al (2006) Schizophrenia-associated reduction of neuronal and oligodendrocyte numbers in the anterior principal thalamic nucleus. Schizophr Res 85:245–253
Association with neuroinflammation (2014) Catts, V.S., Wong, J., Fillman, S.G., Fung, S.J. and Shannon Weickert, C. Increased expression of astrocyte markers in schizophrenia. Aust N Z J Psychiatry 48:722–734
Benes, F., McSparren, J., Bird, E., San Giovanni, J. and Vincent, S. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry 48, 996–1001 (1991).
Chana G, Landau S, Beasley C, Everall IP, Cotter D (2003) Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry 53:1086–1098
Byne W, Dracheva S et al (2008) Schizophrenia and sex associated differences in the expression of neuronal and oligodendrocyte-specific genes in individual thalamic nuclei. Schizophr Res 98:118–128
Clark SM, Pocivavsek A et al (2016) Reduced kynurenine pathway metabolism and cytokine expression in the prefrontal cortex of depressed individuals. J Psychiatry Neurosci 41:386–394
Cobb J, O’Neill K et al (2016) Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience 316:209–220
Cotter D, Hudson L, Landau S (2005) Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disord 7:358–369
Cotter, D., Mackay, D. et al. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex (New York, N.Y. : 1991) 12, 386–394 (2002).
Cotter D, Mackay D, Frangou S, Hudson L, Landau S (2004) Cell density and cortical thickness in Heschl’s gyrus in schizophrenia, major depression and bipolar disorder. British J Psychiatry : J Ment Sci 185:258–259
Cotter D, Mackay D, Landau S, Kerwin R, Everall I (2001) Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry 58:545–553
Davis S, Thomas A et al (2002) Glial fibrillary acidic protein in late life major depressive disorder: an immunocytochemical study. J Neurol Neurosurg Psychiatry 73:556–560
Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S (2010) Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry 67:155–166
Durrenberger, P., Fernando, F. et al. Common mechanisms in neurodegeneration and neuroinflammation: a BrainNet Europe gene expression microarray study. J Neural Transm (Vienna, Austria : 1996) 122, 1055–1068 (2015).
Falkai P, Bogerts B (1986) Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci 236:154–161
Falkai P, Bogerts B, Rozumek M (1988) Limbic pathology in schizophrenia: the entorhinal region–a morphometric study. Biol Psychiat 24:515–521
Radewicz K, Garey LJ, Gentleman SM, Reynolds R (2000) Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol 59:137–150
Falkai, P., Malchow, B. et al. Decreased oligodendrocyte and neuron number in anterior hippocampal areas and the entire hippocampus in schizophrenia: a stereological postmortem study. Schizophr Bull S4-S12 (2016).
Farkas N, Lendeckel U et al (2010) Reduced density of ADAM 12-immunoreactive oligodendrocytes in the anterior cingulate white matter of patients with schizophrenia. World J Biol Psychiatry : Off J World Fed Soc Biol Psychiatry 11:556–566
Fatemi S, Laurence J et al (2004) Glial fibrillary acidic protein is reduced in cerebellum of subjects with major depression, but not schizophrenia. Schizophr Res 69:317–323
Feresten A, Barakauskas V, Ypsilanti A, Barr A, Beasley C (2013) Increased expression of glial fibrillary acidic protein in prefrontal cortex in psychotic illness. Schizophr Res 150:252–257
Gos T, Schroeter M et al (2013) S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: a postmortem study. J Psychiatr Res 47:1694–1699
Gos T, Myint AM et al (2014) Reduced microglial immunoreactivity for endogenous NMDA receptor agonist quinolinic acid in the hippocampus of schizophrenia patients. Brain Behav Immun 41:59–64
Hamidi M, Drevets W, Price J (2004) Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiat 55:563–569
Hercher C, Chopra V, Beasley CL (2014) Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J Psychiatry Neurosci 39:376–385
Hof P, Haroutunian V et al (2003) Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiat 53:1075–1085
Höistad M, Heinsen H, Wicinski B, Schmitz C, Hof P (2013) Stereological assessment of the dorsal anterior cingulate cortex in schizophrenia: absence of changes in neuronal and glial densities. Neuropathol Appl Neurobiol 39:348–361
Karson C, Casanova M, Kleinman J, Griffin W (1993) Choline acetyltransferase in schizophrenia. Am J Psychiatry 150:454–459
Kerns D, Vong G et al (2010) Gene expression abnormalities and oligodendrocyte deficits in the internal capsule in schizophrenia. Schizophr Res 120:150–158
Khundakar A, Morris C, Oakley A, McMeekin W, Thomas A (2009) Morphometric analysis of neuronal and glial cell pathology in the dorsolateral prefrontal cortex in late-life depression. The British journal of psychiatry : the journal of mental science 195:163–169
Khundakar A, Morris C, Oakley A, Thomas A (2011) A morphometric examination of neuronal and glial cell pathology in the orbitofrontal cortex in late-life depression. Int Psychogeriatr 23:132–140
Khundakar A, Morris C, Oakley A, Thomas A (2011) Morphometric analysis of neuronal and glial cell pathology in the caudate nucleus in late-life depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 19:132–141
Khundakar A, Morris C, Oakley A, Thomas A (2011) Cellular pathology within the anterior cingulate cortex of patients with late-life depression: a morphometric study. Psychiatry Res 194:184–189
Kolomeets N, Uranova N (2019) Reduced oligodendrocyte density in layer 5 of the prefrontal cortex in schizophrenia. Eur Arch Psychiatry Clin Neurosci 269:379–386
Kolomeets NS, Uranova NA (2020) Numerical density of oligodendrocytes and oligodendrocyte clusters in the anterior putamen in major psychiatric disorders. Eur Arch Psychiatry Clin Neurosci 270:841–850
Lopez-Gonzalez I, Pinacho R et al (2019) Neuroinflammation in the dorsolateral prefrontal cortex in elderly chronic schizophrenia. Eur Neuropsychopharmacol 29:384–396
Martins-de-Souza D, Gattaz W et al (2009) Proteomic analysis of dorsolateral prefrontal cortex indicates the involvement of cytoskeleton, oligodendrocyte, energy metabolism and new potential markers in schizophrenia. J Psychiatr Res 43:978–986
Mauney S, Pietersen C, Sonntag K, Woo T (2015) Differentiation of oligodendrocyte precursors is impaired in the prefrontal cortex in schizophrenia. Schizophr Res 169:374–380
Miguel-Hidalgo J, Baucom C et al (2000) Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiat 48:861–873
Miguel-Hidalgo J, Waltzer R et al (2010) Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord 127:230–240
Nagy C, Suderman M et al (2015) Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry 20:320–328
Nasrallah H, McCalley-Whitters M, Bigelow L, Rauscher F (1983) A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res 8:251–260
Nishioka N, Arnold SE (2004) Evidence for oxidative DNA damage in the hippocampus of elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry 12:167–175
O'Leary, L.A., Belliveau, C. et al. Widespread decrease of cerebral vimentin-immunoreactive astrocytes in depressed suicides. Front Psychiatry 12, 640963 (2021).
Petrasch-Parwez, E., Schöbel, A. et al. Lateralization of increased density of Iba1-immunopositive microglial cells in the anterior midcingulate cortex of schizophrenia and bipolar disorder. European archives of psychiatry and clinical neuroscience (2020).
Qi XR, Kamphuis W, Shan L (2019) Astrocyte changes in the prefrontal cortex from aged non-suicidal depressed patients. Front Cell Neurosci 13:503
Rajkowska G, Halaris A, Selemon L (2001) Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiat 49:741–752
Rajkowska G, Miguel-Hidalgo JJ et al (2002) Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophr Res 57:127–138
Rajkowska G, Miguel-Hidalgo J, Dubey P, Stockmeier C, Krishnan K (2005) Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol Psychiat 58:297–306
Rajkowska G, Mahajan G et al (2015) Oligodendrocyte morphometry and expression of myelin - Related mRNA in ventral prefrontal white matter in major depressive disorder. J Psychiatr Res 65:53–62
Rao J, Harry G, Kim H (2010) Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry 15:384–392
Di Rosa E, Crow T, Walker M, Black G, Chance S (2009) Reduced neuron density, enlarged minicolumn spacing and altered ageing effects in fusiform cortex in schizophrenia. Psychiatry Res 166:102–115
Schmitt A, Steyskal C et al (2009) Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol 117:395–407
Schmitt A, Leonardi-Essmann F et al (2011) Regulation of immune-modulatory genes in left superior temporal cortex of schizophrenia patients: a genome-wide microarray study. World J Biol Psychiatry 12:201–215
Segal D, Schmitz C, Hof P (2009) Spatial distribution and density of oligodendrocytes in the cingulum bundle are unaltered in schizophrenia. Acta Neuropathol 117:385–394
Selemon L, Mrzljak J, Kleinman J, Herman M, Goldman-Rakic P (2003) Regional specificity in the neuropathologic substrates of schizophrenia: a morphometric analysis of Broca’s area 44 and area 9. Arch Gen Psychiatry 60:69–77
Selemon L, Begovic A (2007) Stereologic analysis of the lateral geniculate nucleus of the thalamus in normal and schizophrenic subjects. Psychiatry Res 151:1–10
Seredenina T, Sorce S et al (2017) Decreased NOX2 expression in the brain of patients with bipolar disorder: association with valproic acid prescription and substance abuse. Transl Psychiatry 7:e1206
Shimamoto-Mitsuyama C, Nakaya A et al (2021) Lipid pathology of the corpus callosum in schizophrenia and the potential role of abnormal gene regulatory networks with reduced microglial marker expression. Cereb Cortex 31:448–462
Si X, Miguel-Hidalgo J, O’Dwyer G, Stockmeier C, Rajkowska G (2004) Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 29:2088–2096
Smiley J, Hackett T et al (2016) Reduced GABA neuron density in auditory cerebral cortex of subjects with major depressive disorder. J Chem Neuroanat 76:108–121
Snijders G, van Zuiden W et al (2021) A loss of mature microglial markers without immune activation in schizophrenia. Glia 69:1251–1267
Steiner J, Bielau H et al (2008) Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res 42:151–157
Steiner J, Mawrin C et al (2006) Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol 112:305–316
Stockmeier C, Mahajan G et al (2004) Cellular changes in the postmortem hippocampus in major depression. Biol Psychiat 56:640–650
Tanti A, Kim J et al (2018) Child abuse associates with an imbalance of oligodendrocyte-lineage cells in ventromedial prefrontal white matter. Mol Psychiatry 23:2018–2028
Tkachev D, Mimmack ML et al (2003) Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 362:798–805
Torii, Y., Iritani, S. et al. Morphological alteration of myelin-oligodendrocytes in a schizophrenic patient with 22q11.2 deletion syndrome Schizophr Res 223:353–355
Torres-Platas S, Cruceanu C, Chen G, Turecki G, Mechawar N (2014) Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun 42:50–59
Torres-Platas S, Hercher C et al (2011) Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 36:2650–2658
Torres-Platas SG, Nagy C, Wakid M, Turecki G, Mechawar N (2016) Glial fibrillary acidic protein is differentially expressed across cortical and subcortical regions in healthy brains and downregulated in the thalamus and caudate nucleus of depressed suicides. Mol Psychiatry 21:509–515
Tzioras M, Stevenson AJ, Boche D, Spires-Jones TL (2021) Microglial contribution to synaptic uptake in the prefrontal cortex in schizophrenia. Neuropathol Appl Neurobiol 47:346–351
Uranova N, Orlovskaya D et al (2001) Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull 55:597–610
Uranova N, Vikhreva O, Rakhmanova V, Orlovskaya D (2018) Ultrastructural pathology of oligodendrocytes adjacent to microglia in prefrontal white matter in schizophrenia. NPJ Schizophr 4:26
Uranova N, Vikhreva O, Rakhmanova V, Orlovskaya D (2020) Dystrophy of oligodendrocytes and adjacent microglia in prefrontal gray matter in schizophrenia. Front Psych 11:204
Uranova N, Vostrikov V, Orlovskaya D, Rachmanova V (2004) Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res 67:269–275
Vikhreva O, Rakhmanova V, Orlovskaya D, Uranova N (2016) Ultrastructural alterations of oligodendrocytes in prefrontal white matter in schizophrenia: A post-mortem morphometric study. Schizophr Res 177:28–36
Vostrikov V, Orlovskaya D, Uranova N (2008) Deficit of pericapillary oligodendrocytes in the prefrontal cortex in schizophrenia. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry 9:34–42
Walker C, Roche J, Sinha V, Roberts R (2018) Substantia nigra ultrastructural pathology in schizophrenia. Schizophr Res 197:209–218
Webster MJ, O’Grady J, Kleinman JE, Weickert CS (2005) Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience 133:453–461
Wesseling, H., Gottschalk, M. and Bahn, S. Targeted multiplexed selected reaction monitoring analysis evaluates protein expression changes of molecular risk factors for major psychiatric disorders. The international journal of neuropsychopharmacology 18(2014).
Williams MR, Galvin K et al (2014) Neuropathological changes in the substantia nigra in schizophrenia but not depression. Eur Arch Psychiatry Clin Neurosci 264:285–296
Zhang L, Verwer RWH, Lucassen PJ, Huitinga I, Swaab DF (2020) Sex difference in glia gene expression in the dorsolateral prefrontal cortex in bipolar disorder: Relation to psychotic features. J Psychiatr Res 125:66–74
Farnsworth B, Radomska KJ et al (2017) QKI6B mRNA levels are upregulated in schizophrenia and predict GFAP expression. Brain Res 1669:63–68
Marui T, Torii Y et al (2018) The neuropathological study of myelin oligodendrocyte glycoprotein in the temporal lobe of schizophrenia patients. Acta neuropsychiatrica 30:232–240
Müller M, Lucassen P et al (2001) Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci 14:1603–1612
Rajkowska G, Selemon L, Goldman-Rakic P (1998) Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry 55:215–224
Rubinow M, Mahajan G et al (2016) Basolateral amygdala volume and cell numbers in major depressive disorder: a postmortem stereological study. Brain Struct Funct 221:171–184
Sneeboer MAM, van der Doef T et al (2020) Microglial activation in schizophrenia: is translocator 18kDa protein (TSPO) the right marker? Schizophr Res 215:167–172
Sneeboer M, Snijders G et al (2019) Microglia in post-mortem brain tissue of patients with bipolar disorder are not immune activated. Transl Psychiatry 9:153
Cullen T, Walker M et al (2006) Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia. The British journal of psychiatry : the journal of mental science 188:26–31
Rajkowska G, Stockmeier C (2013) Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14:1225–1236
Chandley M, Szebeni K et al (2013) Gene expression deficits in pontine locus coeruleus astrocytes in men with major depressive disorder. Journal of psychiatry & neuroscience : JPN 38:276–284
Falkai P, Honer WG et al (1999) No evidence for astrogliosis in brains of schizophrenic patients. A post-mortem study Neuropathol Appl Neurobiol 25:48–53
Damadzic R, Bigelow LB et al (2001) A quantitative immunohistochemical study of astrocytes in the entorhinal cortex in schizophrenia, bipolar disorder and major depression: absence of significant astrocytosis. Brain Res Bull 55:611–618
Malchow, B., Strocka, S. et al. Stereological investigation of the posterior hippocampus in affective disorders. Journal of neural transmission (Vienna, Austria : 1996) 122, 1019–1033 (2015).
Hercher C, Chopra V, Beasley C (2014) Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. Journal of psychiatry & neuroscience : JPN 39:376–385
Czeh, B, Di Benedetto, B. Antidepressants act directly on astrocytes Eur Neuropsychopharmacol 23:171–185
Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E (2006) Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology 31:1616–1626
Czeh B, Muller-Keuker JI et al (2007) Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology 32:1490–1503
Kodama M, Fujioka T, Duman RS (2004) Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry 56:570–580
Toro CT, Hallak JE, Dunham JS, Deakin JF (2006) Glial fibrillary acidic protein and glutamine synthetase in subregions of prefrontal cortex in schizophrenia and mood disorder. Neurosci Lett 404:276–281
Hendrickx DAE, van Eden CG, Schuurman KG, Hamann J, Huitinga I (2017) Staining of HLA-DR, Iba1 and CD68 in human microglia reveals partially overlapping expression depending on cellular morphology and pathology. J Neuroimmunol 309:12–22
Pandey GN, Rizavi HS et al (2012) Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res 46:57–63
Voineskos AN, Felsky D et al (2013) Oligodendrocyte genes, white matter tract integrity, and cognition in schizophrenia. Cereb Cortex 23:2044–2057
Takahashi N, Sakurai T, Davis KL, Buxbaum JD (2011) Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol 93:13–24
Fields RD (2008) White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31:361–370
Kaufman J, Charney D (2000) Comorbidity of mood and anxiety disorders. Depress Anxiety 12(Suppl 1):69–76
Funding
This study was supported by the National Science Foundation of China (82071676, 81703492).
Author information
Authors and Affiliations
Contributions
All authors have read and agree with the published version of the manuscript. Yong Cheng conceived and designed this study; Shu-Han Liu and Yang Du searched database and identified eligible studies; Shu-Han Liu and Lei Chen extracted the data; all authors critically reviewed the manuscript; Shu-Han Liu drafted the manuscript with critical revisions from Yong Cheng.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, SH., Du, Y., Chen, L. et al. Glial Cell Abnormalities in Major Psychiatric Diseases: A Systematic Review of Postmortem Brain Studies. Mol Neurobiol 59, 1665–1692 (2022). https://doi.org/10.1007/s12035-021-02672-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02672-8