Abstract
Parkinson’s disease (PD) ranks first in the world as a neurodegenerative movement disorder and occurs most commonly in an idiopathic form. PD patients may have motor symptoms, non-motor symptoms, including cognitive and behavioral changes, and symptoms related to autonomic nervous system (ANS) failures, such as gastrointestinal, urinary, and cardiovascular symptoms. Unfortunately, the diagnostic accuracy of PD by general neurologists is relatively low. Currently, there is no objective molecular or biochemical test for PD; its diagnosis is based on clinical criteria, mainly by cardinal motor symptoms, which manifest when patients have lost about 60–80% of dopaminergic neurons. Therefore, it is urgent to establish a panel of biomarkers for the early and accurate diagnosis of PD. Once the disease is accurately diagnosed, it may be easier to unravel idiopathic PD’s pathogenesis, and ultimately, finding a cure. This review discusses several biomarkers’ potential to set a panel for early idiopathic PD diagnosis and future directions.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s but ranks first in the world as neurodegenerative movement disorder [1]. The various monogenic forms of PD account for a minority of PD cases. PD most commonly occurs in an idiopathic form, where genetics, environmental exposure, and aging are related risk factors [2, 3].
PD’s pathological characteristics include dopaminergic neuronal loss in the substantia nigra at the central nervous system (CNS), the subsequent dopamine (DA) level reduction affecting motor function, and Lewy bodies’ presence [4, 5]. Also, mitochondrial dysfunction, oxidative stress (OS), and abnormal protein accumulation are involved in PD’s pathogenesis [6].
PD patients may suffer from motor and non-motor symptoms (cognitive and behavioral) and those related to the autonomic nervous system (ANS) [7]. PD cardinal motor symptoms are tremor, bradykinesia, rigidity, and postural instability [8]. Some pathologic changes that may appear up to 20 years before the motor symptoms onset [7] are related to ANS failures, including gastrointestinal, urinary, and cardiovascular symptoms, which increase with age, the severity of the disease, and higher dopaminergic medication doses [9]. Constipation is an early gastrointestinal symptom that precedes motor symptoms. Men with less than one bowel movement per day have 4.1 times the risk of developing PD compared to men who have two bowel movements per day, and the risk increases 4.5 times compared to men who have more than two bowel movements per day [10]. There, the importance of non-motor symptoms as a potential warning for PD progression since they can precede motor symptoms by decades.
Paradoxically, although the current technological advances are rapidly growing, the generation of new effective disease-modifying treatment is not yet available in the clinic. The current dopaminergic replacement therapy for PD prevails 60 years after the dopaminergic deficit discovery [11]. Although there are several FDA-approved medications for PD (anticholinergics, carbidopa/levodopa, catechol-o-methyl transferase (COMT) inhibitors, dopamine agonists, MAO-B inhibitors, and NMDA receptor inhibitor), all of them target symptomatology, primarily motor symptoms [12].
Several biomolecules, including non-enzymatic antioxidants, and synthetic drugs, have yielded positive outcomes in PD models. However, they have failed to reproduce such results in clinical trials [13]. Besides, numerous drugs are still under evaluation in clinical trials [14]. Nonetheless, there are currently no PD-modifying treatments [15].
Current Diagnosis of Parkinson’s Disease
Despite advances in neurodegenerative disease research and improved neuroimaging and genetic studies, PD diagnosis remains mainly dependent on observational clinical criteria, mostly clinical motor symptoms [16] manifesting when patients have lost about 60–80% of dopaminergic neurons [17].
Since the development of the Unified Parkinson’s Disease Rating Scale (UPDRS) in the 1980s, the Movement Disorder Society (MDS) has evaluated [18] and updated the diagnostic criteria to include also the non-motor symptoms [19]. An update for the research criteria for prodromal PD has also been published to achieve an early diagnosis [20]. Prodromal PD encompasses patients in a stage where they do not fulfill diagnostic criteria for PD but exhibit nonmotor signs and symptoms, increasing the risk of developing motor skills symptoms and PD in the future. Among prodromal PD symptoms are hyposmia, constipation, mood disorders, and REM sleep behavior disorder (RBD), which significantly impact the life quality early and once the disease has progressed to motor PD [21]. However, these criteria only allow a phenotypic clinical classification of PD that includes the subtypes of tremor dominant (TD), postural instability-gait disorder (PIGD), nonmotor mild cognitive impairment (PD-MCI), and dementia (PDD).
Notwithstanding the effort to improve the diagnosis accuracy, it is not yet satisfying. The diagnosis accuracy is 75% for general neurologists [22] and 79% for movement disorders experts. The overall validity of clinical diagnosis has not improved in the last 25 years [23].
Currently, clinical diagnosis is supported by imaging studies, including (1) computed tomography (CT) scan, consisting of passing X-rays from different angles to obtain brain cross-sectional images, helps to rule out other diseases with similar symptoms to those of PD [24]. (2) Magnetic resonance imaging (MRI) scan obtains weighted images by measuring the tissues’ water diffusion speed. Because of the dopaminergic neuronal death and the reduction of the region’s volume in PD, a greater water molecule diffusivity occurs, helping to distinguish PD from similar diseases [25]. (3) Dopamine transporter single-photon emission computed tomography (DaTSCAN™ SPECT) scan requires the intravenous administration of ioflupane 123I as 123I-FP-CIT, which binds to the DA transporter (DAT) in the dopaminergic neurons presynaptic membrane, making evident the loss of dopaminergic neurons in PD [26]. (4) Positron emission tomography (PET) scan uses radiotracers that emit positrons to evaluate the presynaptic DAT presence. The radiotracers employed are 18F or 11C radiolabeled DA analogs. Besides, the vesicular monoamine transporter 2, which also transports DA, can be assessed using radiolabeled dihydrotetrabenazine (DTBZ) [25]. Since the specific patterns of regional glucose metabolism are known, the 18F-FDG radiotracer is used to evaluate the brain’s glucose metabolism as a neuronal function marker to detect PD’s specific alterations due to synaptic dysfunction, allowing differential diagnosis between parkinsonism and PD [27]. (5) Scintigraphy of cardiac 123I-metaiodobenzylguanidina (123I-MIBG), which is a synthetic analog of norepinephrine, allows differential diagnosis of PD and MSA (multiple system atrophy) and is based on postganglionic sympathetic neurons integrity. In PD, pre and postganglionic autonomic neurons are affected by α-synuclein; therefore, cardiac 123I-MIBG uptake is disrupted. In contrast, in MSA, where autonomic deterioration is mainly preganglionic, uptake of 123I-MIBG is assumed to be preserved [28].
However, all these imaging techniques have some disadvantages when used in PD diagnosis. For instance, CT and MRI scans are used for differential diagnosis but not to confirm PD. DaTSCAN™ SPECT and PET scan evaluate DATs. Therefore, they can detect the first signs of dopaminergic damage but cannot differentiate PD from atypical parkinsonism with dopaminergic dysfunction [25]. Furthermore, DaTSCAN™ SPECT and PET scans are costly, and of limited access, so the latter is mainly used in research. In the case of cardiac MIBG scintigraphy, its specificity is limited in the presence of cardiac damage such as cardiomyopathy and myocardial infarction, in addition to peripheral nerve diseases such as diabetes and other polyneuropathies [29].
The main obstacles to finding a cure for PD are inaccurate diagnosis and deficient availability of high-quality human tissues [22]. Therefore, pursuing the identification of biomarkers for early PD diagnosis is critical; with effective, not invasive biomarkers, a chemical test could be created, so those at risk could be identified and treated before any overt signs or symptoms.
Biomarkers
Biomarkers are defined as a characteristic that can be objectively measured and evaluated as an indicator of normal or pathogenic processes or pharmacological responses to therapies [30]. Biomarkers can be cells, lipids, proteins, genes, or metabolites; their presence; variation in concentration; or even their molecular modification [31]. Biomarkers can be detected and measured in biological samples such as tissues, cells, or ideally in body fluids (blood, plasma, saliva, urine) due to their easy access [32].
Pursuing an ideal PD biomarker is challenging, mainly when signs and symptoms take decades to appear, and even more, to chase early stages or predictive biomarkers when there are no signs of disease and its origin is idiopathic. Therefore, it is likely that a panel of biomarkers will provide greater diagnostic accuracy than any single marker.
Major concerns related to biomarkers search are individual or group variability, sensitivity, specificity, and effect modifiers. Therefore, reliability is essential, which is usually affected by differences in storage, transport, methods, and instruments [33].
Herein, we discuss integrating multiple candidate biomarkers, proposing those less invasive and more reliable, to form a potential panel of early PD diagnosis and its progression. Therefore, CSF biomarkers are not addressed since CSF sampling is an invasive outpatient procedure that has to be performed by an experienced and specialized physician and may not be performed in developing countries as easily as in developed countries. Besides, CSF biomarkers were recently reviewed by Parnetti et al. [34]. Establishing a validated biomarker panel for early PD diagnosis ideally may allow treatment of patients before or at the earliest stages of a diagnosable form of the disease and interventions targeting specific biological processes associated with disease progression [35].
Potential Biomarkers for the Diagnosis of PD
Is α-Synuclein the Leading PD Candidate Biomarker?
α-Synuclein (α-Syn) is the main component of Lewy bodies, whose presence is one of the primary PD’s pathological characteristics [5]. α-Syn is encoded by the SNCA gene, which has three main domains: (1) the N-terminal domain, which has a multiple repeat consensus sequence (KTKEGV); (2) the central domain, called non-amyloid β component (NAC), which is highly hydrophobic and participates in α-Syn aggregation when it acquires the structure of the β-sheet; and (3) the C-terminal domain, enriched in proline residues and negatively charged, which provides flexibility [36]. α-Syn has dynamic conformations stabilized by long-range interactions between the C-terminal end and the NAC region, and between the N and C-terminal ends, probably due to hydrophobic and electrostatic contacts that may prevent aggregation [37]. SNCA gene multiplication and point mutations, environmental changes, and post-translational modifications can alter the α-Syn native structure, inducing misfolding and aggregation [38, 39]. Among the post-translational modifications, phosphorylation affects the α-Syn function [38], and OS increases phosphorylated α-Syn at S129 (pS129-α-Syn) and inclusion formation [40]. Also, pS129-α-Syn is found abundantly in various synucleopathies, including PD [41]. It has been reported that α-Syn is not a sensitive biomarker for PD diagnosis in the early stages when analyzed using the immunohistochemical staining method in the skin and submandibular gland (invasive examination), obtaining a sensitivity of 24.1% and 56.1%, respectively [42]. Interestingly, an increase of α-Syn has been found in peripheral tissues and fluids using other methods, including real-time quaking-induced conversion (RT-QuIC) and protein misfolding cyclic amplification (PMCA), which are described below, along with other promising PD biomarkers (Fig. 1).
Skin Biomarkers
The skin is a promising organ for the biomarkers search for diagnosing PD because it shares the embryological origin (ectoderm) with neural tissue, is highly innervated, easily accessible, and its fibroblasts reflect environmental and aging changes [43, 44].
The association between the brain and the skin was evidenced by detecting α-Syn inclusions in the epidermis and skin appendages of PD patients while they were absent in control subjects. Besides, relatively high α-Syn levels were observed in PD patients’ skin, while atypical parkinsonism patients had few inclusions, providing a guideline to diagnose PD and differentiate between these two movement disorders [45].
Recently, α-Syn has also been detected in the skin through the RT-QuIC and PMCA techniques. In the RT-QuIC, a soluble recombinant prion protein (rPrP-sen) is used as a substrate to amplify minute amounts of the abnormal prion protein (PrPSc) with vigorous intermittent shaking that induces rPrP-sen aggregation, forming fibrils. Then, protease-resistant rPrP fibrils (rPrP-res) are detected with thioflavin T fluorescent dye [46].
Similarly, the PMCA takes advantage of the nucleation-dependent prion replication process to accelerate the conversion of cellular prion protein (PrPC) to PrPSc but using ultrasound waves to fragment the PrPSc polymers and increasing the number of seeds present in the infected sample without affecting its ability to act as a conversion nucleus. The final result is detected by western blot [47].
Through RT-QuIC, α-Syn differences between postmortem skin samples of PD patients and control subjects were detected with high sensitivity and specificity, varying depending on the sample fixation method. Formalin-fixed and paraffin-embedded skin sections showed 75% sensitivity and 83% specificity, whereas frozen skin tissues had 96% sensitivity and specificity [48]. In another study, skin samples from PD patients were analyzed by the RT-QuIC and PMCA techniques, obtaining 95% sensitivity and 100% specificity for RT-QuIC, and 80% sensitivity and 90% specificity for PMCA [49]. In addition, PMCA has a lower sensitivity and specificity; as a disadvantage, it allows the detection of approximately 1 attogram (10−21 kg) of PrPSc in approximately 3 weeks, while RTQuIC can detect approximately one lethal prion dose in one day [50].
Additionally, increased α-Syn deposition was found in cutaneous sympathetic adrenergic and cholinergic peripheral nerve fibers of PD patients related to higher autonomic dysfunction in advanced PD [51]. Also, skin fibroblasts from PD patients could be used to monitor alterations in morphology, growth patterns, mitochondrial function, autophagy, and OS [44].
Recently, a hyperosmic or “super smeller” individual contributed to identifying a distinctive signature of volatile metabolites from the skin’s sebum in PD patients. This PD distinctive signature consists of the perillic aldehyde decrease and the eicosane increase compared to the controls, resulting in a slight modification in people’s aroma [52]. Therefore, skin biopsies may be a non-invasive alternative for PD diagnosis.
Digestive System Biomarkers
The search for biomarkers in the digestive system is crucial because PD patients can suffer early intestinal inflammation and dysfunction [53]. Up to 30% of PD patients report gastrointestinal symptoms, commonly observed at all stages of the disease but frequently precede motor symptoms, indicating an early digestive tract involvement in the pathological process [54]. Indeed, there is a direct relationship between the gut and the CNS through the “gut-brain axis,” which is bidirectional [55].
Exosomal α-Syn
Exosomes are cell-derived vesicles (30–100 nm) generated by the endosomal pathway and released through exocytosis to the extracellular space and circulation [56]. Exosomes enclose specific macromolecules mirroring the cytosol content of their cellular origin [57]. They are secreted by many cells, including neurons, astrocytes, endothelial [58, 59], and abundant in distant cells and different body fluids, such as saliva [60].
Most proteins involved in neurodegenerative diseases are transported in exosomes [61]. Cerebrospinal fluid (CSF) α-Syn was transported towards blood, with a small portion being contained in exosomes relatively specific to the CNS [62]. α-Syn oligomers were found outside and inside exosomes, probably as a clearance mechanism, particularly when autophagy is altered. These exosomes are more likely to be internalized by recipient cells and are more toxic than free α-Syn oligomers [63]. Exosomes found in saliva samples from PD patients contained a higher proportion of oligomeric α-Syn than controls [64]. Another study showed that total α-Syn levels in saliva from early, moderate, and advanced PD patients were not significantly different between PD and healthy groups [42].
Oral Microbiota
The oral cavity has a moist and warm, suitable environment for microbiota adhesion and colonization [65]. Salivary antimicrobials are critical to maintaining a symbiotic relationship between the host and its resident microbiota [66]. Some oral bacteria routinely survive transit to the gut, and their functional activity is significantly reduced [67]. From oral to the gut, the increased microbial transmission was observed in diseased patients [68]. Early-stage PD patients have shown changes in the oral cavity’s microbiome, most frequently in the taxa abundance. Among the significantly increased bacteria were Acidaminococcus, Bifidobacterium, Brucella, Cellulosimicrobium, Clavibacter, Gardnerella, Lactobacillus, Methylobacterium, Rhodococcus, and Scardovia, while Buchnera, Chryseobacterium, and Wenyingzhuangia were significantly decreased compared to the controls. The bacteriophage Streptococcus phage PhiSpn 200 was significantly decreased, while the yeasts Candida albicans, C. dubliniensis, and Saccharomyces cerevisiae were increased in PD subjects. The microbial metabolic pathways related to serine metabolism, glycolysis, and pentose phosphate showed an increased expression, while the tryptophan’s metabolism and tricarboxylic acid cycle pathways decreased in PD subjects [69]. A strong separation of groups was achieved using a microbiota data set transformed in 5 proportions of 11 taxa in total, and only 13 subjects were misclassified from a total of 84 subjects, with an overall precision of 84.5%. A partial least squares discriminant analysis (PLSDA) showed almost complete separation of PD and control subjects when examined in a multidimensional setting [69]. Saliva might then be a feasible alternative for studying oral microbiota in health and disease [70]. However, there are currently few studies on the analysis of the oral microbiota in PD, so there is a long way to go before they can be used for the early diagnosis of PD.
Intestinal Permeability and Inflammation
Intestinal inflammation has been detected in PD patients in ascending colon biopsies [71]. The inflammatory process and intestinal barrier dysfunction are interrelated. The intestinal mucosal barrier’s integrity depends mainly on the epithelium tight junctions (TJ), which regulate the biomolecules’ paracellular transport. When the intestinal epithelium is damaged, serum proteins diffuse into the gut lumen. Intestinal permeability is reversibly regulated by zonulin (pre-haptoglobin 2) modulating TJ [72]. Upon intestinal exposure to enteric bacteria, zonulin is secreted to the lumen, followed by an increased intestinal permeability because of the zonula occludens 1 protein dissociation from the TJ complex [73]. The protease α-1 antitrypsin is also produced in the liver; it plays an immunomodulatory function by inhibiting neutrophil elastase and may reach the intestinal lumen, mainly when there is damage [74]. Therefore, elevated concentrations of fecal zonulin and α-1 antitrypsin reflect an increased intestinal permeability.
The loss of the intestinal barrier’s integrity leads to inflammation. Neutrophils are the first to arrive at the inflammation sites; monocytes/macrophages follow them and together orchestrate immune responses against infections [75]. Upon stimulation, neutrophils and monocytes/macrophages release calprotectin, a calcium binding-protein that belongs to the S100 protein family [76, 77]. Calprotectin regulates myeloid cell adhesion to endothelium and extracellular matrix and has antimicrobial properties by sequestering zinc [78, 79]. Likewise, lactoferrin is an iron-binding glycoprotein that belongs to the transferrin family; it is also secreted by activated neutrophils and has a critical role in the innate immunity as bactericidal [80]. Since calprotectin and lactoferrin stability and presence in feces are proportional to neutrophil migration to the gastrointestinal tract and the inflammation degree, both are considered inflammation biomarkers [81, 82].
The four biomarkers described above were evaluated in PD patients’ stool samples. Zonulin, α-1-antitrypsin, and calprotectin were significantly elevated in PD patients than age-matched controls, while lactoferrin showed a non-significant trend towards elevated concentrations. However, none of the four fecal biomarkers correlated with disease severity, subtype, dopaminergic therapy, or constipation [83]. No significant differences were observed in zonulin in another study, but calprotectin levels were significantly higher in PD patients than in healthy subjects. However, there was no correlation between the calprotectin level and the disease duration [84].
α-Syn in the Gastrointestinal-Nervous System
Lewy bodies containing α-Syn have been found in the mucosa, submucosal, and myenteric plexus in the enteric nervous system (ENS) of PD patients [85, 86]. α-Syn presence in the ENS was higher in the stomach, intestine, and appendix biopsies from PD patients than controls [87]. PD patients’ intestinal hyperpermeability significantly correlated with bacterial invasion, OS, and α-Syn accumulation in the intestinal mucosa [88]. Besides, a large-scale study found that α-Syn accumulation in the gastrointestinal tract occurs before the motor symptoms onset and principally consists of pS129-α-Syn [86].
Intestinal Microbiota
The brain and gut are connected through the ENS, the vagus nerve, the immune system, and the microbiota’s metabolites. Intestinal microbiota affects the development and function of the CNS through the microbiota-gut-brain axis [55]. Upon vagotomy, the therapeutic effects on the brain caused by probiotic bacteria such as Lactobacillus rhamnosus and Bifidobacterium longum are absent [89, 90]. Using transgenic mice overexpressing α-Syn, it was demonstrated that gut microbiota is essential for motor deficits, microglia activation, and α-Syn accumulation. Antibiotic treatment mitigated, while microbial recolonization promoted the pathology. Besides, oral administration of specific microbial metabolites to germ-free mice promoted neuroinflammation and motor symptoms. Also, colonization of α-Syn-overexpressing mice with microbiota from PD patients intensified physical impairments compared to microbiota transplants from healthy donors [91]. The intestinal microbiota also plays an essential role in the microglia maturation and proper function in the CNS. A study showed that germ-free mice had microglia with an immature phenotype and altered cell ratios, resulting in an impaired innate immune response. Microglia also were defective in mice in which the microbiota was temporarily depleted or had limited complexity. On the contrary, after recolonization with complex intestinal microbiota, the microglia were partially restored. Furthermore, these effects are regulated by the short-chain fatty acids (SCFA) that are produced from the dietary fibers during bacterial fermentation in the large intestine [92].
Interestingly, the elderly has unusual microbiota proportions and extreme variability [93]. Modifications in the intestinal microbiota profile in PD patients have also been reported. So far, due to the intestinal microbiota’s complexity, it has not been possible to establish a specific composition as a biomarker for this disease. However, few studies in different populations have shown modifications in the intestinal microbiota composition in PD patients (Table 1). Among the more consistent modifications of the intestinal microbiota, the families Lactobacillaceae, Enterococcaceae, Christensenellaceae, Verrucomicrobiaceae, and Enterobacteriaceae were increased.
In contrast, Bacteroidetes, the family Prevotellaceae, and Lachnospiraceae were decreased in PD patients compared to control subjects (Table 1), and the fecal SCFA were also reduced [94]. Fecal SCFA includes acetate, propionate, and butyrate [95]. Butyrate plays a vital role in intestinal mucosa homeostasis as it reduces OS by increasing glutathione and exerts an effect on the ENS [96, 97].
Interestingly, modifications in the microbiota are correlated with mitochondrial metabolism and mitochondrial DNA (mtDNA) haplogroup variants [98, 99], which may be explained because both mtDNA and microbiota are inherited from the mother [100, 101]. Also, mitochondrial redox status and reactive oxygen species (ROS) production changes are associated with intestinal microbiota modifications, indicating that mitochondrial function controls the microbiota composition [102].
Blood Biomarkers
Seeking blood biomarkers has excellent advantages like easy sample obtention, minimal invasion, and low cost [103].
Erythrocyte α-Syn
α-Syn can be detected in the blood, which is higher in individuals with SNCA gene multiplication [104]. More than 99% of α-Syn in the blood is localized in erythrocytes [105]. Total and aggregated α-Syn is higher in the erythrocyte membrane, while pS129-α-Syn is higher in the cytosolic compartment of PD patients than in control subjects [106]. However, a recent study found that total α-Syn levels in blood from early, moderate, and advanced PD patients were not significantly different between PD and healthy groups [42].
Nonmercaptalbumin
Albumin is one of the primary antioxidants in human serum (HAS) capable of scavenging hydroxyl radicals through its reduced cysteine residue (Cys34). Human albumin with the reduced cysteine thiol group is known as human mercaptalbumin (HMA), while the oxidized form is called human nonmercaptalbumin (HNA). Because albumin is widely distributed intravascularly and extravascularly, its oxidation reflects a systemic oxidative state [107].
Therefore, the redox ratio of HNA to HSA, defined as % HNA, was investigated in patients with idiopathic PD and autosomal recessive familial PD due to parkin mutations (PARK2). Patients with idiopathic PD had a higher % HNA than healthy controls, while patients with PARK2 mutations did not show a significant difference compared to controls [108].
Neurofilament Light Chain
Neurofilament light chain (NfL) is a neuronal cytoplasmic protein highly expressed in large-caliber myelinated axons [109]. Under normal conditions, axons constantly release NfL in an age-dependent manner to release the highest levels at older ages [110, 111]. However, NfL release increases in response to axonal damage due to inflammatory or neurodegenerative injury in the CNS [109]. Therefore, NfL levels have been evaluated in several studies in the blood of PD and atypical parkinsonism patients. Unfortunately, there is no consensus because of the inconsistency of the results. In some studies, differences were found in blood NfL levels between PD patients and healthy subjects [112, 113], while others did not find differences [111, 114, 115]. However, an increase in NfL was reported in patients with atypical parkinsonism (such as progressive supranuclear palsy, multiple system atrophy, and corticobasal syndrome) compared to healthy subjects and even to PD patients [111, 114, 115]. Differences between the results may be due to variations between populations, as demonstrated in a study where NfL levels were analyzed in PD patients from Sweden and London. Significant differences were found between PD patients and control subjects in the London population, while no significant differences were detected in the Switzerland population [116]. Furthermore, NfL levels are directly related to the prognosis of PD patients [111, 117].
Mesencephalic Astrocyte-Derived Neurotrophic Factor
Mesencephalic astrocyte-derived neurotrophic factor (MANF) is an 18-kDa arginine-rich soluble protein localized in the endoplasmic reticulum (ER), but it is also secreted upon ER stress-induction [118]. MANF exerts a selective protective role on dopaminergic neurons [119]. Its neuroprotective effects may occur through the ER stress pathway [120] induced by unfolded protein accumulation and leading to unfolded protein response (UPR). The UPR increases the protein folding capacity and the misfolded proteins’ degradation to counterbalance ER stress [121]. Importantly, MANF has immune-modulatory properties. Its levels decline with age in serum [122], which correlates with some PD pathogenesis features, like misfolded protein aggregation and inflammation. However, MANF serum concentration was elevated in PD patients [123], probably to counteract misfolded protein aggregation and inflammation.
Resolvin D1
Pro-resolving mediators, including resolvins, stimulate the inflammation resolution in PD models and modulate the disease progression of PD patients [124]. The resolvin D1 (RvD1) is an anti-inflammatory lipid mediator [125]. The evidence suggests a strong link between RvD1 and mitochondrial homeostasis. In a recent study, where mice with traumatic brain injury were treated with RvD1, damaged mitochondria and mitochondrial ROS were eliminated by mitophagy in astrocytes [126]. Also, plasma RvD1 levels were drastically reduced in early PD patients (duration of symptoms 13 ± 5 months) compared to control subjects [127]. However, it needs to be validated in a larger group.
Urate and Homocysteine
Since OS plays a crucial role in PD dopaminergic neuronal loss [128], it is not surprising that antioxidant levels are altered in PD patients. In humans, urate is the end product of purine metabolism and is present intracellularly and in body fluids as the anionic form of uric acid [129]. Uric acid circulates in the blood at high concentrations near its solubility limits with a physiological range between 240 and 350 µM [130] and represents 60% of the antioxidant capacity of plasma [131]. Its decrease has been related to a greater susceptibility to OS and is a high-risk predictor for developing PD [132]. Also, postmortem substantia nigra of PD patients showed 54% less uric acid levels than control subjects [133]. However, the association of low urate levels with a higher risk of PD and the faster progression of the disease have been observed only in men [134, 135] but not in women [136, 137]. Therefore, this metabolite may not be useful in the diagnosis of PD in women.
On the other hand, homocysteine (Hcy) is an amino acid that contains a thiol group generated through methionine demethylation. Methionine reacts with ATP to produce S-adenosyl-L-methionine, which is then demethylated to form Hcy [138]. It is present in plasma in three forms: bound to proteins through disulfide linkage (~ 70–80%), as free Hcy in a reduced form (~ 2%), and the remaining portion correspond to Hcy-cysteine disulfide [139]. Total Hcy ranges between 5 and 15 µmol/L in healthy individuals [140]. Elevated Hcy in plasma has been associated with cognitive impairment and dementia [141]. Notably, the total mean Hcy was significantly elevated in idiopathic PD patients’ plasma than controls [142]. Recently, low urate and high Hcy serum levels were associated with decreased motor function, while only high Hcy serum levels were associated with cognitive decline in early PD progression [143].
Mitochondrial Creatine Kinase
Creatine kinase (CK) is a central controller of cellular energy homeostasis by catalyzing a phosphoryl group’s reversible transfer from MgATP to creatine, producing phosphocreatine and MgADP [144]. There are four major CK isoenzymes: two cytosolic forms, the muscle (MMCK) and brain (BBCK) forms [145], and two mitochondrial forms (MtCK), the sarcomeric (sMtCK) and the ubiquitous (uMtCK) forms [146]. MtCK is highly susceptible to reactive oxygen and nitrogen species damage since most of them originate from the mitochondrial respiratory chain [147]. Since the pathogenesis of PD is linked to mitochondrial dysfunction, MtCK is a potential PD biomarker [148]. A recent study found no differences in PD patient’s serum sMtCK activity. In contrast, a significant decrease in uMtCK activity was observed compared to the control group, associated with the disease progression rate [149].
Metabolomic Profiling
The metabolomic profile comprises a large-scale analysis of metabolites and is widely applied as a comprehensive diagnostic tool because it is a chemical reflection of a phenotype of a particular biological system. Therefore, it is implemented to understand the pathophysiological processes involved in the progression of the disease and search for new diagnostic or prognostic biomarkers of various diseases [150].
Several studies have evaluated the metabolomic profile in serum of PD patients. Among the metabolites that have found increased in PD patients compared to healthy subjects are glutathione, which is a non-enzymatic antioxidant [151]; pyruvate, which is involved in the energy metabolism [152]; amino acids, such as methionine, threonine, alanine, and serine [153]; bile acids like cholic acid, deoxycholic acid, and lithocholic acid [154, 155]; and pyroglutamate, which is a metabolite of glutathione [153]. The metabolites that have been found decreased in PD patients are uric acid, which is a non-enzymatic antioxidant [151, 156]; hypoxanthine, a metabolite of purine [156]; galactitol, glycerol, methylamine, trimethylamine, ethanolamine, suberate, glutarate, malate, methylmalonate, succinate, acetate, gluconate, threonate, glucolate, ascorbate, isocitrate, and citrate, which is involved in the energy metabolism [152]; caffeine metabolites [155]; and C16-C18 saturated and unsaturated fatty acids [153].
Also, fatty acid 14:1 was associated with disease severity, while its levels and those of phosphatidylcholine 34:2, and indolelactic acid were associated with the disease duration [155].
MicroRNAs
MicroRNAs (miRNAs) are endogenous non-coding RNA molecules, approximately 21 to 24 nucleotides in length, that play a role in the post-transcriptional regulation of gene expression during development [157]. The first miRNA (lin-4) was discovered in Caenorhabditis elegans [158]. Several miRNAs are essential neuroinflammation regulators. Inflammatory and neurodegenerative disorders have display variations in microRNA [159, 160]. Therefore, a study evaluated miRNAs’ expression profiles related to aging and cellular senescence in peripheral blood mononuclear cells from PD patients. The expression of miR-885 and miR-17 increased along with PD’s severity.
In contrast, the expression of miR-361 decreased in PD patients compared to controls. Furthermore, the lowest miR-361 levels were observed in the initial stages, while the highest levels were detected in the disease’s advanced stages. Combining detection of these three miRNAs provides a set of biomarkers that allows discrimination of PD patients from healthy subjects [161].
Urine Biomarkers
The urine is another non-invasive sample with diagnostic value for searching PD biomarkers. Importantly, urine is not subject to homeostatic mechanisms as blood and accumulates many changes that may reflect the body’s status [162].
As mentioned before, OS is involved in the loss of dopaminergic neurons. The increase in ROS causes oxidative modifications in lipids, proteins, and DNA. Heterocyclic bases in DNA are prone to oxidative damage, particularly guanine, which is more susceptible to form 8-hydroxydeoxyguanosine (8-OHdG) [163]. Then, 8-OHdG was evaluated in PD patients’ urine, whose concentration was higher and dependent on disease progression than control subjects [164]. Also, there was a positive correlation between urine 8-OHdG levels and the hallucinations presented in PD patients [165].
Another potential urine biomarker associated with OS is kynurenine, a tryptophan metabolite. In mammalian cells, tryptophan is degraded mainly by the kynurenine pathway, where the following metabolites are included: kynurenic acid (KYNA), quinolinic acid (QUIN), 3-hydroxyquinurenine (3-HK), and picolinic acid (PIC). KYNA and QUIN are considered neuroactive, while 3-HK and PIC have pro-oxidant and antioxidant properties. Therefore, the kynurenine pathway may play an essential role in PD pathogenesis [166]. Kynurenine concentration in PD patients’ urine was significantly higher than in control samples, and it was associated with the severity of the disease [167].
Also, the metabolic phenotype analysis in urine samples using high-performance liquid chromatography coupled with high-resolution mass spectrometry has yielded significant differences between PD patients and control subjects. Differences in related metabolic pathways were observed, including steroidogenesis, β-oxidation of fatty acids, metabolism of histidine, phenylalanine, tryptophan, tyrosine, and nucleotide, generating a panel of altered metabolites associated with PD [168]. On the other hand, proteomic analysis of urine exosomes from patients has identified synaptosomal-associated protein 23 (SNAP23), a component of the SNARE complex, and calbindin, a calcium-binding protein, as novel PD biomarkers [169]. Perturbations of some metabolites and protein levels in PD patients’ urine indicate that these can be used as a potential biomarker signature and may help in PD diagnosis and searching for a potential therapeutic target.
Concluding Remarks
The early PD diagnosis biomarker pursuing is an arduous task that still has a long way to go. Several biomarkers can be used to evaluate the prognosis and development of the disease. Some of them are related to the disease’s predominant pathophysiological characteristics, including mitochondrial damage, OS, and protein accumulation (Fig. 2), which have been detected in both the CNS and systemically in a wide variety of samples in PD patients, indicating that the study and diagnosis of PD should not only focus on the CNS but should be approached systemically. Many potential biomarkers in non-invasive samples have been reported and are summarized in this review.
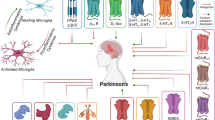
Potential biomarkers and their relationship with predominant pathophysiological characteristics of Parkinson’s disease. Mitochondrial dysfunction, OS, and abnormal protein accumulation are involved in dopaminergic neuronal death in PD pathogenesis. Biomarkers related to these events are shown, including the sample type for their detection. Created with Biorender.com
α-Syn seems to be the leading candidate biomarker for PD detection, as it can be found in diverse tissue and fluid samples, including skin, saliva, blood, and urine. However, there is still controversy regarding its sensitivity and specificity. Increasing evidence supports that microbiota influences α-Syn accumulation in the gut, from where it travels through the vagus nerve to the CNS; there, it starts aggregating and forming Lewy bodies until they cross the blood–brain barrier and disseminate systemically.
Significant progress has been achieved on the human metabolome in the last decade. Without a doubt, metabolomic profiles are a promising diagnostic tool of a diseased state as they reflect the metabolic state. Changes in biofluid metabolites seem to be a potential biomarker profile given the ease of obtaining the sample. However, these profiles have only been analyzed in patients who already present one or more motor symptoms. Still, there is a long way to find a biomarker profile for early idiopathic PD diagnosis.
PD patients suffer from early intestinal inflammation and dysfunction. In agreement with the gut-brain axis bidirectionality and its implication in PD pathogenesis, it is reasonable to hypothesize that the biomarkers with the best potential for early idiopathic PD diagnosis are those found in feces. Feces represent a non-invasive sample, where zonulin, α-1 antitrypsin, and calprotectin are stable and reflect increased intestinal permeability, damage, and inflammation. However, these biomolecules are not specific for PD by themselves, but they are connected to the gut modifications that PD patient suffers years before clinical symptoms are present.
The intestinal microbiota plays a crucial role in PD development. Despite intestinal microbiota’s complexity, some bacteria families have been found to increase or decrease in two or three PD patients’ population groups compared to control subjects (Table 1). Microbiota variations in the reported populations (German, Chinese, Italian, American, and Finnish) need to be validated in more diverse populations; other and additional differences could be present and be population-specific. To deploy a more precise medicine, potential confounders such as medications, diet, gastrointestinal symptoms, demographics, and others, can be controlled through a systematic approach [170]. Further studies are crucial to assure this biomarker panel sensitivity, specificity, repeatability, and reliability.
Can We Exploit These Biomarkers as a Diagnostic Approach?
Probably, a panel consisting of zonulin, α-1 antitrypsin, and calprotectin, which are related to early intestinal inflammation and dysfunction, along with Lactobacillaceae, Enterococcaceae, Christensenellaceae, Verrucomicrobiaceae, Enterobacteriaceae, Prevotellaceae, Lachnospiraceae, and Bacteroidetes quantification, all of which can be detected from feces, have an excellent potential for early idiopathic PD diagnosis. Protein biomarker modification can be detected with specific antibodies, while microbiota alterations can be simultaneously detected by real-time multiplex PCRs of the 16S rRNA gene with family-specific oligonucleotide sets. Further studies are crucial to assure this biomarker panel sensitivity, specificity, repeatability, and reliability. Additionally, particular caution must be exercised in PD patients since substantial variability was observed in PD subtype classification within 1 year of analysis in a Parkinson’s Progression Markers Initiative (PPMI) [171]. Hopefully, this biomarker panel may help identify subjects in the early stages of the illness and reduce the heterogeneity of disease patients in clinical trials or epidemiologic studies, leading to a better understanding of PD pathogenesis.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
References
Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, Larsen JP (2008) Epidemiology of Parkinson’s disease. J Neurol 255(Suppl 5):18–32. https://doi.org/10.1007/s00415-008-5004-3
Horowitz MP, Greenamyre JT (2010) Gene-environment interactions in Parkinson’s disease: the importance of animal modeling. Clin Pharmacol Ther 88(4):467–474. https://doi.org/10.1038/clpt.2010.138[pii]
Allam MF, Del Castillo AS, Navajas RF-C (2005) Parkinson’s disease risk factors: genetic, environmental, or both? Neurol Res 27(2):206–208. https://doi.org/10.1179/016164105X22057
Hornykiewicz O (2006) The discovery of dopamine deficiency in the parkinsonian brain. J Neural Transm Suppl 70:9–15. https://doi.org/10.1007/978-3-211-45295-0_3
Shults CW (2006) Lewy bodies. Proc Natl Acad Sci U S A 103(6):1661–1668. https://doi.org/10.1073/pnas.0509567103
Levy OA, Malagelada C, Greene LA (2009) Cell death pathways in Parkinson’s disease: proximal triggers, distal effectors, and final steps. Apoptosis 14(4):478–500. https://doi.org/10.1007/s10495-008-0309-3
Beitz JM (2014) Parkinson’s disease: a review. Front Biosci (Schol Ed) 6:65–74. https://doi.org/10.2741/s415
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56(1):33–39. https://doi.org/10.1001/archneur.56.1.33
Löhle M, Storch A, Reichmann H (2009) Beyond tremor and rigidity: non-motor features of Parkinson’s disease. J Neural Transm (Vienna) 116(11):1483–1492. https://doi.org/10.1007/s00702-009-0274-1
Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, Grandinetti A, Blanchette PL, et al (2001) Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57(3):456–462. https://doi.org/10.1212/wnl.57.3.456
Hornykiewicz O (2002) Dopamine miracle: from brain homogenate to dopamine replacement. Mov Disord 17(3):501–508. https://doi.org/10.1002/mds.10115
Rao SS, Hofmann LA, Shakil A (2006) Parkinson’s disease: diagnosis and treatment. Am Fam Physician 74(12):2046–2054
Duarte-Jurado AP, Gopar-Cuevas Y, Saucedo-Cardenas O, Loera-Arias MJ, Montes-de-Oca-Luna R, Garcia-Garcia A, Rodriguez-Rocha H (2021) Antioxidant therapeutics in Parkinson’s disease: current challenges and opportunities. Antioxidants 10(3):453. https://doi.org/10.3390/antiox10030453
Stoker TB, Barker RA (2020) Recent developments in the treatment of Parkinson’s disease. F1000Res 9:862. https://doi.org/10.12688/f1000research.25634.1
Balestrino R, Schapira AHV (2020) Parkinson disease. Eur J Neurol 27(1):27–42. https://doi.org/10.1111/ene.14108
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376. https://doi.org/10.1136/jnnp.2007.131045
Wu Y, Yao Q, Jiang GX, Wang G, Cheng Q (2020) Identification of distinct blood-based biomarkers in early stage of Parkinson’s disease. Neurol Sci 41(4):893–901. https://doi.org/10.1007/s10072-019-04165-y
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, et al (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–2170. https://doi.org/10.1002/mds.22340
Chaudhuri KR, Schrag A, Weintraub D, Rizos A, Rodriguez-Blazquez C, Mamikonyan E, Martinez-Martin P (2020) The movement disorder society nonmotor rating scale: initial validation study. Mov Disord 35(1):116–133. https://doi.org/10.1002/mds.27862
Heinzel S, Berg D, Gasser T, Chen H, Yao C, Postuma RB, Disease MTFotDoPs (2019) Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord 34(10):1464–1470. https://doi.org/10.1002/mds.27802
Marsili L, Rizzo G, Colosimo C (2018) Diagnostic criteria for Parkinson’s disease: from James Parkinson to the concept of prodromal disease. Front Neurol 9:156. https://doi.org/10.3389/fneur.2018.00156
Joutsa J, Gardberg M, Röyttä M, Kaasinen V (2014) Diagnostic accuracy of parkinsonism syndromes by general neurologists. Parkinsonism Relat Disord 20(8):840–844. https://doi.org/10.1016/j.parkreldis.2014.04.019
Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G (2016) Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 86(6):566–576. https://doi.org/10.1212/WNL.0000000000002350
Kalender WA (2006) X-ray computed tomography. Phys Med Biol 51(13):R29-43. https://doi.org/10.1088/0031-9155/51/13/R03
Emamzadeh FN, Surguchov A (2018) Parkinson’s disease: biomarkers, treatment, and risk factors. Front Neurosci 12:612. https://doi.org/10.3389/fnins.2018.00612
Lotankar S, Prabhavalkar KS, Bhatt LK (2017) Biomarkers for Parkinson’s disease: recent advancement. Neurosci Bull 33(5):585–597. https://doi.org/10.1007/s12264-017-0183-5
Meyer PT, Frings L, Rücker G, Hellwig S (2017) F-FDG PET in Parkinsonism: differential diagnosis and evaluation of cognitive impairment. J Nucl Med 58(12):1888–1898. https://doi.org/10.2967/jnumed.116.186403
Skowronek C, Zange L, Lipp A (2019) Cardiac 123I-MIBG scintigraphy in neurodegenerative Parkinson syndromes: performance and pitfalls in clinical practice. Front Neurol 10:152. https://doi.org/10.3389/fneur.2019.00152
Rascol O, Schelosky L (2009) 123I-metaiodobenzylguanidine scintigraphy in Parkinson’s disease and related disorders. Mov Disord 24(Suppl 2):S732–741. https://doi.org/10.1002/mds.22499
Group BDW (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69(3):89–95 https://doi.org/10.1067/mcp.2001.113989
Huss R (2015) Biomarkers. In: Atala A, Allickson JG (eds) Translational regenerative medicine. pp 235–241. https://doi.org/10.1016/b978-0-12-410396-2.00019-0
Strimbu K, Tavel JA (2010) What are biomarkers? Curr Opin HIV AIDS 5(6):463–466. https://doi.org/10.1097/COH.0b013e32833ed177
Mayeux R (2004) Biomarkers: potential uses and limitations. NeuroRx 1(2):182–188. https://doi.org/10.1602/neurorx.1.2.182
Parnetti L, Gaetani L, Eusebi P, Paciotti S, Hansson O, El-Agnaf O, Mollenhauer B, Blennow K, et al (2019) CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol 18(6):573–586. https://doi.org/10.1016/S1474-4422(19)30024-9
Espay AJ, Schwarzschild MA, Tanner CM, Fernandez HH, Simon DK, Leverenz JB, Merola A, Chen-Plotkin A, et al (2017) Biomarker-driven phenotyping in Parkinson’s disease: a translational missing link in disease-modifying clinical trials. Mov Disord 32(3):319–324. https://doi.org/10.1002/mds.26913
Breydo L, Wu JW (1822) Uversky VN (2012) Α-synuclein misfolding and Parkinson’s disease. Biochim Biophys Acta 2:261–285. https://doi.org/10.1016/j.bbadis.2011.10.002
Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M (2005) Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proc Natl Acad Sci U S A 102(5):1430–1435. https://doi.org/10.1073/pnas.0407146102
Villar-Piqué A, Lopes da Fonseca T, Outeiro TF (2016) Structure, function and toxicity of alpha-synuclein: the Bermuda triangle in synucleinopathies. J Neurochem 139(Suppl 1):240–255. https://doi.org/10.1111/jnc.13249
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, et al (2003) alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302(5646):841. https://doi.org/10.1126/science.1090278
Smith WW, Margolis RL, Li X, Troncoso JC, Lee MK, Dawson VL, Dawson TM, Iwatsubo T, et al (2005) Alpha-synuclein phosphorylation enhances eosinophilic cytoplasmic inclusion formation in SH-SY5Y cells. J Neurosci 25(23):5544–5552. https://doi.org/10.1523/JNEUROSCI.0482-05.2005
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, et al (2002) alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4(2):160–164. https://doi.org/10.1038/ncb748
Chahine LM, Beach TG, Brumm MC, Adler CH, Coffey CS, Mosovsky S, Caspell-Garcia C, Serrano GE, et al (2020) In vivo distribution of α-synuclein in multiple tissues and biofluids in Parkinson disease. Neurology 95(9):e1267–e1284. https://doi.org/10.1212/WNL.0000000000010404
Castanedo-Cazares JP, Rodriguez-Leyva I (2015) Skin biomarkers for neurodegenerative disease: a future perspective. Neurodegener Dis Manag 5(6):465–467. https://doi.org/10.2217/nmt.15.51
Teves JMY, Bhargava V, Kirwan KR, Corenblum MJ, Justiniano R, Wondrak GT, Anandhan A, Flores AJ, et al (2017) Parkinson’s disease skin fibroblasts display signature alterations in growth, redox homeostasis, mitochondrial function, and autophagy. Front Neurosci 11:737. https://doi.org/10.3389/fnins.2017.00737
Rodríguez-Leyva I, Calderón-Garcidueñas AL, Jiménez-Capdeville ME, Rentería-Palomo AA, Hernandez-Rodriguez HG, Valdés-Rodríguez R, Fuentes-Ahumada C, Torres-Álvarez B, et al (2014) α-Synuclein inclusions in the skin of Parkinson’s disease and parkinsonism. Ann Clin Transl Neurol 1(7):471–478. https://doi.org/10.1002/acn3.78
Atarashi R, Sano K, Satoh K, Nishida N (2011) Real-time quaking-induced conversion: a highly sensitive assay for prion detection. Prion 5(3):150–153. https://doi.org/10.4161/pri.5.3.16893
Barria MA, Gonzalez-Romero D, Soto C (2012) Cyclic amplification of prion protein misfolding. Methods Mol Biol 849:199–212. https://doi.org/10.1007/978-1-61779-551-0_14
Manne S, Kondru N, Jin H, Serrano GE, Anantharam V, Kanthasamy A, Adler CH, Beach TG, et al (2020) Blinded RT-QuIC Analysis of α-Synuclein biomarker in skin tissue from Parkinson’s disease patients. Mov Disord 35(12):2230–2239. https://doi.org/10.1002/mds.28242
Wang Z, Becker K, Donadio V, Siedlak S, Yuan J, Rezaee M, Incensi A, Kuzkina A, et al (2020) Skin α-synuclein aggregation seeding activity as a novel biomarker for Parkinson disease. JAMA Neurol 78(1):30–40. https://doi.org/10.1001/jamaneurol.2020.3311
Atarashi R, Wilham JM, Christensen L, Hughson AG, Moore RA, Johnson LM, Onwubiko HA, Priola SA, et al (2008) Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat Methods 5(3):211–212. https://doi.org/10.1038/nmeth0308-211
Wang N, Gibbons CH, Lafo J, Freeman R (2013) α-Synuclein in cutaneous autonomic nerves. Neurology 81(18):1604–1610. https://doi.org/10.1212/WNL.0b013e3182a9f449
Trivedi DK, Sinclair E, Xu Y, Sarkar D, Walton-Doyle C, Liscio C, Banks P, Milne J, et al (2019) Discovery of volatile biomarkers of Parkinson’s disease from Sebum. ACS Cent Sci 5(4):599–606. https://doi.org/10.1021/acscentsci.8b00879
Kim YJ, Lee CM, Kim S, Jang JW, Lee SY, Lee SH (2019) Risk of Parkinson’s disease after colectomy: longitudinal follow-up study using a national sample cohort. J Neurol. https://doi.org/10.1007/s00415-019-09617-1
Felice VD, Quigley EM, Sullivan AM, O’Keeffe GW, O’Mahony SM (2016) Microbiota-gut-brain signalling in Parkinson’s disease: implications for non-motor symptoms. Parkinsonism Relat Disord 27:1–8. https://doi.org/10.1016/j.parkreldis.2016.03.012
Zhu X, Han Y, Du J, Liu R, Jin K, Yi W (2017) Microbiota-gut-brain axis and the central nervous system. Oncotarget 8 (32):53829–53838. https://doi.org/10.18632/oncotarget.17754
Vlassov AV, Magdaleno S, Setterquist R (1820) Conrad R (2012) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 7:940–948. https://doi.org/10.1016/j.bbagen.2012.03.017
Mathivanan S, Fahner CJ, Reid GE, Simpson RJ (2012) ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res 40 (Database issue):D1241–1244. https://doi.org/10.1093/nar/gkr828
Proia P, Schiera G, Mineo M, Ingrassia AM, Santoro G, Savettieri G, Di Liegro I (2008) Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. Int J Mol Med 21(1):63–67
Schiera G, Proia P, Alberti C, Mineo M, Savettieri G, Di Liegro I (2007) Neurons produce FGF2 and VEGF and secrete them at least in part by shedding extracellular vesicles. J Cell Mol Med 11(6):1384–1394. https://doi.org/10.1111/j.1582-4934.2007.00100.x
Pant S, Hilton H, Burczynski ME (2012) The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol 83(11):1484–1494. https://doi.org/10.1016/j.bcp.2011.12.037
Howitt J, Hill AF (2016) Exosomes in the pathology of neurodegenerative diseases. J Biol Chem 291(52):26589–26597. https://doi.org/10.1074/jbc.R116.757955
Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, Li Y, Aro P, et al (2014) Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol 128(5):639–650. https://doi.org/10.1007/s00401-014-1314-y
Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, Vanderburg CR, McLean PJ (2012) Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 7:42. https://doi.org/10.1186/1750-1326-7-42
Cao Z, Wu Y, Liu G, Jiang Y, Wang X, Wang Z, Feng T (2019) α-Synuclein in salivary extracellular vesicles as a potential biomarker of Parkinson’s disease. Neurosci Lett 696:114–120. https://doi.org/10.1016/j.neulet.2018.12.030
Lynge Pedersen AM, Belstrøm D (2019) The role of natural salivary defences in maintaining a healthy oral microbiota. J Dent 80(Suppl 1):S3–S12. https://doi.org/10.1016/j.jdent.2018.08.010
Marsh PD, Do T, Beighton D (2000) Devine DA (2016) Influence of saliva on the oral microbiota. Periodontol 70(1):80–92. https://doi.org/10.1111/prd.12098
Franzosa EA, Morgan XC, Segata N, Waldron L, Reyes J, Earl AM, Giannoukos G, Boylan MR, et al (2014) Relating the metatranscriptome and metagenome of the human gut. Proc Natl Acad Sci U S A 111(22):E2329-2338. https://doi.org/10.1073/pnas.1319284111
Schmidt TS, Hayward MR, Coelho LP, Li SS, Costea PI, Voigt AY, Wirbel J, Maistrenko OM, et al (2019) Extensive transmission of microbes along the gastrointestinal tract. Elife 8. https://doi.org/10.7554/eLife.42693
Mihaila D, Donegan J, Barns S, LaRocca D, Du Q, Zheng D, Vidal M, Neville C, et al (2019) The oral microbiome of early stage Parkinson’s disease and its relationship with functional measures of motor and non-motor function. PLoS ONE 14(6):e0218252. https://doi.org/10.1371/journal.pone.0218252
Belibasakis GN, Bostanci N, Marsh PD, Zaura E (2019) Applications of the oral microbiome in personalized dentistry. Arch Oral Biol 104:7–12. https://doi.org/10.1016/j.archoralbio.2019.05.023
Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H, Coron E, Bruley des Varannes S, et al (2013) Colonic inflammation in Parkinson’s disease. Neurobiol Dis 50:42–48. https://doi.org/10.1016/j.nbd.2012.09.007
Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, et al (2009) Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A 106(39):16799–16804. https://doi.org/10.1073/pnas.0906773106
El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A (2002) Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 123(5):1607–1615. https://doi.org/10.1053/gast.2002.36578
Wilson CM, McGilligan K, Thomas DW (1988) Determination of fecal alpha 1-antitrypsin concentration by radial immunodiffusion: two systems compared. Clin Chem 34(2):372–376
Silva MT (2010) When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol 87(1):93–106. https://doi.org/10.1189/jlb.0809549
Hessian PA, Edgeworth J, Hogg N (1993) MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J Leukoc Biol 53(2):197–204
Roth J, Goebeler M, Wrocklage V, van den Bos C, Sorg C (1994) Expression of the calcium-binding proteins MRP8 and MRP14 in monocytes is regulated by a calcium-induced suppressor mechanism. Biochem J 301(Pt 3):655–660. https://doi.org/10.1042/bj3010655
Eue I, König S, Pior J, Sorg C (2002) S100A8, S100A9 and the S100A8/A9 heterodimer complex specifically bind to human endothelial cells: identification and characterization of ligands for the myeloid-related proteins S100A9 and S100A8/A9 on human dermal microvascular endothelial cell line-1 cells. Int Immunol 14(3):287–297. https://doi.org/10.1093/intimm/14.3.287
Clohessy PA, Golden BE (1995) Calprotectin-mediated zinc chelation as a biostatic mechanism in host defence. Scand J Immunol 42(5):551–556. https://doi.org/10.1111/j.1365-3083.1995.tb03695.x
Masson PL, Heremans JF, Schonne E (1969) Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med 130(3):643–658. https://doi.org/10.1084/jem.130.3.643
Summerton CB, Longlands MG, Wiener K, Shreeve DR (2002) Faecal calprotectin: a marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol 14(8):841–845. https://doi.org/10.1097/00042737-200208000-00005
Guerrant RL, Araujo V, Soares E, Kotloff K, Lima AA, Cooper WH, Lee AG (1992) Measurement of fecal lactoferrin as a marker of fecal leukocytes. J Clin Microbiol 30(5):1238–1242. https://doi.org/10.1128/JCM.30.5.1238-1242.1992
Schwiertz A, Spiegel J, Dillmann U, Grundmann D, Bürmann J, Faßbender K, Schäfer KH, Unger MM (2018) Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord 50:104–107. https://doi.org/10.1016/j.parkreldis.2018.02.022
Mulak A, Koszewicz M, Panek-Jeziorna M, Koziorowska-Gawron E, Budrewicz S (2019) Fecal calprotectin as a marker of the gut immune system activation is elevated in Parkinson’s disease. Front Neurosci 13:992. https://doi.org/10.3389/fnins.2019.00992
Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F (1988) Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 76(3):217–221. https://doi.org/10.1007/BF00687767
Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, Broughton E, Hagan H, et al (2014) Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol 127(2):235–241. https://doi.org/10.1007/s00401-013-1214-6
Yan F, Chen Y, Li M, Wang Y, Zhang W, Chen X, Ye Q (2018) Gastrointestinal nervous system α-synuclein as a potential biomarker of Parkinson disease. Medicine (Baltimore) 97(28):e11337. https://doi.org/10.1097/MD.0000000000011337
Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, et al (2011) Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 6(12):e28032. https://doi.org/10.1371/journal.pone.0028032
Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, et al (2011) The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil 23(12):1132–1139. https://doi.org/10.1111/j.1365-2982.2011.01796.x
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 108(38):16050–16055. https://doi.org/10.1073/pnas.1102999108
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, et al (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167(6):1469-1480.e1412. https://doi.org/10.1016/j.cell.2016.11.018
Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, et al (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18(7):965–977. https://doi.org/10.1038/nn.4030
Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, et al (2011) Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A 108(Suppl 1):4586–4591. https://doi.org/10.1073/pnas.1000097107
Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, et al (2016) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32:66–72. https://doi.org/10.1016/j.parkreldis.2016.08.019
Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28(10):1221–1227. https://doi.org/10.1136/gut.28.10.1221
Hamer HM, Jonkers DM, Bast A, Vanhoutvin SA, Fischer MA, Kodde A, Troost FJ, Venema K, et al (2009) Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr 28(1):88–93. https://doi.org/10.1016/j.clnu.2008.11.002
Neunlist M, Dobreva G, Schemann M (1999) Characteristics of mucosally projecting myenteric neurones in the guinea-pig proximal colon. J Physiol 517(Pt 2):533–546. https://doi.org/10.1111/j.1469-7793.1999.0533t.x
Clark A, Mach N (2017) The crosstalk between the gut microbiota and mitochondria during exercise. Front Physiol 8:319. https://doi.org/10.3389/fphys.2017.00319
Ma J, Coarfa C, Qin X, Bonnen PE, Milosavljevic A, Versalovic J, Aagaard K (2014) mtDNA haplogroup and single nucleotide polymorphisms structure human microbiome communities. BMC Genomics 15:257. https://doi.org/10.1186/1471-2164-15-257
Giles RE, Blanc H, Cann HM, Wallace DC (1980) Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A 77(11):6715–6719. https://doi.org/10.1073/pnas.77.11.6715
Moeller AH, Suzuki TA, Phifer-Rixey M, Nachman MW (2018) Transmission modes of the mammalian gut microbiota. Science 362(6413):453–457. https://doi.org/10.1126/science.aat7164
Yardeni T, Tanes CE, Bittinger K, Mattei LM, Schaefer PM, Singh LN, Wu GD, Murdock DG, et al (2019) Host mitochondria influence gut microbiome diversity: A role for ROS. Sci Signal 12(588). https://doi.org/10.1126/scisignal.aaw3159
Chahine LM, Stern MB, Chen-Plotkin A (2014) Blood-based biomarkers for Parkinson’s disease. Parkinsonism Relat Disord 20(Suppl 1):S99-103. https://doi.org/10.1016/S1353-8020(13)70025-7
Miller DW, Hague SM, Clarimon J, Baptista M, Gwinn-Hardy K, Cookson MR, Singleton AB (2004) Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology 62(10):1835–1838. https://doi.org/10.1212/01.wnl.0000127517.33208.f4
Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Fox M, Goldstein JM, et al (2008) Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis 5(2):55–59. https://doi.org/10.1159/000112832
Tian C, Liu G, Gao L, Soltys D, Pan C, Stewart T, Shi M, Xie Z, et al (2019) Erythrocytic α-Synuclein as a potential biomarker for Parkinson’s disease. Transl Neurodegener 8:15. https://doi.org/10.1186/s40035-019-0155-y
Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E (2008) The antioxidant properties of serum albumin. FEBS Lett 582(13):1783–1787. https://doi.org/10.1016/j.febslet.2008.04.057
Ueno SI, Hatano T, Okuzumi A, Saiki S, Oji Y, Mori A, Koinuma T, Fujimaki M, et al (2020) Nonmercaptalbumin as an oxidative stress marker in Parkinson’s and PARK2 disease. Ann Clin Transl Neurol 7(3):307–317. https://doi.org/10.1002/acn3.50990
Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H (2019) Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 90(8):870–881. https://doi.org/10.1136/jnnp-2018-320106
Disanto G, Barro C, Benkert P, Naegelin Y, Schädelin S, Giardiello A, Zecca C, Blennow K, et al (2017) Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 81(6):857–870. https://doi.org/10.1002/ana.24954
Lin YS, Lee WJ, Wang SJ, Fuh JL (2018) Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci Rep 8(1):17368. https://doi.org/10.1038/s41598-018-35766-w
Lin CH, Chiu MJ (2020) Author response: Blood NfL: a biomarker for disease severity and progression in Parkinson disease. Neurology 95(14):658. https://doi.org/10.1212/WNL.0000000000010664
Sampedro F, Pérez-González R, Martínez-Horta S, Marín-Lahoz J, Pagonabarraga J, Kulisevsky J (2020) Serum neurofilament light chain levels reflect cortical neurodegeneration in de novo Parkinson’s disease. Parkinsonism Relat Disord 74:43–49. https://doi.org/10.1016/j.parkreldis.2020.04.009
Marques TM, van Rumund A, Oeckl P, Kuiperij HB, Esselink RAJ, Bloem BR, Otto M, Verbeek MM (2019) Serum NFL discriminates Parkinson disease from atypical parkinsonisms. Neurology 92(13):e1479–e1486. https://doi.org/10.1212/WNL.0000000000007179
Chung CC, Chan L, Chen JH, Bamodu OA, Hong CT (2020) Neurofilament light chain level in plasma extracellular vesicles and Parkinson’s disease. Ther Adv Neurol Disord 13:1756286420975917. https://doi.org/10.1177/1756286420975917
Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, et al (2017) Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology 88(10):930–937. https://doi.org/10.1212/WNL.0000000000003680
Bäckström D, Linder J, Jakobson Mo S, Riklund K, Zetterberg H, Blennow K, Forsgren L, Lenfeldt N (2020) NfL as a biomarker for neurodegeneration and survival in Parkinson disease. Neurology 95(7):e827–e838. https://doi.org/10.1212/WNL.0000000000010084
Mizobuchi N, Hoseki J, Kubota H, Toyokuni S, Nozaki J, Naitoh M, Koizumi A, Nagata K (2007) ARMET is a soluble ER protein induced by the unfolded protein response via ERSE-II element. Cell Struct Funct 32(1):41–50. https://doi.org/10.1247/csf.07001
Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire AE, Shridhar V, et al (2003) MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci 20(2):173–188. https://doi.org/10.1385/jmn:20:2:173
Zhang GL, Wang LH, Liu XY, Zhang YX, Hu MY, Liu L, Fang YY, Mu Y, et al (2018) Cerebral dopamine neurotrophic factor (CDNF) has neuroprotective effects against cerebral ischemia that may occur through the endoplasmic reticulum stress pathway. Int J Mol Sci 19(7):1905. https://doi.org/10.3390/ijms19071905
Volmer R, van der Ploeg K, Ron D (2013) Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc Natl Acad Sci U S A 110(12):4628–4633. https://doi.org/10.1073/pnas.1217611110
Sousa-Victor P, Neves J, Cedron-Craft W, Ventura PB, Liao CY, Riley RR, Soifer I, van Bruggen N, et al (2019) MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat Metab 1(2):276–290. https://doi.org/10.1038/s42255-018-0023-6
Galli E, Planken A, Kadastik-Eerme L, Saarma M, Taba P, Lindholm P (2019) Increased serum levels of mesencephalic astrocyte-derived neurotrophic factor in subjects with Parkinson’s disease. Front Neurosci 13:929. https://doi.org/10.3389/fnins.2019.00929
Shang P, Zhang Y, Ma D, Hao Y, Wang X, Xin M, Zhu M, Feng J (2019) Inflammation resolution and specialized pro-resolving lipid mediators in CNS diseases. Expert Opin Ther Targets 23(11):967–986. https://doi.org/10.1080/14728222.2019.1691525
Abdolmaleki F, Kovanen PT, Mardani R, Gheibi-Hayat SM, Bo S, Sahebkar A (2020) Resolvins: emerging players in autoimmune and inflammatory diseases. Clin Rev Allergy Immunol 58(1):82–91. https://doi.org/10.1007/s12016-019-08754-9
Ren YZ, Zhang BZ, Zhao XJ, Zhang ZY (2020) Resolvin D1 ameliorates cognitive impairment following traumatic brain injury via protecting astrocytic mitochondria. J Neurochem 154(5):530–546. https://doi.org/10.1111/jnc.14962
Krashia P, Cordella A, Nobili A, La Barbera L, Federici M, Leuti A, Campanelli F, Natale G, et al (2019) Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat Commun 10(1):3945. https://doi.org/10.1038/s41467-019-11928-w
Jenner P, Olanow CW (1996) Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 47(6 Suppl 3):S161-170. https://doi.org/10.1212/wnl.47.6_suppl_3.161s
Cipriani S, Chen X, Schwarzschild MA (2010) Urate: a novel biomarker of Parkinson’s disease risk, diagnosis and prognosis. Biomark Med 4(5):701–712. https://doi.org/10.2217/bmm.10.94
Hediger MA, Johnson RJ, Miyazaki H, Endou H (2005) Molecular physiology of urate transport. Physiology (Bethesda) 20:125–133. https://doi.org/10.1152/physiol.00039.2004
Yeum KJ, Russell RM, Krinsky NI, Aldini G (2004) Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch Biochem Biophys 430(1):97–103. https://doi.org/10.1016/j.abb.2004.03.006
de Lau LM, Koudstaal PJ, Hofman A, Breteler MM (2005) Serum uric acid levels and the risk of Parkinson disease. Ann Neurol 58(5):797–800. https://doi.org/10.1002/ana.20663
Church WH, Ward VL (1994) Uric acid is reduced in the substantia nigra in Parkinson’s disease: effect on dopamine oxidation. Brain Res Bull 33(4):419–425. https://doi.org/10.1016/0361-9230(94)90285-2
Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM (1996) Observations on serum uric acid levels and the risk of idiopathic Parkinson’s disease. Am J Epidemiol 144(5):480–484. https://doi.org/10.1093/oxfordjournals.aje.a008954
Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A (2007) Plasma urate and risk of Parkinson’s disease. Am J Epidemiol 166(5):561–567. https://doi.org/10.1093/aje/kwm127
O’Reilly EJ, Gao X, Weisskopf MG, Chen H, Schwarzschild MA, Spiegelman D, Ascherio A (2010) Plasma urate and Parkinson’s disease in women. Am J Epidemiol 172(6):666–670. https://doi.org/10.1093/aje/kwq195
Gao X, O’Reilly É, Schwarzschild MA, Ascherio A (2016) Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology 86(6):520–526. https://doi.org/10.1212/WNL.0000000000002351
Cantoni GL (1953) The nature of the active methyl donor formed enzymatically from L-methionine and adenosinetriphosphate. J Amer Chem Soc 74:2942–2943. https://doi.org/10.1021/ja01131a519
Silla Y, Varshney S, Ray A, Basak T, Zinellu A, Sabareesh V, Carru C, Sengupta S (2019) Hydrolysis of homocysteine thiolactone results in the formation of Protein-Cys-S-S-homocysteinylation. Proteins 87(8):625–634. https://doi.org/10.1002/prot.25681
Hasan T, Arora R, Bansal AK, Bhattacharya R, Sharma GS, Singh LR (2019) Disturbed homocysteine metabolism is associated with cancer. Exp Mol Med 51(2):1–13. https://doi.org/10.1038/s12276-019-0216-4
Quadri P, Fragiacomo C, Pezzati R, Zanda E, Forloni G, Tettamanti M, Lucca U (2004) Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am J Clin Nutr 80(1):114–122. https://doi.org/10.1093/ajcn/80.1.114
Kuhn W, Roebroek R, Blom H, van Oppenraaij D, Przuntek H, Kretschmer A, Büttner T, Woitalla D, et al (1998) Elevated plasma levels of homocysteine in Parkinson’s disease. Eur Neurol 40(4):225–227. https://doi.org/10.1159/000007984
Sleeman I, Lawson RA, Yarnall AJ, Duncan GW, Johnston F, Khoo TK, Burn DJ (2019) Urate and homocysteine: predicting motor and cognitive changes in newly diagnosed Parkinson’s disease. J Parkinsons Dis 9(2):351–359. https://doi.org/10.3233/JPD-181535
McLeish MJ, Kenyon GL (2005) Relating structure to mechanism in creatine kinase. Crit Rev Biochem Mol Biol 40(1):1–20. https://doi.org/10.1080/10409230590918577
Eppenberger HM, Dawson DM, Kaplan NO (1967) The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. J Biol Chem 242(2):204–209
Wyss M, Smeitink J, Wevers RA, Wallimann T (1992) Mitochondrial creatine kinase: a key enzyme of aerobic energy metabolism. Biochim Biophys Acta 1102(2):119–166. https://doi.org/10.1016/0005-2728(92)90096-k
Schlattner U, Tokarska-Schlattner M, Wallimann T (2006) Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta 1762(2):164–180. https://doi.org/10.1016/j.bbadis.2005.09.004
Beal MF (2000) Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci 23(7):298–304. https://doi.org/10.1016/s0166-2236(00)01584-8
Xu J, Fu X, Pan M, Zhou X, Chen Z, Wang D, Zhang X, Chen Q, et al (2019) Mitochondrial creatine kinase is decreased in the serum of idiopathic Parkinson’s disease patients. Aging Dis 10(3):601–610. https://doi.org/10.14336/AD.2018.0615
Bujak R, Struck-Lewicka W, Markuszewski MJ, Kaliszan R (2015) Metabolomics for laboratory diagnostics. J Pharm Biomed Anal 113:108–120. https://doi.org/10.1016/j.jpba.2014.12.017
Bogdanov M, Matson WR, Wang L, Matson T, Saunders-Pullman R, Bressman SS, Flint Beal M (2008) Metabolomic profiling to develop blood biomarkers for Parkinson’s disease. Brain 131(Pt 2):389–396. https://doi.org/10.1093/brain/awm304
Ahmed SS, Santosh W, Kumar S, Christlet HT (2009) Metabolic profiling of Parkinson’s disease: evidence of biomarker from gene expression analysis and rapid neural network detection. J Biomed Sci 16:63. https://doi.org/10.1186/1423-0127-16-63
Trupp M, Jonsson P, Ohrfelt A, Zetterberg H, Obudulu O, Malm L, Wuolikainen A, Linder J, et al (2014) Metabolite and peptide levels in plasma and CSF differentiating healthy controls from patients with newly diagnosed Parkinson’s disease. J Parkinsons Dis 4(3):549–560. https://doi.org/10.3233/JPD-140389
Yakhine-Diop SMS, Morales-García JA, Niso-Santano M, González-Polo RA, Uribe-Carretero E, Martinez-Chacon G, Durand S, Maiuri MC, et al (2020) Metabolic alterations in plasma from patients with familial and idiopathic Parkinson’s disease. Aging (Albany NY) 12(17):16690–16708. https://doi.org/10.18632/aging.103992
Shao Y, Li T, Liu Z, Wang X, Xu X, Li S, Xu G, Le W (2021) Comprehensive metabolic profiling of Parkinson’s disease by liquid chromatography-mass spectrometry. Mol Neurodegener 16(1):4. https://doi.org/10.1186/s13024-021-00425-8
Johansen KK, Wang L, Aasly JO, White LR, Matson WR, Henchcliffe C, Beal MF, Bogdanov M (2009) Metabolomic profiling in LRRK2-related Parkinson’s disease. PLoS ONE 4(10):e7551. https://doi.org/10.1371/journal.pone.0007551
Grosshans H, Slack FJ (2002) Micro-RNAs: small is plentiful. J Cell Biol 156(1):17–21. https://doi.org/10.1083/jcb.200111033
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75(5):843–854. https://doi.org/10.1016/0092-8674(93)90529-y
Junker A, Krumbholz M, Eisele S, Mohan H, Augstein F, Bittner R, Lassmann H, Wekerle H, et al (2009) MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain 132(Pt 12):3342–3352. https://doi.org/10.1093/brain/awp300
Femminella GD, Ferrara N, Rengo G (2015) The emerging role of microRNAs in Alzheimer’s disease. Front Physiol 6:40. https://doi.org/10.3389/fphys.2015.00040
Behbahanipour M, Peymani M, Salari M, Hashemi MS, Nasr-Esfahani MH, Ghaedi K (2019) Expression profiling of blood microRNAs 885, 361, and 17 in the patients with the Parkinson’s disease: integrating interaction data to uncover the possible triggering age-related mechanisms. Sci Rep 9(1):13759. https://doi.org/10.1038/s41598-019-50256-3
Wu J, Gao Y (2015) Physiological conditions can be reflected in human urine proteome and metabolome. Expert Rev Proteomics 12(6):623–636. https://doi.org/10.1586/14789450.2015.1094380
Singh A, Kukreti R, Saso L, Kukreti S (2019) Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 24(8). https://doi.org/10.3390/molecules24081583
Sato S, Mizuno Y, Hattori N (2005) Urinary 8-hydroxydeoxyguanosine levels as a biomarker for progression of Parkinson disease. Neurology 64(6):1081–1083. https://doi.org/10.1212/01.WNL.0000154597.24838.6B
Hirayama M, Nakamura T, Watanabe H, Uchida K, Hama T, Hara T, Niimi Y, Ito M, et al (2011) Urinary 8-hydroxydeoxyguanosine correlate with hallucinations rather than motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 17(1):46–49. https://doi.org/10.1016/j.parkreldis.2010.11.004
Tan L, Yu JT (2012) The kynurenine pathway in neurodegenerative diseases: mechanistic and therapeutic considerations. J Neurol Sci 323(1–2):1–8. https://doi.org/10.1016/j.jns.2012.08.005
Bai JH, Zheng YL, Yu YP (2020) Urinary kynurenine as a biomarker for Parkinson’s disease. Neurol Sci. https://doi.org/10.1007/s10072-020-04589-x
Luan H, Liu LF, Meng N, Tang Z, Chua KK, Chen LL, Song JX, Mok VC, et al (2015) LC-MS-based urinary metabolite signatures in idiopathic Parkinson’s disease. J Proteome Res 14(1):467–478. https://doi.org/10.1021/pr500807t
Wang S, Kojima K, Mobley JA, West AB (2019) Proteomic analysis of urinary extracellular vesicles reveal biomarkers for neurologic disease. EBioMedicine 45:351–361. https://doi.org/10.1016/j.ebiom.2019.06.021
Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, et al (2017) Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32(5):739–749. https://doi.org/10.1002/mds.26942
Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Tanner C, Marek K, Investigators P (2016) How stable are Parkinson’s disease subtypes in de novo patients: Analysis of the PPMI cohort? Parkinsonism Relat Disord 28:62–67. https://doi.org/10.1016/j.parkreldis.2016.04.027
Acknowledgements
Y.G.C. (No. CVU: 856246) and A.P.D.J. (No. CVU: 708195) received a scholarship from the National Council of Science and Technology (Consejo Nacional de Ciencia y Tecnología, CONACYT). All figures were created with Biorender.com.
Funding
This research has been funded by Programa de Apoyo a la Investigación Científica y Tecnológica (PAICyT) 2021: SA1872-21 (A.G.G), and SA1891-21 (H.R.R).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gopar-Cuevas, Y., Duarte-Jurado, A.P., Diaz-Perez, R.N. et al. Pursuing Multiple Biomarkers for Early Idiopathic Parkinson’s Disease Diagnosis. Mol Neurobiol 58, 5517–5532 (2021). https://doi.org/10.1007/s12035-021-02500-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02500-z