Abstract
Background and purpose
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by deposition of intraneural inclusion bodies in the brain as well as the enteric nervous system. Emerging concepts regarding the brain–gut axis have been proposed for neurological disorders. Thus, the present study investigated the associations between colectomy and developing PD.
Methods
We conducted a retrospective cohort study using National Health Insurance Service–National Sample Cohort of Korea. This study included patients who underwent colectomy during 2003–2009, and up to 10 individuals per patient, matched in terms of age and sex, who did not undergo colectomy. The colectomy group was subdivided by the causes and surgical methods of colectomy. The risk of PD occurrence was evaluated over a follow-up period of at least 6 years using Cox regression analyses.
Results
Colectomy was associated with a higher risk of developing PD (adjusted hazard ratio [HR]: 1.962; 95% confidence interval [CI] 1.002–3.840). There was no significant difference in the occurrence of PD among the subgroups classified by the causes or surgical methods of colectomy.
Conclusions
Colectomy was associated with the development of PD, suggesting that colon issues play an important role in the pathophysiological mechanisms of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disease with primary clinical features of resting tremor, rigidity, bradykinesia, and postural instability. In addition to the motor symptoms of PD, there are also non-motor symptoms such as dysfunctions of smell, sleep, somatic sense, the autonomic nerve system, and cognition. Although Lewy bodies (LBs)/Lewy neurites (LNs) deposits and dopaminergic cell loss in the substantia nigra are key components of the pathology of PD, various etiologies have also been proposed as pathophysiological mechanisms underlying the development of PD, including genetics, endogenous factors, and environmental risk factors [1,2,3,4]. Recently, evidence of an association between PD and the gut has been reported, such as the appearance of LB in the gut, gut dysbiosis, and the presence of inflammation in the enteric nervous system (ENS).
The well-known pathological findings of PD include intracellular deposits of LB/LN that are composed primarily of alpha-synuclein. The medulla oblongata and olfactory bulb are the initial regions affected by inclusion bodies during the progression of PD [5, 6]. It has been proposed that unmyelinated vagal preganglionic neurons might play an important role in PD by providing a route for the pathology to affect the peripheral nervous system (PNS) as well as central nervous system (CNS) [7]. Furthermore, unidentified pathogens might enter the CNS via the PNS along the so-called “gut-to-brain” axis [8, 9]. Many studies have demonstrated that PD-related lesions, such as deposits of LBs/LNs, occur in the CNS and autonomic nerve system in the gastrointestinal tract, heart, and bladder [1, 10,11,12,13,14,15]. Gastrointestinal dysfunction is a prominent non-motor symptom of PD. For example, weight loss, oropharyngeal dysfunction, dysphagia, gastric dysfunction, colonic dysmotility, and anorectal dysfunction are common gastrointestinal complications of PD [16]. Alpha-synuclein deposits in the ENS can be detected before the onset of PD, even in normal people [11, 17, 18].
There are over 100 trillion microbes in the human gut. The normal microbiome in the gut is believed to play a role in the barrier function of the intestine without acting as pathogens. Recent studies have suggested that there is a bidirectional pathway between the gut and the brain, the so called “brain–gut–microbiome (BGM) axis”, which could explicitly affect brain activity [19]. For example, changes in bidirectional interactions within the BGM axis may result in gastrointestinal and neurological disorders, such as PD [20, 21]. Moreover, inflammatory bowel disease (IBD), such as ulcerative colitis and Crohn’s disease, is also linked to PD in terms of involving cytokines, related animal models, and LRRK2 gene studies [22,23,24].
Based on the results of previous studies of the gut and PD, we speculated that colectomy may be associated with the development of PD by potentially altering the BMG axis. To assess this hypothesis, we investigated the risk of PD associated with colectomy, using data from the Korea National Health Insurance Service (NHIS) program collected from 2002 to 2015, and the risk of PD.
Materials and methods
Data acquisition
The NHIS is a universal health insurance program that offers medical care coverage to all residents of South Korea (http://nhiss.nhis.or.kr); almost all Korean residents are registered. Likewise, all medical care institutions in South Korea must be registered in this program for insurance claims. The NHIS–National Sample Cohort (NHIS-NSC) involves approximately 1 million NHIS and Medical Aid program subscribers, approximately 2% of all Koreans, who were extracted by a stratified sampling method in 2002. This 14-year cohort (2002–2015) of NSC subscribers was tracked in terms of socioeconomic variables (residence, year and month of death, cause of death, and income level) and medical treatment details (health examinations, medical care history, and medical care institutions), allowing for long-term observations and investigations of causal relationships among the variables. Within the NSC, diseases are registered using the Korean Classification of Disease, sixth edition (KCD-6), which was modified from the International Classification of Disease, 10th revision (ICD-10), for use in the NHIS and medical care institutions in South Korea [25]. For medical institutions to make a claim to the NHIS for a specific medical practice, the corresponding distinct treatment and procedure codes for the claim, which correspond to individual KCD-6 codes, must be used.
This population-based matched cohort study conducted using the NHIS-NSC dataset (NHIS-2018-2-197) was approved by the Institutional Review Board of Kangwon National University Hospital (KNUH-2018-06-004).
Analysis strategy
Colectomy was identified using the procedure codes for claims that included total colectomy (Q2672 and QA672), subtotal colectomy (Q1261 and Q1262), hemi-colectomy (Q2671 and QA671), colectomy-segmental resection (Q2673 and QA673), and/or colectomy with colostomy (Q2679 and QA679) from 2002 to 2009. The colectomy group was subdivided into five groups according to the surgical methods described above. The index date for these subjects in the colectomy group was the date the colectomy was performed.
The possible causes of colectomy were inferred manually by two investigators (SH Lee and CM Lee) based on the disease codes registered at the time point adjacent (± 50 days) to the colectomy. Based on the causes of surgery, the colectomy group was also subdivided into six groups: malignant neoplasm, benign tumor, disease of appendix, non-infective inflammation, injury, and other disease of the intestines.
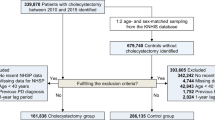
To ensure an observation period of at least 6 years, the colectomy group included subjects aged 40 years or older who underwent colectomy between 2003 and 2009. Patients who underwent colectomy in 2002 or 2010–2015 (n = 105), died within 1 year (n = 4), or had a previous PD diagnosis prior to the index date or within 1 year after colectomy (n = 23) were excluded from the analyses.
The NHIS-NSC database contains information on 1,108,369 subscribers from 2002 to 2015. Since PD is a progressive neurodegenerative disorder influenced by age, we selected subscribers who were aged 40 years or older in 2003 (n = 588,235). The occurrence of PD was defined by the code G20 based on the ICD-10 protocol; subscribers with secondary parkinsonism (G21) or atypical parkinsonism (G22 and G23) were excluded.
In addition to information about the diagnosis and surgical procedures of interest, data regarding the sex, age, body mass index (BMI), and diabetes and hypertension statuses of each subject were extracted [26, 27]. When multiple BMI measurements were available for a single subscriber, the BMI closest to the time of registration was used for the present analyses. The BMI measurements were subcategorized as underweight (< 18.5 kg/m2), normal weight (18.5–2.9 kg/m2), or overweight/obese (≥ 23 kg/m2) [28]. Of the subjects in the exposed group, 185 did not have BMI information and were excluded. Thus, the present study analyzed the data of 511 subjects who underwent colectomy (Fig. 1).
For each subject in the colectomy group, 10 control subjects with no history of colectomy were selected randomly and matched in terms of age, sex, and index date. Subjects in the control group were given the same index date as the subjects with a history of colectomy. Subjects in both groups were followed from the index date until the occurrence of PD, death, or December 31, 2015.
Statistical analyses
The present study estimated the prevalence rates of colectomy and PD in 2003 and the incidence rates of colectomy per 100,000 person-years from 2003 to 2009.
Pearson’s Chi square test was conducted to compare differences in PD occurrence between the colectomy and control groups. Fisher’s exact tests were performed to compare the difference in PD occurrence among the subgroups according to the causes and surgical methods of colectomy. A Cox proportional hazards model was used to calculate adjusted hazard ratios (HR) and 95% confidence intervals (CI) to determine whether colectomy is an independent risk factor for PD, after adjusting for sex, age, diabetes, hypertension, and BMI. Survival was defined as the time from the index date until the diagnosis of PD, censoring due to death, or the end of the study (December 31, 2015); subjects who were never diagnosed with PD by the end of the study were treated as censored. Cumulative incidence curves with 95% confidential intervals for the colectomy and control groups were estimated.
All statistical analyses were performed using the statistical package SAS for Windows, ver. 9.4 (SAS; Cary, NC).
Results
In 2003, the prevalence of patients with PD per 100,000 individuals aged 40 years or older was 155.75. The prevalence rate of PD according to age and sex gradually increased up to 80 years of age (Fig. 2). The crude incidence rates of colectomy and PD per 100,000 person-years were 18.39 and 57.88, respectively, in 2003. Table 1 summarizes the incidence rates of colectomy and PD according to age from 2003 to 2015.
According to NSC, 511 people underwent colectomy from 2003 to 2009. Of these, 359 underwent hemi-colectomy, 104 colectomy-segmental resection, 30 colectomy with colostomy, 16 total colectomy, and two subtotal colectomy. The causes of colectomy were 395 malignant neoplasms, followed by 35 benign tumors, 16 disease of the appendix, 8 non-infective inflammation, 3 injuries, and 54 other diseases of the intestines. However, there was no significant difference in the frequency of PD according to the methods or causes of colectomy (p > 0.05, Table 2).
The frequency of colectomy was higher in subjects with diabetes than in those without diabetes (p < 0.05). There were no other significant differences in age, sex, hypertension, or BMI between the colectomy and control groups (p > 0.05). Throughout the entire observational period, there were 10 (2.0%) total occurrences of PD in the colectomy group and 61 (1.2%) in the control group (p = 0.14). Although there was no significant difference between the two groups in terms of PD occurrence, there tended to be more cases of PD in the colectomy group compared with the control (Table 3, Fig. 3). A higher risk of PD was found in subjects with colectomy than in those without colectomy, after adjusting for sex, age, and BMI with a multivariate Cox regression model (adjusted HR 1.987; 95% CI 1.015–3.891). Further adjustment for sex, age, BMI, diabetes, and hypertension also showed a higher risk associated with the adjusted HR (1.962; 95% CI 1.002–3.840) (Table 4).
Discussion
This study examined a population-based matched cohort of approximately 1 million subscribers to medical insurance and aid in Korea. Without exception, all medical service providers must register with the NHIS program for reimbursement, and thus, the frequencies of colectomy and PD identified during the observational period in the present cohort are likely reliable because medical care institutions are required to submit a claim to the NHIS for colectomy. In 2003, the prevalence of patients with PD per 100,000 individuals aged 40 years or older was 155.75. The prevalence of PD in the present study was somewhat different than those reported previously in South Korea and other Asian countries [29,30,31,32]. These discrepancies may be due to differences in diagnostic criteria and/or statistical approaches. In comparing subjects with and without colectomy, the occurrence of PD tended to be more frequent in the colectomy group. In addition, after adjusting for confounding factors such as hypertension and diabetes, this study found that a history of colectomy was significantly associated with a higher risk of PD.
This study had a large sample size and a 6–12-year observation period, which allowed the investigation of causal relationships between colectomy and PD occurrence. It is possible that the observation period was insufficient for the development of clinical PD, seeing that constipation could precede PD diagnosis by 10 years with wide range of the interval in previous studies of constipation of PD [33,34,35,36]. However, cohort studies with relatively short follow-up durations (about 6 years) reported a positive association between constipation and PD [37,38,39]. In addition, a neuropathology study predicted a 4.6-year preclinical state [40], and a neuroimaging study predicted that the mean preclinical period in PD is unlikely to exceed 7 years, although this report was mainly about CNS disease [41]. Therefore, we believe that our follow-up period was sufficient for assessing the risk of developing PD after colectomy.
There have been many conflicting reports regarding the relationships between the risk of PD and vascular risk factors, including hypertension and diabetes [42,43,44]. Diabetes, hypertension, and obesity are also reported to be associated with colorectal cancer [26, 27]. Regarding the association between PD and colorectal cancer risk, some reported that PD had a negative association with the risk of colorectal cancer [45,46,47], while others reported the opposite [48] or no association [49]. Therefore, it is possible that colon cancer or risk factors for colon cancer, rather than colectomy itself, are related to the development of PD: colectomy is likely to be an interim outcome that reflects several related prior diseases. However, we could not find a difference in the subgroup analysis among the groups according to the causes of colectomy. Moreover, the association between colectomy and PD was confirmed after adjusting for diabetes, hypertension, and BMI.
The highlight of this study is that we examined a hypothesis regarding the brain–gut and BGM axes with real-world data [19,20,21]. This analysis revealed that colectomy was associated with the development of PD for the first time.
Although the pathogenic mechanisms of PD are not fully understood, recent studies suggest that the protective effect of vagotomy on PD based on hypotheses of the transmission of causative agents from the PNS along the vagus nerve [50, 51]. Similarly, it seems reasonable to assume that a colectomy would block the spread of causative agents to the CNS and thereby lower the risk of developing PD. Interestingly, however, the results were the opposite of what was expected by the hypothesis mentioned above.
Two important inferences can be made based on the present results. First, we did not find a significant difference in the occurrence of PD according the method, depending on the extent of gut removal. This result did not support the possibility that the extent of gut removal may influence the spread of the causative agent of PD. Based on this result, we thought that there might be other mechanisms in the gut besides the direct propagation of a causative agent. Interestingly, some studies have shown an increased risk of PD associated with appendectomy, suggesting direct invasion by a neurotrophic enteric pathogen or trigger processes for retrograde propagation of alpha-synuclein to the brain [52], while there was one report with the opposite result [53]. It is unclear whether there is a shared mechanism between appendectomy and colectomy in the development of PD. However, it may suggest that changes in the anatomical structures of the colon due to surgical procedure play an important role in the brain–gut interaction.
Second, colectomy may result in changes in the environment of the colon, which could affect the composition and habitat of the microbiome. Several current studies have investigated the CNS, behaviors, neurotransmitters, and neurotrophic factors based on hypotheses regarding the BGM axis [19,20,21, 54,55,56,57]. One study analyzed fecal microbiomes and found that the abundance of Prevotellaceae in feces was significantly lower in PD patients than in controls, whereas the abundance of Enterobacteriaceae was associated with the severity of gait impairments [58]. Another study reported that PD patients exhibit alterations in colonic microbiota and dysbiosis [59]. Furthermore, another study revealed the presence of small intestinal bacterial overgrowth (SIBO) in patients with PD [60]. Alterations in intestinal anatomy or motility could result in SIBO [61], and some studies have reported an association between SIBO and motor fluctuations in PD, including poor motor function, prolonged off latencies, and delayed on latencies [62, 63]. A higher prevalence of SIBO was observed in patients with colectomy responding to antibiotics [64] and in an experimental rat model [65]. Based on the abovementioned studies, we speculate that alterations in the microbiome resulting from colectomy might be associated with the development of PD.
This study was limited in terms of a lack of clinical PD information related to medical history, social history, and clinical manifestations such as severity and clinical stage in the NHIS-NSC data. According to previous studies, the diagnostic accuracy of PD is approximately 75–90%, which was also a potential bias of selection of PD [66,67,68,69,70]. Additionally, depending on the date of surgery, the duration of observation in the case group was 6–12 years, which may necessitate caution in interpreting our results because of long duration until the development of PD.
In conclusion, we demonstrated that colectomy is associated with the development of PD. This suggests that the colon plays an important role in the pathophysiological mechanisms of PD.
References
Sakakibara R, Hattori T, Uchiyama T et al (2000) Micturitional disturbance in pure autonomic failure. Neurology 54:499–501
Brown TP, Rumsby PC, Capleton AC et al (2006) Pesticides and Parkinson’s disease—is there a link? Environ Health Perspect 114:156–164
Gupta A, Dawson VL, Dawson TM (2008) What causes cell death in Parkinson’s disease? Ann Neurol 64(Suppl 2):S3–S15. https://doi.org/10.1002/ana.21573
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
Braak H, Tredici KD, Rüb U et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211. https://doi.org/10.1016/S0197-4580(02)00065-9
Palma J-A, Kaufmann H (2014) Autonomic disorders predicting Parkinson’s disease. Parkinsonism Relat Disord 20:S94–S98. https://doi.org/10.1016/S1353-8020(13)70024-5
Cersosimo MG, Benarroch EE (2012) Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol Dis 46:559–564. https://doi.org/10.1016/j.nbd.2011.10.014
Braak H, Rüb U, Gai WP, Del Tredici K (2003) Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm Vienna Austria 1996 110:517–536. https://doi.org/10.1007/s00702-002-0808-2
Olanow CW, Prusiner SB (2009) Is Parkinson’s disease a prion disorder? Proc Natl Acad Sci USA 106:12571–12572. https://doi.org/10.1073/pnas.0906759106
Abbott RD, Petrovitch H, White LR et al (2001) Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57:456–462
Shannon KM, Keshavarzian A, Dodiya HB et al (2012) Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord 27:716–719. https://doi.org/10.1002/mds.25020
Postuma RB, Gagnon J-F, Pelletier A, Montplaisir J (2013) Prodromal autonomic symptoms and signs in Parkinson’s disease and dementia with Lewy bodies. Mov Disord 28:597–604. https://doi.org/10.1002/mds.25445
Orimo S, Uchihara T, Nakamura A et al (2008) Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain J Neurol 131:642–650. https://doi.org/10.1093/brain/awm302
Orimo S, Takahashi A, Uchihara T et al (2007) Degeneration of cardiac sympathetic nerve begins in the early disease process of Parkinson’s disease. Brain Pathol Zurich Switz 17:24–30. https://doi.org/10.1111/j.1750-3639.2006.00032.x
Goldstein DS (2006) Orthostatic hypotension as an early finding in Parkinson’s disease. Clin Auton Res 16:46–54. https://doi.org/10.1007/s10286-006-0317-8
Pfeiffer RF (2018) Gastrointestinal Dysfunction in Parkinson’s Disease. Curr Treat Options Neurol 20:54. https://doi.org/10.1007/s11940-018-0539-9
Derkinderen P, Rouaud T, Lebouvier T et al (2011) Parkinson disease: the enteric nervous system spills its guts. Neurology 77:1761–1767. https://doi.org/10.1212/WNL.0b013e318236ef60
Miki Y, Mori F, Wakabayashi K et al (2009) Incidental Lewy body disease restricted to the heart and stellate ganglia. Mov Disord 24:2299–2301. https://doi.org/10.1002/mds.22775
Mulak A (2015) Brain–gut–microbiota axis in Parkinson’s disease. World J Gastroenterol 21:10609. https://doi.org/10.3748/wjg.v21.i37.10609
Parashar A, Udayabanu M (2017) Gut microbiota: implications in Parkinson’s disease. Parkinsonism Relat Disord 38:1–7. https://doi.org/10.1016/j.parkreldis.2017.02.002
Sampson TR, Debelius JW, Thron T et al (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167:1469–1480.e12. https://doi.org/10.1016/j.cell.2016.11.018
Leal MC, Casabona JC, Puntel M, Pitossi FJ (2013) Interleukin-1β and tumor necrosis factor-α: reliable targets for protective therapies in Parkinson’s disease? Front Cell Neurosci 7:53. https://doi.org/10.3389/fncel.2013.00053
Mogi M, Harada M, Riederer P et al (1994) Tumor necrosis factor-α (TNF-α) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett 165:208–210. https://doi.org/10.1016/0304-3940(94)90746-3
Hui KY, Fernandez-Hernandez H, Hu J et al (2018) Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aai7795
An SJ, Lee SH, Lee SY et al (2017) Femur fractures in Parkinsonism: analysis of a national sample cohort in South Korea. J Clin Neurol 13:380–386. https://doi.org/10.3988/jcn.2017.13.4.380
Hanyuda A, Ogino S, Qian ZR et al (2016) Body mass index and risk of colorectal cancer according to tumor lymphocytic infiltrate. Int J Cancer 139:854–868. https://doi.org/10.1002/ijc.30122
Ahmadi A, Mobasheri M, Hashemi-Nazari SS et al (2014) Prevalence of hypertension and type 2 diabetes mellitus in patients with colorectal cancer and their median survival time: a cohort study. J Res Med 19:850–854
Anuurad E, Shiwaku K, Nogi A et al (2003) The new BMI criteria for asians by the regional office for the western pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J Occup Health 45:335–343
Chen RC, Chang SF, Su CL et al (2001) Prevalence, incidence, and mortality of PD A door-to-door survey in Ilan County, Taiwan. Neurology 57:1679–1686
Tan LCS, Venketasubramanian N, Hong CY et al (2004) Prevalence of Parkinson disease in Singapore: Chinese vs Malays vs Indians. Neurology 62:1999–2004
Seo W-K, Koh S-B, Kim B-J et al (2007) Prevalence of Parkinson’s disease in Korea. J Clin Neurosci 14:1155–1157. https://doi.org/10.1016/j.jocn.2006.09.005
Yamawaki M, Kusumi M, Kowa H, Nakashima K (2009) Changes in prevalence and incidence of Parkinson’s disease in Japan during a quarter of a century. Neuroepidemiology 32:263–269. https://doi.org/10.1159/000201565
Schrag A, Horsfall L, Walters K et al (2015) Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. Lancet Neurol 14:57–64. https://doi.org/10.1016/S1474-4422(14)70287-X
Pont-Sunyer C, Hotter A, Gaig C et al (2015) The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD study). Mov Disord 30:229–237. https://doi.org/10.1002/mds.26077
Abbott RD, Ross GW, Petrovitch H et al (2007) Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord 22:1581–1586. https://doi.org/10.1002/mds.21560
Savica R, Carlin JM, Grossardt BR et al (2009) Medical records documentation of constipation preceding Parkinson disease: a case-control study. Neurology 73:1752–1758. https://doi.org/10.1212/WNL.0b013e3181c34af5
Gao X, Chen H, Schwarzschild MA, Ascherio A (2011) A prospective study of bowel movement frequency and risk of Parkinson’s disease. Am J Epidemiol 174:546–551. https://doi.org/10.1093/aje/kwr119
Lin C-H, Lin J-W, Liu Y-C et al (2014) Risk of Parkinson’s disease following severe constipation: a nationwide population-based cohort study. Parkinsonism Relat Disord 20:1371–1375. https://doi.org/10.1016/j.parkreldis.2014.09.026
Cersosimo MG, Raina GB, Pecci C et al (2013) Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J Neurol 260:1332–1338. https://doi.org/10.1007/s00415-012-6801-2
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain J Neurol 114(Pt 5):2283–2301. https://doi.org/10.1093/brain/114.5.2283
Morrish PK, Rakshi JS, Bailey DL et al (1998) Measuring the rate of progression and estimating the preclinical period of Parkinson’s disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry 64:314–319. https://doi.org/10.1136/jnnp.64.3.314
Powers KM, Smith-Weller T, Franklin GM et al (2006) Diabetes, smoking, and other medical conditions in relation to Parkinson’s disease risk. Parkinsonism Relat Disord 12:185–189. https://doi.org/10.1016/j.parkreldis.2005.09.004
Giulio Scigliano, Massimo Musicco, Paola Soliveri et al (2006) Reduced risk factors for vascular disorders in Parkinson disease patients. Stroke 37:1184–1188. https://doi.org/10.1161/01.STR.0000217384.03237.9c
Miyake Y, Tanaka K, Fukushima W et al (2010) Case–control study of risk of Parkinson’s disease in relation to hypertension, hypercholesterolemia, and diabetes in Japan. J Neurol Sci 293:82–86. https://doi.org/10.1016/j.jns.2010.03.002
Guttman M, Slaughter PM, Theriault M-E et al (2004) Parkinsonism in Ontario: comorbidity associated with hospitalization in a large cohort. Mov Disord 19:49–53. https://doi.org/10.1002/mds.10648
Kareus SA, Figueroa KP, Cannon-Albright LA, Pulst SM (2012) Shared predispositions of parkinsonism and cancer: a population-based pedigree-linked study. Arch Neurol 69:1572–1577. https://doi.org/10.1001/archneurol.2012.2261
Peretz C, Gurel R, Rozani V et al (2016) Cancer incidence among Parkinson’s disease patients in a 10-years time-window around disease onset: a large-scale cohort study. Parkinsonism Relat Disord 28:68–72. https://doi.org/10.1016/j.parkreldis.2016.04.028
Lin P-Y, Chang S-N, Hsiao T-H et al (2015) Association between parkinson disease and risk of cancer in Taiwan. JAMA Oncol 1:633–640. https://doi.org/10.1001/jamaoncol.2015.1752
Wirdefeldt K, Weibull CE, Chen H et al (2014) Parkinson’s disease and cancer: a register-based family study. Am J Epidemiol 179:85–94. https://doi.org/10.1093/aje/kwt232
Svensson E, Horváth-Puhó E, Thomsen RW et al (2015) Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol 78:522–529. https://doi.org/10.1002/ana.24448
Liu B, Fang F, Pedersen NL et al (2017) Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology 88:1996–2002
Svensson E, Horváth-Puhó E, Stokholm MG et al (2016) Appendectomy and risk of Parkinson’s disease: a nationwide cohort study with more than 10 years of follow-up. Mov Disord 31:1918–1922. https://doi.org/10.1002/mds.26761
Gray MT, Munoz DG, Gray DA et al (2014) Alpha-synuclein in the appendiceal mucosa of neurologically intact subjects. Mov Disord 29:991–998. https://doi.org/10.1002/mds.25779
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701–712. https://doi.org/10.1038/nrn3346
Tillisch K, Labus J, Kilpatrick L et al (2013) Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144:1394–1401. https://doi.org/10.1053/j.gastro.2013.02.0431394–1401.e1
Heijtz RD, Wang S, Anuar F et al (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci 108:3047–3052. https://doi.org/10.1073/pnas.1010529108
Bercik P, Denou E, Collins J et al (2011) The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141:599–609.e3. https://doi.org/10.1053/j.gastro.2011.04.052
Scheperjans F, Aho V, Pereira PAB et al (2015) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30:350–358. https://doi.org/10.1002/mds.26069
Keshavarzian A, Green SJ, Engen PA et al (2015) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30:1351–1360. https://doi.org/10.1002/mds.26307
Gabrielli M, Bonazzi P, Scarpellini E et al (2011) Prevalence of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord 26:889–892. https://doi.org/10.1002/mds.23566
Rana SV, Bhardwaj SB (2008) Small intestinal bacterial overgrowth. Scand J Gastroenterol 43:1030–1037. https://doi.org/10.1080/00365520801947074
Tan AH, Mahadeva S, Thalha AM et al (2014) Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat Disord 20:535–540. https://doi.org/10.1016/j.parkreldis.2014.02.019
Fasano A, Bove F, Gabrielli M et al (2013) The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord 28:1241–1249. https://doi.org/10.1002/mds.25522
Rao SSC, Tan G, Abdulla H et al (2018) Does colectomy predispose to small intestinal bacterial (SIBO) and fungal overgrowth (SIFO)? Clin Transl Gastroenterol 9:146. https://doi.org/10.1038/s41424-018-0011-x
van Minnen LP, Nieuwenhuijs VB, de Bruijn MT et al (2006) Effects of subtotal colectomy on bacterial translocation during experimental acute pancreatitis. Pancreas 32:110–114
Hughes AJ, Daniel SE, Lees AJ (2001) Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology 57:1497–1499
Rajput AH, Rozdilsky B, Rajput A (1991) Accuracy of clinical diagnosis in parkinsonism—a prospective study. Can J Neurol Sci J Can Sci Neurol 18:275–278
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184. https://doi.org/10.1136/jnnp.55.3.181
Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ (2002) The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125:861–870. https://doi.org/10.1093/brain/awf080
Joutsa J, Gardberg M, Röyttä M, Kaasinen V (2014) Diagnostic accuracy of parkinsonism syndromes by general neurologists. Parkinsonism Relat Disord 20:840–844. https://doi.org/10.1016/j.parkreldis.2014.04.019
Author information
Authors and Affiliations
Contributions
Conceptualization: Y-JK, S-YL, and S-HL. Data curation: Y-JK, C-ML, and S-HL. Formal analysis: Y-JK, C-ML, and S-HL. Investigation: Y-JK, C-ML, S-YL, and S-HL. Methodology: Y-JK, SK, J-WJ, and S-HL. Project administration: Y-JK and S-HL. Resources: Y-JK and S-HL. Software: Y-JK and C-ML. Supervision: Y-JK and S-HL. Validation: SK, J-WJ, S-YL, and S-HL. Writing: original draft: Y-JK, C-ML, and S-HL. Writing, review and editing: Y-JK, SK, J-WJ, S-YL, and S-HL.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Institutional Review Board of Kangwon National University Hospital (KNUH-2018-06-004).
Rights and permissions
About this article
Cite this article
Kim, YJ., Lee, CM., Kim, S. et al. Risk of Parkinson’s disease after colectomy: longitudinal follow-up study using a national sample cohort. J Neurol 267, 513–521 (2020). https://doi.org/10.1007/s00415-019-09617-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09617-1