Abstract
Physical exercise (PE) is an effective method for improving cognitive function among patients with traumatic brain injury (TBI). We previously demonstrated that PE with an infrared-sensing running wheel (ISRW) system provides strong neuroprotection in an experimental animal model of stroke. In this study, we used fluid percussion injury in rats to simulate mild TBI. For rats, we used both passive avoidance learning and the Y-maze tests to evaluate cognitive function. We investigated whether PE rehabilitation attenuated cognitive deficits in rats with TBI and determined the contribution of hippocampal and cortical expression of heat shock protein 20 (HSP20) to PE-mediated cognitive recovery. In addition to increasing hippocampal and cortical expression of HSP20, brain-derived neurotrophic factor (BDNF), and the tropomyosin receptor kinase B (TrkB) ratio, PE rehabilitation significantly attenuated brain contusion and improved cognitive deficits in the rat model. Furthermore, reducing hippocampal and cortical expression of HSP20 with an intracerebral injection of pSUPER hsp20 small interfering RNA significantly diminished the PE-induced overexpression of hippocampal and cortical BDNF and the TrkB ratio and also reversed the beneficial effect of PE in reducing neurotrauma and the cognitive deficits. A positive Pearson correlation was found between HSP20 and BDNF, as well as between HSP20 and TrkB, in the hippocampal and cortical tissues. We thus conclude that post-ischaemic ISRW exercise rehabilitation attenuates cognitive deficits, as well as brain contusions, in TBI rats by stimulating the cerebral HSP20/BDNF/TrkB signalling axis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 75% of all cases of traumatic brain injury (TBI) are mild but cause cognitive deficits [1,2,3]. The improvements induced by physical exercise (PE) regimens in brain plasticity and neurocognitive performance are evident in those afflicted by TBI [4]. Indeed, early exercise rehabilitation improves cognitive function among patients with TBI [5, 6]. In rats with TBI, exercise attenuates cognitive deficits by stimulating hippocampal brain-derived neurotrophic factor (BDNF)/tropomyosin receptor kinase B (TrkB) activation [7, 8]. Compared to the control group, rats with TBI in the exercise group have a shorter latency to locating a platform and demonstrate a marked improvement in spatial memory in the Morris water maze [8, 9]. However, it is not known whether PE training (or rehabilitation) improves both passive avoidance learning and spatial memory in the Y-maze in rats with TBI, and via what neural mechanisms.
Heat shock protein 20 (HSP20), one of the small HSPs, plays an important role in modulating physiological conditions and pathological processes [10]. HSP20 protects against the Golgi fragmentation and apoptosis induced by oxygen-glucose deprivation/reperfusion in mouse neuroblastoma cells [11]. In addition, pre-ischaemic treadmill exercise improves the outcomes of ischaemic stroke in rats by increasing the numbers of HSP20-containing neurons and glia [12]. However, it is unknown whether PE training or rehabilitation attenuates TBI-induced cognitive deficits by stimulating hippocampal and cortical HSP20/BDNF/TrkB activation.
A recent study by our research group demonstrated that for a given exercise intensity, PE training with an infrared-sensing running wheel (ISRW) system with an effective exercise activity indicator provided greater neuroprotection in an animal stroke model than did a conventional treadmill or motorised running wheel [13]. In the present study, we extend this previous work by elucidating whether TBI causes cognitive deficits and associated decreases in hippocampal and cortical expression of HSP20/BDNF/TrkB in rats. In addition, we investigated whether PE rehabilitation with an ISRW system reduces cognitive deficits in rats following TBI, and whether any such result is mediated by an increase in hippocampal and cortical expression of HSP20/BDNF/TrkB. Finally, we investigated whether the beneficial effects of PE training in attenuating cognitive deficits following TBI can be reversed by depleting hippocampal and cortical expression of HSP20 by gene silencing [12].
A recent double-blind, placebo-controlled study has demonstrated that N-acetyl cysteine ameliorates acute sequelae of blast-induced mild traumatic brain injury [14]. A more recent study, using fluid percussion injury (FPI), N-acetyl cysteine also produces significant cognitive recovery after TBI in rats [15]. Therefore, in the present study, FPI was chosen to simulate mild TBI. For rats, we used Y-maze and passive avoidance learning to evaluate cognitive function.
Methods
Animals
Male Sprague-Dawley rats, each weighing 251–278 g, were housed in standard cages and were provided food and water ad libitum. The Institutional Animal Care and Use Committee at Chi Mei Medical Center approved all experimental procedures (IACUC approved number 105110328). All protocols were designed to minimise pain and discomfort during both the injury procedure and recovery.
Fluid Percussion Injury
All rats were surgically prepared for lateral FPI in accordance with the methodology detailed previously [16,17,18]. Eighty animals underwent sham procedures, and the remaining 80 received mild (1.8 to 2.0 atm) FPI. All animals were surgically prepared for lateral FPI under a mixture of ketamine (44 mg/kg, intramuscularly (i.m.); Nankuang Pharmaceutical, Tainan, Taiwan), atropine (0.062633 mg/kg, i.m.; Sintong Chemical Ind. Co., Taoyuan, Taiwan), and xylazine (6.77 mg/kg, i.m.; Bayer, Leverkusen, Germany) anaesthesia. A 2-mm diameter burr hole was performed 4 mm posterior to bregma and 3 mm from sagittal sutures in the right parietal cortex, and a Luer-lock hub was affixed to the perimeter of the burr hole using cyanoacrylate. Dental acrylic and two small nickel-plated screws were used to anchor a hut to the stull. One day later, the rat was anaesthetized at the time of injury, the surgical site was exposed, and the rat was connected to the injury device. The force of the FPI caused a mild degree of contusion volume [~ 150 mm3] in the ipsilateral brain. Post impact seizures were observed immediately after the induction of lateral FPI in ∼ 30% of rats and lasted for 10–15 s. Other aspects of TBI were reproduced by lateral FPI, such as haematoma which occurred at the grey-white matter interface, acute apnoea (typically 10–60 s), intracranial hypertension, bradycardia, hyperglycaemia, and suppressed electroencephalogram amplitude [19]. Sham operation was connected to the injury device, but no injury was delivered. NSAIDs were used for postoperative analgesia. No animals died during the procedure.
Depletion of Cerebral HSP20 with Gene Silencing
During FPI surgery, a microinfusion pump injected an intracerebral dose of recombinant pSUPER plasmid expressing HSP20-small interfering RNA (HSP20-RNAi; 5 μg/rat) in 5 μL of pSUPER-small interfering RNA delivery media (Vector) into the cerebral cortex at a rate of 0.5 μL/min [12]. We lowered an injecting cannula into the ipsilateral cerebral cortex based on the coordinates of 1.2 mm anterior to bregma, 4.6 mm lateral to the midline, and 3.0 mm ventral to the skull surface [15].
Infrared-Sensing Running Wheel System
The ISRW system is an effective exercise activity indicator that provides superior exercise training to commercially available traditional animal running platforms [13]. All animals were pre-trained with the ISRW at a speed of 20 m/min for three consecutive days (10 min/day) before surgery. Automatic mode training began on the fourth day after induction of TBI and lasted for 3 weeks. In the first, second, and third weeks, PE-treated rats were taught to run for 30 min at 20 m/min, for 30 min at 30 m/min, and for 60 min at 30 m/min, respectively. The sedentary control groups were placed on a stationary ISRW for individual durations but did not run.
Experimental Groups and Procedures
As shown in Table 1, we randomly assigned the animals into one of the following eight groups (n = 10 for each group): (i) sedentary (Sed)-treated sham controls that received siRNA-vector (Sham+Vector+Sed); (ii) PE-treated sham controls that received siRNA-vector (Sham+Vector+PE); (iii) PE-treated sham controls that received HSP20-RNAi (Sham+HSP20-RNAi+PE); (iv) Sed-treated sham controls that received HSP20-RNAi (Sham+HSP20-RNAi+Sed); (v) Sed-treated TBI rats that received siRNA-vector (TBI+Vector+Sed); (vi) PE-treated TBI rats that received siRNA-vector (TBI+Vector+PE); (vii) PE-treated TBI rats that received HSP20-RNAi (TBI+HSP20-RNAi+ PE); and (viii) Sed-treated TBI rats that received HSP20-RNAi (TBI+HSP20-RNAi+Sed).
In experiment 1, which was conducted in all groups, we performed both passive avoidance and spatial memory tests to evaluate cognitive function.
In experiment 2, the optical density values for HSP20, BDNF, and TrkB in the ipsilateral hippocampal and cortical tissues were determined 28 days after a TBI or sham operation in all groups.
In experiment 3, we evaluated the cerebral contusion volumes with the triphenyl tetrazolium chloride (TTC) staining procedure.
Passive Avoidance Learning Test and Y-Maze Test
At 1 day after each rat had become habituated in a training apparatus, we performed an acquisition trial for each rat, as previously described [20]. A single, inescapable scrambled electric shock was delivered for 3 s after the rat entered the dark chamber. The latency to entering the dark compartment from the light compartment was defined as the testing latency. Each rat was scored on day 28 post-FPI. The Y-maze test was performed at 28 days post-injury in rats as previously described [21]. For each rat during one Y-maze testing session, the latency to enter the correct arm (time of reaction) was measured and the number of wrong entries (error number) was counted.
Western Blotting
We homogenised the cortical and the hippocampal tissue sample obtained on day 28 after FPI in a lysis buffer at 4 °C and determined the total protein concentration using a bovine serum standard curve as a reference. Sample protein concentrations were measured using the Lowry method (DC protein assay kit, Bio-Rad, Hercules, CA, USA). We normalised each blot to its corresponding β-actin value. A total of 40 μg of protein was evaluated via 10% SDS-PAGE and electrotransferred onto a nitrocellulose membrane. The membrane was incubated with anti-HSP20 (1:1000; catalog # ab13491, Abcam Inc., Boston, MA, USA), anti-BDNF (1:1000; catalog # ab108319, Abcam Inc., Boston, MA, USA), anti-TrkB (1:500; catalog # AF1494, R&D Systems, Inc., Minneapolis, MN, USA), or anti-β-actin (1:3000; catalog # MAB1501, EMD Millipore, Billerica, MA, USA) antibodies overnight at 4 °C, followed by a horseradish peroxidase (HRP)-conjugated anti-goat IgG antibody (1:2000 dilution; Cat# HAF109; R&D Systems) or rabbit IgG HRP-conjugated antibody (1:2000 dilution; Cat# HAF008; R&D Systems). The ratios of the actin values expressed the relative values of the band intensity. Relative expression was expressed as a percentage of the Sham+Vector+Sed group, as presented in the histograms.
Cerebral Contusion Assay
At 28 days after the rats had undergone FPI, we removed brain tissues from deeply anaesthetised animals, immersed the tissues in cold saline for 5 min, and sliced them into 2.0-mm sections with a tissue slicer. We adopted TTC staining procedures to evaluate the cerebral contusion volume as described previously [22], which was expressed in mm3 for all brain slices.
Statistical Analysis
Results are expressed as the mean ± SD. Behavioural data were tested for normality (D’Agostino and Pearson omnibus normality test) and skewness using GraphPad Prism 7.01 (Graph Pad Sofware, San Diego, CA, USA). The statistical analysis was carried out using repeated measures ANOVA and non-parametric Kruskal-Wallis and Mann-Whitney tests. The relationship between HSP20 and BDNF were analysed by calculating Pearson product-moment correlation coefficient. A statistical P value less than 0.05 was considered significant.
Results
HSP20, BDNF, and TrkB Levels Were Higher in PE Rats but Lower in TBI Rats
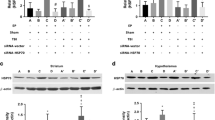
Western blotting showed that the hippocampal and cortical levels of HSP20, BDNF, and TrkB in the Sham+Vector+PE group were significantly higher than those in the Sham+Vector+Sed group (all comparisons, P < 0.05). In the TBI+Vector+Sed group, the hippocampal and cortical levels of HSP20, BDNF, and TrkB were significantly lower than those in the Sham+Vector+Sed group rats (all, P < 0.05; Fig. 1). The TBI-induced reduction in the hippocampal and cortical levels of HSP20, BDNF, and TrkB was significantly attenuated by PE (all, P < 0.05; Fig. 1) but significantly exacerbated by gene silencing with HSP20-RNAi (all, P < 0.05; Fig. 1). In addition, the beneficial effects of PE in attenuating the reduction in hippocampal and cortical HSP20, BDNF, and TrkB levels following TBI were significantly reversed by gene silencing with HSP20-RNAi (all, P < 0.05; Fig. 1).
Effect of physical exercise (PE) and/or traumatic brain injury (TBI) on HSP20, BDNF, and TrkB expression in both the hippocampus and the frontal cortex of the ipsilateral brain in rats with or without traumatic brain injury (TBI). Please see the “Experimental Groups and Procedures” section for the explanation of the group abbreviations. Values represent the mean ± SD of three separate experiments 28 days post-TBI. *P < 0.05, Sham+Vector+PE group vs. Sham+Vector+Sed group or TBI + Vector + PE group vs. TBI+Vector+Sed group. +P < 0.05, Sham+HSP20-RNAi+PE group vs. Sham+Vector+PE group; or TBI+HSP20-RNAi+PE group vs. TBI+Vector+PE group. #P < 0.05, Sham+HSP20-RNAi +Sed group vs. Sham+Vector+Sed group; or TBI+HSP20-RNAi+Sed group vs. Sham+Vector+Sed group. §P < 0.05, TBI+HSP20-RNAi+Sed group vs. Sham+HSP20-RNAi+Sed group; or TBI+HSP20-RNAi+Sed group vs. TBI+Vector+Sed group rats. Each group contains 10 animals
HSP20-Mediated PE Prevented Cognitive Deficits in TBI Rats
The results of behavioural testing are shown in both Figs. 2 and 3. Figure 2 shows both the pre-shock latency (acquisition time) and 24-h post-shock latency (retention time) for all groups in passive avoidance learning test. No between-group differences were found for acquisition time (P > 0.05) suggesting short-term memory (1.5-h post-test) was unaffected by TBI. However, both the Sham+HSP20-RNAi+Sed and TBI+Vector+Sed groups had significantly shorter retention times than the Sham+Vector+Sed group (P < 0.05). Additionally, the TBI+HSP20-RNAi+Sed group had a significantly shorter retention time than did the Sham+HSP20-RNAi+PE or the TBI+HSP20-RNAi+PE groups. The results revealed that depletion of HSP20 or TBI alone significantly attenuated passive avoidance learning, whereas combined HSP20-RNAi and TBI caused a more severe impairment in passive avoidance learning. The TBI-induced passive avoidance learning deficits were significantly attenuated by PE. However, the beneficial effects of PE in improving passive avoidance learning at 28 days post-TBI were significantly abolished by depleting brain HSP20 with gene silence (Fig. 2). Figure 3 shows the effect of HSP20-RNAi and/or PE on spatial memory function in rats 28 days after TBI. The Y-maze test results showed that the TBI rats or the rats treated with HSP20-RNAi had a deficit in spatial memory (time of reaction prolonged in Fig. 3a and error number increased in Fig. 3b) compared with the sham controls at 28 days after injury. Combined TBI and HSP20-RNAi caused an additional impairment in spatial memory deficits. The spatial memory impairment caused by TBI could be prevented by PE. Furthermore, attenuating the beneficial effects exerted by PE by HSP20-RNAi indicates that PE improved cognitive deficits in TBI rats by increasing cortical HSP20, BDNF, and TrkB signalling pathways.
Comparison of latency to entering the dark chamber before receiving the foot shock (acquisition time) and 24 h after receiving the foot shock (retention time). Each column and bar represent the mean ± SD of 20 rats per group. *P < 0.05 for the Sham+HSP20-RNAi+Sed group or the TBI+Vector+Sed group vs. the Sham+Vector+Sed group rats. +P < 0.05 for the TBI+Vector+PE group vs. the TBI+Vector+Sed group. #P < 0.01 for the TBI+HSP20-RNAi+PE group vs. TBI+Vector+PE group. §P < 0.01 for the TBI+HSP20-RNAi+Sed group vs. the Sham+HSP20-RNAi+Sed or the TBI+HSP20-RNAi+PE group. Please see the “Experimental Groups and Procedures” section for the explanations of the group abbreviations
Effect of PE and/or HSP20-RNAi on learning and spatial memory in rats 28 days after TBI. We determined the a latency to enter the correct arm (time of reaction) and b the number of wrong entries (error number) in the Y-maze. Each column and bar denote the mean ± SD of 20 rats per group. *P < 0.05 for the Sham+HSP20-RNAi+Sed group or the TBI+Vector+Sed group vs. the Sham+Vector+Sed group rats. +P < 0.05 for the TBI+Vector+PE group vs. the TBI+Vector+Sed group. #P < 0.01 for the TBI+HSP20-RNAi+PE group vs. TBI+Vector+PE group. §P < 0.01 for the TBI+HSP20-RNAi+Sed group vs. the TBI+Vector+Sed or the TBI+HSP20-RNAi+PE group. Please see the “Experimental Groups and Procedures” section for the explanations of the group abbreviations
HSP20-Mediated PE Attenuated Cerebral Contusion Volume in TBI Rats
Figure 4 shows between-group differences in cerebral contusion volumes of the ipsilateral side but not in those of the contralateral side. The TBI+Vector+Sed group had significantly larger cerebral contusion volumes in the ipsilateral side than did the sham counterparts (P < 0.01). Compared to the TBI+Vector+Sed group, the corresponding TBI+Vector+PE group had significantly smaller cerebral contusion volumes. The TBI+HSP20-RNAi+PE group had significantly larger cerebral contusion volumes than did the TBI+Vector+PE group (P < 0.05). However, the TBI+HSP20-RNAi+Sed group had significantly larger cerebral contusion volumes than did the TBI+Vector+PE group. These results reveal that PE attenuated the cerebral contusion volumes and that hippocampal depletion of HSP20 with gene silencing increased the volumes. In addition, depletion of hippocampal HSP20 with gene silencing reversed the beneficial effects of PE in reducing the cerebral contusion volumes.
TBI-induced brain contusion. a The tetrazolium chloride (TTC) stains are representative of the brain contusion results. b Data are presented as the mean ± SD (n = 10 per group). *P < 0.001 for TBI+Vector+Sed group rats vs. the Sham+Vector+Sed group rats. #P < 0.001 for TBI+Vector+PE vs. TBI+Vector+Sed. +P < 0.001 for the TBI+HSP20-RNAi+PE group rats vs. the TBI+Vector+PE group rats. §P < 0.001 for the TBI+HSP20-RNAi+ Sed group rats vs. the TBI+Vector+PE group rats
Correlations Between HSP20/BDNF and HSP20/TrkB
We found a positive correlation (Fig. 5) between the hippocampal and cortical levels of HSP20 and BDNF, as well as between levels of HSP20 and TrkB (measured by western blotting, Fig. 1) in the ipsilateral brain regions.
Discussion
The majority of individuals who experience TBI are mild [17]. In rats, mild (1.8–2.2 atm) FPI has been found to cause profound impairment of spatial learning and short-term memory accompanied by visible neuronal loss in the hippocampus or neocortex [17, 18]. Examination of fractional anisotropy colour maps after diffusion tensor imaging suggested axonal injury in rats with FPI [23]. The present study showed that FBI caused brain contusion and cognitive dysfunction evidenced by passive avoidance learning and spatial memory impairments in rats. Generation of a protocol, similar to a recent clinical trial with N-acetyl cysteine in blast-induced mild TBI in a battlefield setting [14], produces significant behavioural recovery after mild fluid percussion injury in rats [15]. These findings are consistent with the neuropathologies and cognitive dysfunctions associated with mild TBI among humans [24].
The most striking findings of the present study were that TBI may cause both brain contusion and cognitive deficits in rats by reducing hippocampal expression of HSP20 and BDNF. We found that PE with an improved ISRW system with an effective exercise activity indicator [13], in addition to inducing hippocampal and cortical expression of HSP20/BDNF/TrkB, significantly attenuated the brain contusion volume and cognitive deficits in a rat model of TBI. By contrast, depletion of the cerebral levels of HSP20/BDNF/TrkB with an intracerebral injection of pSUPER plasmid expressing HSP20-small interfering RNA (HSP20-RNAi) significantly reversed the beneficial effects of physical rehabilitation on TBI. Our data therefore indicate that post-TBI running wheel exercise attenuates cognitive deficits following mild TBI in rats by increasing hippocampal and cortical expression of HSP20/BDNF/TrkB. The present results are partly consistent with previous findings that low-intensity treadmill exercise attenuated spatial memory deficits following a severe controlled cortical impact in rats [8, 9]. It is believed that treadmill exercise may improve cognitive function by increasing the expression of BDNF in the hippocampus in rats with TBI [8, 25, 26].
Brain-derived neurotrophic factor (BDNF) and its functional receptor tropomyosin-related kinase B (TrkB) have been proposed to promote neuronal survival and synaptic plasticity in neurodegenerative disease [27, 28]. Systemic administration of BDNF and intracerebral injection of BDNF before and after cerebral ischaemia all significantly reduced infarct volumes [29, 30]. Another line of evidence from both humans and experimental animal models indicates that, following TBI, amyloid-β (Aβ) peptides and tau proteins accumulate in the brain and cerebrospinal fluid [31]. Smile plaques, cerebral amyloid, and cerebral amyloid angiopathies mainly consist of aggregated Aβ [32, 33]. Thus, TBI acts an important risk factor for Alzheimer’s disease [31]. In Alzheimer’s disease brains, HSP20 inhibits amyloid-beta protein aggregation and cerebrovascular amyloid-beta protein toxicity [33, 34]. The phosphorylation of HSP20 enhances its association with Aβ to increase protection against neuronal cell death [35]. TrkB receptors and BDNF function can be downregulated by Aβ accumulation [28]. Our present results further demonstrate that increasing cortical expression of HSP20 with PE attenuates TBI-induced cognitive deficits by increasing cortical BDNF and the TrkB ratio expression in rats. We also find a positive Pearson correlation exists between partial and hippocampal HSP20 and BDNF as well as between cortical and hippocampal HSP20 and TrkB ratio in rats. Putting these observations together, it seems that TBI causes cognitive deficits in rats by increasing cortical and hippocampal expression of Aβ but decreasing cortical and hippocampal expression of HSP20/BDNF/TrkB. Additionally, PE may improve cognitive deficits following TBI in rats by reversing the altered cortical and hippocampal expression of Aβ and HSP20/BDNF/TrkB. Of course, the hypothesis requires further vilification in future.
However, it should be stressed that high-intensity treadmill exercise does not attenuate cognitive deficits in rats following compact cortical injury [8] or FPI [35]. Moreover, wheel-running exercise adopted in the early phase following FPI can impair cognitive performance in rats [26]. It is possible that high-intensity treadmill exercise or running wheel exercise [26] behaves as a stressor and downregulates hippocampal expression of BDNF, as well as hippocampal plasticity. By contrast, ISRW exercise acts as a stimulator and upregulates hippocampal expression of HSP20/BDNF/TrkB, which reverses TBI-induced cognitive deficits, as shown in the present study. This contention is supported by many previous findings. For example, BDNF facilitates the growth, proliferation, and differentiation of hippocampal neurons; synaptic plasticity; and cognitive function [36, 37]. In addition, TBI causes massive neuronal death in the hippocampus, which results in cognitive dysfunction [25].
Our previous study showed that the ISRW system provides more effective exercise training for neuroprotection against and recovery from ischaemic stroke in animal models than does the same training intensity on a treadmill exercise or a motorised running wheel exercise [13]. Therefore, the ISRW system provides a more efficient exercise-training platform for facilitating functional recovery in rats with ischaemic stroke or TBI. The quantitative exercise effectiveness indicator showed a 92% correlation between an increase in effective exercise activity and a decrease in infarct volume [13]. This ISRW system can be used as a non-electrically forced and objective reference in preclinical exercise experiments.
Conclusion
In conclusion, a significant increase in hippocampal and cortical expression of the HSP20/BDNF/TrkB proteins after 3 weeks of exercise coincided with a significant reduction in both brain contusion volume and passive avoidance learning deficits in TBI rats. Reduction of the hippocampal and cortical levels of HSP20/BDNF/TrkB by pSUPER-HSP20 small interfering RNA showed a significant reversal in neuroprotection. There was a positive correlation between the HSP20 and BDNF levels, as well as between the HSP20 and TrkB levels, in both the hippocampus and frontal cortex. Thus, modulating the HSP20/BDNF/TrkB levels in both the hippocampus and the frontal cortex may be a therapeutic option for TBI-induced cerebral contusion and passive avoidance learning impairment. Exercise rehabilitation attenuates cognitive deficits in TBI rats by stimulating the cerebral HSP20/BDNF/TrkB signalling axis.
References
Sterr A, Herron KA, Hayward C, Montaldi D (2006) Are mild head injuries as mild as we think? Neurobehavioral concomitants of chronic post-concussion syndrome. BMC Neurol 6:7. https://doi.org/10.1186/1471-2377-6-7
De Beaumont L, Theoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, Ellemberg D, Lassonde M (2009) Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain 132(Pt 3):695–708. https://doi.org/10.1093/brain/awn347
Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, Jordan BD (2005) Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 57(4):719–726 discussion 719-726
Archer T (2012) Influence of physical exercise on traumatic brain injury deficits: scaffolding effect. Neurotox Res 21(4):418–434. https://doi.org/10.1007/s12640-011-9297-0
Andelic N, Bautz-Holter E, Ronning P, Olafsen K, Sigurdardottir S, Schanke AK, Sveen U, Tornas S et al (2012) Does an early onset and continuous chain of rehabilitation improve the long-term functional outcome of patients with severe traumatic brain injury? J Neurotrauma 29(1):66–74. https://doi.org/10.1089/neu.2011.1811
Franckeviciute E, Krisciunas A (2006) Evaluation of factors influencing effectiveness of kinesitherapy in patients after traumatic brain injury. Medicina (Kaunas) 42(9):732–737
Griesbach GS, Hovda DA, Gomez-Pinilla F (2009) Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res 1288:105–115. https://doi.org/10.1016/j.brainres.2009.06.045
Shen X, Li A, Zhang Y, Dong X, Shan T, Wu Y, Jia J, Hu Y (2013) The effect of different intensities of treadmill exercise on cognitive function deficit following a severe controlled cortical impact in rats. Int J Mol Sci 14(11):21598–21612. https://doi.org/10.3390/ijms141121598
Itoh T, Imano M, Nishida S, Tsubaki M, Hashimoto S, Ito A, Satou T (2011) Exercise inhibits neuronal apoptosis and improves cerebral function following rat traumatic brain injury. J Neural Transm (Vienna) 118(9):1263–1272. https://doi.org/10.1007/s00702-011-0629-2
Edwards HV, Cameron RT, Baillie GS (2011) The emerging role of HSP20 as a multifunctional protective agent. Cell Signal 23(9):1447–1454. https://doi.org/10.1016/j.cellsig.2011.05.009
Zhong B, Hu Z, Tan J, Lu T, Lei Q, Chen C, Zeng L (2015) Hsp20 protects against oxygen-glucose deprivation/reperfusion-induced Golgi fragmentation and apoptosis through Fas/FasL pathway. Oxidative Med Cell Longev 2015:606934–606910. https://doi.org/10.1155/2015/606934
Lin CM, Chang CK, Chang CP, Hsu YC, Lin MT, Lin JW (2015) Protecting against ischaemic stroke in rats by heat shock protein 20-mediated exercise. Eur J Clin Investig 45(12):1297–1305. https://doi.org/10.1111/eci.12551
Chen CC, Chang MW, Chang CP, Chang WY, Chang SC, Lin MT, Yang CL (2015) Improved infrared-sensing running wheel systems with an effective exercise activity indicator. PLoS One 10(4):e0122394. https://doi.org/10.1371/journal.pone.0122394
Hoffer ME, Balaban C, Slade MD, Tsao JW, Hoffer B (2013) Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One 8(1):e54163. https://doi.org/10.1371/journal.pone.0054163
Eakin K, Baratz-Goldstein R, Pick CG, Zindel O, Balaban CD, Hoffer ME, Lockwood M, Miller J et al (2014) Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One 9(4):e90617. https://doi.org/10.1371/journal.pone.0090617
Sanders MJ, Dietrich WD, Green EJ (1999) Cognitive function following traumatic brain injury: effects of injury severity and recovery period in a parasagittal fluid-percussive injury model. J Neurotrauma 16(10):915–925. https://doi.org/10.1089/neu.1999.16.915
Hylin MJ, Orsi SA, Zhao J, Bockhorst K, Perez A, Moore AN, Dash PK (2013) Behavioral and histopathological alterations resulting from mild fluid percussion injury. J Neurotrauma 30(9):702–715. https://doi.org/10.1089/neu.2012.2630
Kabadi SV, Hilton GD, Stoica BA, Zapple DN, Faden AI (2010) Fluid-percussion-induced traumatic brain injury model in rats. Nat Protoc 5(9):1552–1563. https://doi.org/10.1038/nprot.2010.112
Eakin K, Rowe RK, Lifshitz J (2015) Frontiers in neuroengineering modeling fluid percussion injury: relevance to human traumatic brain injury. In: Kobeissy FH (ed) Brain neurotrauma: molecular, neuropsychological, and rehabilitation aspects. CRC Press/Taylor & Francis (c) 2015 by Taylor & Francis Group, LLC., Boca Raton
Hosseini N, Alaei H, Reisi P, Radahmadi M (2013) The effect of treadmill running on passive avoidance learning in animal model of Alzheimer disease. Int J Prev Med 4(2):187–192
Wei J, Pan X, Pei Z, Wang W, Qiu W, Shi Z, Xiao G (2012) The beta-lactam antibiotic, ceftriaxone, provides neuroprotective potential via anti-excitotoxicity and anti-inflammation response in a rat model of traumatic brain injury. J Trauma Acute Care Surg 73(3):654–660. https://doi.org/10.1097/TA.0b013e31825133c0
Tsai MC, Chang CP, Peng SW, Jhuang KS, Fang YH, Lin MT, Tsao TC (2015) Therapeutic efficacy of Neuro AiD (MLC 601), a traditional Chinese medicine, in experimental traumatic brain injury. J NeuroImmune Pharmacol 10(1):45–54. https://doi.org/10.1007/s11481-014-9570-0
Laitinen T, Sierra A, Bolkvadze T, Pitkanen A, Grohn O (2015) Diffusion tensor imaging detects chronic microstructural changes in white and gray matter after traumatic brain injury in rat. Front Neurosci 9:128. https://doi.org/10.3389/fnins.2015.00128
d’Hemecourt P (2011) Subacute symptoms of sports-related concussion: outpatient management and return to play. Clin Sports Med 30(1):63–72, viii. https://doi.org/10.1016/j.csm.2010.08.008
Griesbach GS, Gomez-Pinilla F, Hovda DA (2007) Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma 24(7):1161–1171. https://doi.org/10.1089/neu.2006.0255
Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F (2004) Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience 125(1):129–139. https://doi.org/10.1016/j.neuroscience.2004.01.030
Chen A, Xiong LJ, Tong Y, Mao M (2013) The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed Rep 1(2):167–176. https://doi.org/10.3892/br.2012.48
Jeronimo-Santos A, Vaz SH, Parreira S, Rapaz-Lerias S, Caetano AP, Buee-Scherrer V, Castren E, Valente CA et al (2015) Dysregulation of TrkB receptors and BDNF function by amyloid-beta peptide is mediated by calpain. Cereb Cortex (New York, NY : 1991) 25(9):3107–3121. https://doi.org/10.1093/cercor/bhu105
Schabitz WR, Schwab S, Spranger M, Hacke W (1997) Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J Cereb Blood Flow Metab 17(5):500–506. https://doi.org/10.1097/00004647-199705000-00003
Yamashita K, Wiessner C, Lindholm D, Thoenen H, Hossmann KA (1997) Post-occlusion treatment with BDNF reduces infarct size in a model of permanent occlusion of the middle cerebral artery in rat. Metab Brain Dis 12(4):271–280
Sivanandam TM, Thakur MK (2012) Traumatic brain injury: a risk factor for Alzheimer's disease. Neurosci Biobehav Rev 36(5):1376–1381. https://doi.org/10.1016/j.neubiorev.2012.02.013
Wilhelmus MM, Boelens WC, Kox M, Maat-Schieman ML, Veerhuis R, de Waal RM, Verbeek MM (2009) Small heat shock proteins associated with cerebral amyloid angiopathy of hereditary cerebral hemorrhage with amyloidosis (Dutch type) induce interleukin-6 secretion. Neurobiol Aging 30(2):229–240. https://doi.org/10.1016/j.neurobiolaging.2007.06.001
Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, Kusters B, Maat-Schieman ML, de Waal RM, Verbeek MM (2006) Small heat shock protein HspB8: its distribution in Alzheimer’s disease brains and its inhibition of amyloid-beta protein aggregation and cerebrovascular amyloid-beta toxicity. Acta Neuropathol 111(2):139–149. https://doi.org/10.1007/s00401-005-0030-z
Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, de Waal RM, Verbeek MM (2006) Small heat shock proteins inhibit amyloid-beta protein aggregation and cerebrovascular amyloid-beta protein toxicity. Brain Res 1089(1):67–78. https://doi.org/10.1016/j.brainres.2006.03.058
Cameron RT, Quinn SD, Cairns LS, MacLeod R, Samuel ID, Smith BO, Carlos Penedo J, Baillie GS (2014) The phosphorylation of Hsp20 enhances its association with amyloid-beta to increase protection against neuronal cell death. Mol Cell Neurosci 61:46–55. https://doi.org/10.1016/j.mcn.2014.05.002
Poo MM (2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci 2(1):24–32. https://doi.org/10.1038/35049004
Lee J, Duan W, Mattson MP (2002) Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem 82(6):1367–1375
Funding
This work was supported by the Ministry of Science and Technology (Taiwan) grants MOST 104-2314-B-384-003-MY3 (to C.C. Chio), MOST 106-2314-B-384-001-MY3 (to C.C. Chio), and Chi Mei Medical Center (Taiwan) grant CMFHT 10504 (to C.P. Chang).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The Institutional Animal Care and Use Committee at Chi Mei Medical Center approved all experimental procedures (IACUC approved number 105110328).
Conflict of Interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(PDF 204 kb)
Rights and permissions
About this article
Cite this article
Chou, W., Liu, YF., Lin, CH. et al. Exercise Rehabilitation Attenuates Cognitive Deficits in Rats with Traumatic Brain Injury by Stimulating the Cerebral HSP20/BDNF/TrkB Signalling Axis. Mol Neurobiol 55, 8602–8611 (2018). https://doi.org/10.1007/s12035-018-1011-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1011-2