Abstract
Despite recent advancements in the diagnosis and treatment of breast cancer (BC), patient outcomes in terms of survival, recurrence, and disease progression remain suboptimal. A significant factor contributing to these challenges is the cellular heterogeneity within BC, particularly the presence of breast cancer stem cells (BCSCs). These cells are thought to serve as the clonogenic nexus for new tumor growth, owing to their hierarchical organization within the tumor. This descriptive review focuses on the evolving strategies to target BCSCs, which have become a pivotal aspect of therapeutic development. We explore a variety of approaches, including targeting specific tumor surface markers (CD133 and CD44), transporters, heat shock proteins, and critical signaling pathways like Notch, Akt, Hedgehog, KLF4, and Wnt/β-catenin. Additionally, we discuss the modulation of the tumor microenvironment through the CXCR-12/CXCR4 axis, manipulation of pH levels, and targeting hypoxia-inducible factors, vascular endothelial growth factor, and CXCR1/2 receptors. Further, this review focuses on the roles of microRNA expression, strategies to induce apoptosis and differentiation in BCSCs, dietary interventions, dendritic cell vaccination, oncolytic viruses, nanotechnology, immunotherapy, and gene therapy. We particularly focused on studies reporting identification of BCSCs, their unique properties and the efficacy of various therapeutic modalities in targeting these cells. By dissecting these approaches, we aim to provide insights into the complex landscape of BC treatment and the potential pathways for improving patient outcomes through targeted BCSC therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, breast cancer (BC) is considered a significant health burden. Worldwide, BC is the most commonly diagnosed and a leading cause of cancer-related deaths among women [1]. According to WHO estimates, every year, BC impacts almost 2.1 million women, and in 2018, only an estimated 627,000 women died of BC—nearly 15% of all cancer-related deaths in women [2]. With BC incidence, morbidity, and mortality still increasing in developing and developed countries, newer diagnostic and therapeutic modalities are warranted [3]. Evidence suggests that a tumor’s cellular heterogeneity may contribute to the differences in cell proliferation, morphology, genetic signature, and treatment responses [3, 4]. Based on gene expression profiling, clinicians characterize clinically heterogeneous BC into four distinct molecular subgroups: luminal A and B, ER (estrogen receptor) positive, HER2 (human epidermal growth factor receptor 2) positive, with higher expression of HER2 gene, and triple-negative, lacking the expression of ER, progesterone receptor (PR), and HER2 [3, 5]. Two different models have elucidated breast tumor heterogeneity: clonal evolution and cancer stem cell model [6].
According to the cancer stem cell model, breast cancer stem cells (BCSCs) play a critical role in driving tumor hierarchy and cellular diversity within breast tumors. Tumors originating from BCSCs influence the tumor’s response to therapy. Furthermore, similar to normal stem cells, the self-renewal properties of BCSCs alter the balance of signaling pathways in favor of tumor formation [7]. BCSCs exhibit unique properties within the tumor microenvironment (TME), including slow proliferation, enhanced DNA repair mechanisms, expression of drug efflux pumps, and a surrounding TME characterized by hypoxia and acidosis. Of note, these properties are context-dependent and can be influenced by changes in the TME [8]. Thus, cells that can auto-regenerate, proliferate, and induce tumor growth are called BCSCs or cancer-initiating cells.
Based on specific surface markers, BCSCs have been identified and isolated; notable ones include CD44+/CD24-/lowALDH1+ [9, 10]. Additionally, researchers have utilized several other properties to determine BCSCs, such as their capacity to form spheres, their ability to extrude dye due to the overexpression of efflux pumps (ATP binding cassette), and their enzymatic activity of aldehyde dehydrogenase 1 [8]. BCSCs are pluripotent cells that may give rise to tumor cells with diverse phenotypes, resulting in the growth of the primary and new tumors [11]. Thus, for an effective treatment, considering the heterogeneity of tumor cells, all BCSCs should be explicitly targeted and removed to prevent disease recurrence [4]. New breast cancer treatments target only proliferating cells, bypassing the slowly increasing or quiescent BCSCs to avoid target treatments—and this may be one of the critical reasons for BC chemotherapeutic failure [9]. These literature pieces of evidence suggest that the BCSC microenvironment is complex, which makes BCSC therapy a daunting task. This review will thus concentrate on various strategies aimed at targeting BCSCs within their microenvironment, along with the reasons for targeting BCSCs to enhance outcomes.
Need for targeting BCSCs
One of the major obstacles, resistance to current cancer therapy, represents a significant problem for cancer treatment [7]. Chemoresistance and self-renewal, properties often associated with therapy failure, are exhibited by a subset population of tumor cells known as breast cancer stem cells (BCSCs). BCSCs are responsible for cancer initiation, progression, distant metastasis, disease recurrence, and resistance to numerous forms of therapies, including chemotherapy [12]. Besides, BCSCs express genes that respond to oxidative stress and transporters, such as cell membrane ATP-binding cassette (ABC), which may confer resistance to existing anticancer therapies [11, 12].
Hence, cancer therapies may become ineffective because of leftover BCSCs that may be responsible for tumor regeneration [13]. Several intrinsic factors, such as altered receptor sensitivity, drug extruding potential, highly efficient DNA repair mechanisms, dormancy in their niches, deactivation of phosphatase tensin homolog (PTEN), and augmented expression of HER2 confer resistance to BCSCs against conventional therapies [14]. Alas, the current cancer therapeutic approaches are not only expensive but also cause serious side effects [7].
Thus, a targeted approach against BCSCs must involve treatment modalities to restrict tumor maintenance potential and regrowth to attain ample cure. [15]. Novel therapeutic systems plan to eradicate BCSCs by targeting surface markers and signaling cascades involved in BCSCs maintenance and drug resistance. These novel modalities also aim to disrupt signals from the tumor microenvironment capable of sustaining BCSC growth, which may promote tumor regeneration and therapy resistance-associated relapse [8]. Thus, the prime objective of the upcoming therapeutic approaches is to permanently switch off BCSCs in an attempt to completely diminish or eliminate the chance of relapse after effective treatment [15].
Current therapeutic strategies targeting BCSCs
Researchers have recently extensively investigated multiple strategies for eradicating or extinguishing BCSCs and their associated niches. These strategies include targeting tumor cell-specific surface markers, altering signaling pathways, modifying tumor microenvironment, modifying miRNA expression, impeding drug-efflux pumps, and inducing BCSCs apoptosis and differentiation [8]
Identification and targeting of BCSCs surface markers
Targeting surface markers
The surface markers for isolating and identifying BCSCs are key for targeted therapy. In solid tumors, a cell surface glycoprotein CD133/prominin-1 is widely expressed on BCSCs (Fig. 1). Cancers harboring a substantial population of CD133-expressing cells exhibit a poor prognosis and a resistant phenotype. Consequently, researchers have actively pursued testing anti-CD133 therapy. The polymeric nanoparticles loaded with paclitaxel forming antibody–drug conjugates targeting CD133 might be associated with improved drug delivery (Fig. 1), which resulted in enhanced effects on CD133+ BCSCs, promoting their elimination (Table 1) [16].
Being a receptor of hyaluronic acid, CD44, a transmembrane protein, enables cell-to-cell adhesion and interaction with extracellular matrix (ECM). CD44 is involved in cell proliferation, survival, migration, self-renewal, niche groundwork, differentiation, apoptosis, epithelial-mesenchymal transition, and resistance to apoptosis [17]. Many tumor cells, including breast cancer, demonstrated CD44 overexpression. One of the strategies to eliminate BCSCs is to target CD44-expressing cells with monoclonal antibodies. In this context, to prevent the relapse of aggressive BC, anti-human CD44 monoclonal antibodies have been used in combination with doxorubicin and cyclophosphamide [8]. Using MCF-7 breast cancer cell lines, researchers targeted CD44 + cells, which displayed significant cancer stem cell characteristics, by photo-ablating them with anti-CD44 antibody-conjugated gold nanorods. This process involved absorbing near-infrared light at the targeted site, thereby increasing the temperature of the targeted cells [18]. Another approach to target CD44 receptors on BCSCs was to coat hyaluronic acid on salinomycin nanoparticles, resulting in higher cellular uptake and increased toxicity toward BCSCs (Table 1) [19]. Additionally, oligosaccharides hyaluronan (oHA) conjugated with histidine-menthone-1,2-glycerol ketal (oHM) targeting CD44 were assembled with curcumin and paclitaxel (PTX) demonstrated the most robust anti-tumor activity (Fig. 1) [20].
CD47, a transmembrane protein, is a receptor for signal regulatory protein alpha (SIRPα) and the thrombospondin family. It is extensively expressed in cells of nearly all human solid tumors. Two monoclonal antibodies targeting CD47, B6H12.2 and B6H12, have been developed for breast cancer therapy (Fig. 1). The breast tumors were significantly prevented and repressed after administering B6H12.2 [8].
Currently, there are several promising therapies targeting surface markers undergoing clinical investigation. Antibody–drug conjugates (ADCs), where antibodies targeting specific surface markers are combined with cytotoxic drugs of interest, e.g., Herceptin (trastuzumab) to target HER2-positive breast cancer and Kadcyla (ado-trastuzumab emtansine) to target HER2-positive breast and gastric cancers’. Numerous other ADCs targeting various surface markers are progressing through different phases of clinical trials [21]. CAR T cell therapy, a personalized approach involving genetic modification of a patient’s T cells to recognize and eliminate cancer cells expressing specific surface markers, has shown notable success in treating certain blood cancers and is being explored for broader applications across different cancer types [22]. Additionally, bispecific antibodies which bind to two different targets—one on a cancer cell and the other on an immune cell such, as a T cell—thus, bring immune cells closer to cancer cells, thereby facilitating their destruction. Blinatumomab is an approved example for treating acute lymphoblastic leukemia, with ongoing clinical trials investigating bispecific antibodies targeting diverse cancers and their surface markers [23].
Targeting BCSCs expressing transporters
ABC transporter inhibition
ATP-binding cassette (ABC) transporters are membrane transporters that can pump various structurally unrelated and diverse small molecules out of the cells, such as cytotoxic dyes and drugs employing the ATP hydrolysis mechanism. ABC transporters are highly expressed in both standard and cancer stem cells. The transporters-based drug efflux phenomenon might likely contribute toward multidrug resistance (MDR), thereby pumping many anti-tumor drugs out of the cells, thus lowering intracellular drug concentrations, which enables BCSCs to resist current cancer therapies. ABC superfamily includes P-glycoprotein (P-gp/ABCB1), multidrug resistance proteins (MRPs/ABCC), and breast cancer resistance protein (BCRP/ABCG2) [24]. The expression of ABC transporters can be estimated by treating cells with Hoechst 33,342 dye, expelled out by a subset of the tumor population termed side population (SP). Accumulated data suggest that SP exists in cancer cell lines, and tumor cells can initiate tumorigenesis more than non-SP cells [18].
Nonetheless, interventions targeting ABC transporters may evoke adverse effects, owing to heightened drug accumulation within healthy tissues. Therefore, it is imperative to delineate the physiological distribution of ABC transporters, which predominantly encompass cancer cells, normal epithelial cells, and constituents of the blood–brain barrier. However, the strategic targeting/inhibition of cancer cell expressing ABC transporters holds promise in augmenting chemotherapy efficacy by impeding the efflux of anticancer agents from neoplastic cells. This mechanism amplifies intracellular drug concentrations which augment cancer cell targeting—and may improve therapeutic outcomes [25]—and offers an alternative strategy to overcome chemoresistance. More recently, in an in vitro setting, a tyrosine kinase inhibitor, gefitinib, has been shown to reverse drug resistance in multidrug-resistant BC cell lines expressing ABC transporters [26]. A recent study has validated significantly increased expression of ABC transporters, including ABCG1, ABCG2, ABCC2, ABCC3, ABCC4, ABCC6, ABCC7, ABCC9, and ABCA3 in low-density breast cancer cultures, causing chemoresistance against tamoxifen and doxorubicin. Amplified mRNA expression was reduced after treatment of cultures with ABC transporter pan-inhibitor cyclosporin, leading to increased sensitivity of breast cancer cells toward the drugs mentioned above [27].
Lately, a study has shown that up-regulation of ABCA12 leads to increased stemness and chemoresistance in BCSCs by reducing intracellular ceramide levels. Targeted inhibition of ABCA12 can reverse cancer stemness via ceramide homeostasis as it actively suppresses BCSC resistance against chemotherapeutic drugs [28]. Until now, based on natural compounds, second- and third-generation ABC transporter blockers have been explored, and the fourth generation is still in early stage research [29].
First-generation inhibitors with the ability to block MDR1/ABCBC1 are already in use for various conditions; notable ones include calcium channel blockers, e.g., verapamil, antiarrhythmics and neuroleptics, e.g., quinidine reserpine and yohimbine, immuno-suppressants, e.g., cyclosporin A and antiestrogens, e.g., tamoxifen and toremifene, yet their clinical efficacy is overshadowed by their toxic effects. From 1st generation P-gp (p-glycoprotein) modulators come the 2nd generation MDR1 mediators, such as elacridar (GF120918), R-verapamil, dofequidar (MS-209), biricodar (INCEL, VX-710), valspodar (PSC833), or timcodar (VX-853), possessesing less toxicity compared to the first generation due to nonselective inhibition of multiple ABC transporters and variable pharmacokinetic profiles, yet still produce clinically relevant side effects (Fig. 1). Besides, third generation modulators, still in the early stages of investigations as BCSCs sensitizing agents, are more selective inhibitors of ABCG1, ABCB1, and ABCC1, such as oc144093 (ONT-093), Zosuquidar (LY335979), laniquidar (R101933), and tariquidar (XR-9576) (Fig. 1).
Moreover, other small molecule tyrosine kinase inhibitors (TKIs) can also inhibit ABC transporters, such as WHI-P154, telatinib, AST1306, linsitinib, lapatinib, masitinib, motesanib and nilotinib (Fig. 1). Besides, to target ABCG2 expressing cancer stem cells (CSCs), a new candidate, LY294002, a quercetin derivative, also inhibits ABCG2 [8].
Targeting stress response chaperone proteins (heat shock proteins, Hsps)
Heat shock proteins (Hsps), such as Hsp90, are highly expressed in diverse cancers, including MCF-7 BC. Therefore, these cancers can stand the fatal intrinsic oncogenic mutations, making Hsps ideal targets for anticancer therapies. Recently, a report from Taiwan suggested a decrease in the ALDH+ BCSCs population by knocking down Hsp27, employing siRNA gene silencing in combination with benzo-quinoid ansamycin antibiotic, i.e., geldanamycin (GA) [30]
As reported previously, the inhibition of Hsp90 resulted in the induction of heat shock factor-1 (HSF-1) and up-regulation of other Hsps, such as Hsp27 and Hsp70, all contributing to resistance to treatments in blocking Hsp90. The knocking down of Hsp27 by the siRNA approach revealed a reduction of ALDH+ BCSCs (20.3%). It was increased to 63.7% when combined with 40 nM geldanamycin—a possible option for future clinical practice [15].
More recently, it was found that enzymes involved in the mevalonate metabolic pathway, responsible for protein farnesylation, synthesis of cholesterol, and protein geranyl geranylation [GG], were overexpressed in basal/mesenchymal tumorospheres. In studies conducted in both in vitro and in vivo environments, it was discovered that the protein GG plays a crucial role in sustaining BCSCs. This finding was further substantiated by applying GGTI-288, a targeted inhibitor of geranyl transferase (GGTI). GGTI-288 led to a significant decrease in BCSC populations and a notable reduction in the formation of both primary and secondary tumorospheres [15].
Additionally, investigations in a mouse xenograft model of primary human breast cancer (T226, T214), treated either with GGTI (geranylgeranyl transferase I, 100 mg/kg/d for two weeks), docetaxel (10 mg/kg/wk for two weeks), or a combination of both, demonstrated a marked reduction in cell populations of over 70% in tumors treated with GGTI compared to docetaxel alone [15]. Data revealed that the heat shock protein (Hsp) pathway was implicated in the regulation of the mevalonate pathway and its downstream effects on BCSCs. Therefore, it can be inferred that GGTI specifically targeted BCSCs, with a partial contribution of RHoA/P27kip1 signaling, thus underscoring the importance of this pathway in therapeutic targeting, potentially in conjunction with the inhibition of Hsps [15].
Targeting BCSCs signaling cascades
Targeting signal cascades for renewal, proliferation, and differentiation is another therapeutic strategy to eliminate BCSCs. Interestingly, both cancer and normal stem cells share the pathways and components involved in BCSCs self-renewal and differentiation, such as Notch, PTEN, PI3K/Akt, JAK/STAT, hedgehog, Wnt/β-catenin, Bcl-2, and others (Fig. 2). AKT activation is pivotal for tumor progression and transformation [18]. Many studies have reported on the dysregulation of signaling pathways in BCSCs in an attempt to find new ways for cancer therapy. The promising research in targeting signaling pathways is mainly because many cancers exhibit up-regulation or down-regulation. Consequently, these strategies are beneficial for identifying breast cancer stem cells (BCSCs) through surface markers and understanding the signals linked with the tumor microenvironment [8].
Protein kinase Ca signaling
For targeted BCSC therapy, it is imperative and critical to identify the most pivotal regulatory networks that distinguish BCSCs from non-BCSCs. In this regard, in BCSCs, the protein kinase Ca (PKCa) signaling network is activated, which renders them liable to specific therapeutic agents. FRA1 is a crucial transcription factor downstream of PKCa that controls cancer stem cell function. As potential targets of BC, inhibition of PKCa and FRA1 can hamper tumor initiation with improved therapeutic value. The inhibition of protein kinase Ca (PKCa) affects only BCSCs with minimum or no effect on non-BCSCs. Pharmacologic inhibition of PKCa can target BCSCs selectively and may demonstrate a therapeutic edge in treating breast tumors [13].
Targeting notch pathway
Notch signalling is a pivotal pathway in vital cellular processes, such as stem cell differentiation, maintenance, proliferation, and cell fate determination during embryonic development [31]. The notch signalling pathway consists of four Notch receptors (Notch 1, Notch 2, Notch 3, and Notch 4) and five ligands (delta-like 1, delta-like 3, delta-like 4, Jagged 1, and Jagged 2). However, several literature reports have discussed the activation and inhibition of notch receptors, considering cell type-dependent effects [32, 33].
Both Notch4 and Notch1 were activated in BCSCs, while inhibition of the Notch4 receptor leads to reduced BCSCs activity in vitro and reduced tumor development in vivo [34]. A report by Harrison et al. suggests that in vivo Notch1 knock-down decreased the size and number of tumors. Still, complete repression in tumor initiation was observed after Notch4 knock-down, indicating that therapies targeting Notch4 will be more productive than those targeting Notch1 in defeating BC relapse. Data further suggest that the overexpression of Notch4 resulted in basal-like tumors’ development due to poor differentiation. At the same time, gamma-secretase inhibitors block notch signalling and re-sensitize cancer cells toward trastuzumab, tamoxifen, and chemotherapy. These findings indicate that targeting notch-4 will be more practical than notch-1 in quelling BC recurrence originating from BCSCs [34].
More recently, the development of monoclonal antibody tarextumab, either alone or in combination with other chemotherapeutic medications, has been shown to inhibit both Notch2 and Notch3 functions [8]. In 2009, Merck & Co developed MK-0752, a secretase inhibitor that preferentially kills BCSCs resistant to chemotherapy and radiation by blocking the notch pathway. After successful studies in mice, they conducted a phase I clinical trial by enrolling 35 women with advanced BC who were administered MK-0752 (Fig. 2). Data suggested marked attrition in the number of BCSCs [15].
Another drug, Hesperetin, has been shown as a potential candidate for the treatment of BCSCs by blocking the Notch pathway [35]. Furthermore, Metformin, a drug commonly used for type 2 diabetes, has shown potential as an anticancer agent, particularly in breast cancer treatment. While its exact mechanism of action remains unclear, metformin is believed to exert its anticancer effects through multiple signaling pathways, including the AMP-activated protein kinase (AMPK) pathway, the mammalian target of rapamycin (mTOR) pathway, and the insulin/insulin-like growth factor 1 (IGF-1) signaling pathway. These pathways are involved in regulating cellular processes such as metabolism, proliferation, and survival [36]. Additionally, recent studies have suggested a potential link between metformin and the Notch signaling pathway, which plays a critical role in stem cell maintenance and differentiation. Metformin has been reported to modulate Notch signaling in breast cancer cells, leading to the inhibition of Notch receptors and ligands—suppressing BCSCs. Further research is needed to fully understand the molecular mechanisms underlying the interaction between metformin and the Notch signaling pathway [7, 37].
Targeting hedgehog pathway
The hedgehog pathway is another pivotal pathway with a known contribution to stem cell maintenance, embryonic development, proliferation, and differentiation. Hedgehog signaling (HHs) is also implicated in the transition from epithelial to mesenchymal state (EMT), cancer cell invasion, chemotherapeutic resistance, metastasis, and tumor relapse [8]. Several studies have shown that specific inhibition of HHs in diverse cancers reduced drug resistance, regression, and metastasis [15]. Lately, a phase I clinical trial reported by Chia and Ma demonstrated that a drug named GDC-0449 (Fig. 2), when combined with other molecules, inhibited HH pathways for breast cancer treatment [38].
Moreover, an SMO signaling element inhibitor, cyclopamine, has been utilized by several groups to exploit its anticancer potential, which is known to inhibit the hedgehog signaling cascade. A study by Chen et al. further revealed that cyclopamine inhibited breast tumor growth (Fig. 2), invasion, and distant metastasis in vivo and in vitro [18]. Recent literature has reported that the use of Dinaciclib is associated with inhibition of stemness-related properties in two breast cancer cell lines, HCC-1806 and MCF-7, through regulating hedgehog signaling pathway and FoxM1; cell cycle regulator, showcasing the ability of hedgehog pathway to act as a potential therapeutic target in case of breast cancer [39].
Targeting TGF-β/Smad pathway
Most recently, a modified form of harmine (YH677) was found to selectively target breast cancer stem cells by regulating TGF-β/Smad signaling cascades. It efficiently showcased anti-metastatic and anti-proliferative capabilities, reducing tumor growth and migration and suppressing stem cell-related genes and EMT markers in patient-derived organoids, thereby acting as a potent drug for treating triple-negative breast cancer [40].
Targeting Wnt/b catenin pathway
In cancers such as BC, leukemia, colon, and skin, the wnt/β-catenin signaling pathway is one of the most dysregulated [8].
A novel derivative of sulindac, a nonsteroidal anti-inflammatory drug, i.e., phosphosulindac, has been shown to selectively kill BCSCs both in vivo and in vitro settings via blocking EMT and Wnt/β-catenin signaling pathways (Fig. 2) [15]. Moreover, another study utilized a natural compound, sulforaphane, derived from broccoli sprouts, which has been shown to inhibit BCSCs by down-regulating Wnt/β-catenin self-renewal pathway BCSCs [41].
Another compound, salinomycin, derived from streptomyces albus, has been shown to reduce BCSCs more than 100 folds compared to paclitaxel and almost 20-fold decrease in the proportion of CD44+/CD24−/low compared to approximately 18-fold reduction by paclitaxel (Fig. 2) [41]. These effects have been attributed to inhibiting Wnt/β-catenin signaling networks by impeding the phosphorylation of LRP6, the Wnt co-receptor, and promoting its degradation [38].
Targeting NF-κB pathway
The transcription nuclear factor kappa B (NF-kB) is constitutively activated in several cancers. It controls the expression of several proteins involved in apoptosis, cell survival, and proliferation [42], significantly impacting self-renewal and expansion of BCSCs [43].
Furthermore, a study using MCF-7 cells as a model of BC-like stem cells evaluated the effects of specific inhibitors of NF-κB, pyrrolidine-di-thiocarbamate (PDTC), and parthenolide (PTL), and its analog diethyldithiocarbamate (DETC), which specially retard MCF7 sphere cell proliferation (Fig. 2). It was discovered that the inhibitory effect of these compounds was due to the blocking of NF-κB actions in both MCF7 sphere and MCF7 cells, with significantly higher inhibition in MCF7 sphere cells than in MCF7 cells [44], suggesting that the targeted inhibition of BCSCs can be achieved by targeting NF-κB signaling pathway.
A growing body of evidence suggests that specific inhibition of NF-κB can suppress chemoresistance to a large extent. At the same time, inhibitors targeting NF-κB can augment the sensitivity of cancer cells to chemotherapeutic agents. Besides, the combined delivery of NF-κB inhibitor PDTC and DOX successfully overpowers the menace of multidrug resistance [18].
PDTC and DETC are well-acknowledged antioxidants that can hamper the NF-κB pathway by blocking and inhibiting NF-kB and IKK activity, respectively [45, 46]. Furthermore, in the mouse xenograft model, PDTC alone demonstrated marked inhibition of tumor growth but revealed even better tumor growth inhibitory effects when combined with paclitaxel. Besides, parthenolide (PTL), obtained from the extracts of Tanacetum parthenium, can inhibit the NF-κB pathway. In a xenograft model of BC, PTL, in combination with docetaxel, has been shown to reduce metastasis and improve survival significantly (Fig. 2). [44].
Targeting Akt signaling
PI3K/Akt/mTOR signaling pathway is known to be involved in a variety of cancer processes, with a series of phosphorylation reactions involving several vital molecules such as PI3K activating mutations, AKT overexpression or activation in metastasis and invasion, and mTOR are considered pivotal for cancer cell growth, survival, and angiogenesis [47]. This signaling pathway is suggested to maintain BCSC features [8]. In BC, the targeted inhibition of PI3K or Akt resulted in reduced genesis and CD44/CD24 mammospheres growth, which leads to loss of stem cell/mesenchymal stem cell phenotype and retrieval of endothelial phenotype [48].
A pharmacological agent, Perifosine, an Akt inhibitor, has been shown to decrease the serial mammosphere formation (Fig. 2). Nonetheless, the major challenge is their limited toxicity toward selected cell types, i.e., BCSCs [49]. Targeting the mTOR pathway can be promising therapeutic strategy against BCSCs. A steroidal compound, Dioscin, was tested in MDA-MB-231 and MCF-7 cell lines to assess its treatment efficacy against breast cancer. Dioscin-treated BCSCs showed reduced proliferation, migration, and differentiation, possibly due to G0/G1 and G2/M cell cycle arrest. Insights into molecular mechanisms associated with DS treatment showed significant down-regulation of the mTOR pathway, which leads to reduced BCSC proliferation and increased apoptosis [50].
Targeting Kruppel-like factor 4 (KLF4)
The Kruppel-like factors (KLFs), transcription factors, belong to a family of gene regulatory proteins involved in the regulation of a wide variety of cellular processes, such as proliferation, differentiation, inflammation, migration, apoptosis, and tumor formation [51] [52]. Kruppel-like factor 4 (KLF4) is overexpressed in more than 70% of BC and acts as an oncogene, while persistent overexpression of KLF4 leads to an increase in the BCSCs population. Thus, knocking down of KLF4 in BC cells, MDA-MB-231, and MCF-7 resulted in a marked reduction in the proportion of stem/progenitor cells, as evident by a significant decrease in the expression of stem cell-associated surface markers, such as ALDH1 and side population, along with suppression in cell migration and invasion potential of MCF-7 cells [52]. These data suggest that KLF4 plays an imperative role in mammary tumorigenesis, probably by upholding stem cell-like characteristics and supporting cell migration and invasion. Thus, targeting KLF4 may provide an efficacious therapeutic approach to contain tumorigenicity in BC. Moreover, a small molecule inhibitor of KLF4, Kenpaullone, decreases the mRNA and protein expression of KLF4 in both MDA-MB-231 and MCF-7 cells, which might provide a future therapeutic strategy to suppress tumorigenesis [52].
Targeting tumor microenvironment
The BCSCs reside in a “niche” tumor microenvironment, providing essential signals for their self-renewal, maintenance, and homeostatic processes, including angiogenesis, weakly acidic pH, and hypoxia [8]. This involves a variety of factors, receptors, and procedures.
CXCL12-CXCR4 axis
It has been argued that the CXCL12-CXCR4 axis regulates the cross-talk between BCSCs and tumor stroma. This starts with binding chemokine, CXCL12, or stromal-derived factor-1 (SDF-1) to its receptor CXCR4, which is implicated in the invasion, migration, and survival of both standard and malignant cells. In the tumor microenvironment, this binding, the CXCL12-CXCR4 axis, establishes an intricate relation between the cells and tumor stroma that may activate many processes related to cellular growth, metastasis, and chemoresistance (Fig. 3). In human BC, CXCL12 is produced by stromal fibroblasts, thereby exerting two independent effects on the development of a tumor, i.e., an endocrine effect produced by stimulating angiogenesis and a paracrine effect induced by directly acting on cancer cells via CXCR4 [53].
After extensive research, several inhibitors that potentially target CXCR12 or CXCL4 have been studied. One of the inhibitors of CXCR4, AMD3100, also known as plerixafor, has been shown to induce the mobilization of hematopoietic stem and progenitor cells and inhibit the growth of tumors by enhancing apoptosis and reducing the proliferation of tumor cells [54]. CTCE 9908 is another CXCR4 antagonist significantly reducing tumor growth and metastasis in xenograft mouse models of inflammatory BC (Fig. 3) [8]. Besides, 14,003 analogs and Plerixafor (AMD3100), CXCR4 antagonists, destroy the adhesiveness of tumor-stroma interactions (Fig. 3), thus making the cells more vulnerable to cytotoxic drugs [18].
Vascular endothelial growth factor (VEGF)
Another therapeutic strategy is to target BCSCs in their endothelium niche. Enhanced angiogenesis will likely enrich these niches with a blood supply [26]. However, another pivotal mechanism stimulated and controlled by the tumor microenvironment is tumor angiogenesis, which may impact BCSC survival and overall tumor growth. In this context, targeting the vascular endothelial growth factor (VEGF) that regulates optimal vasculogenesis reduces tumor growth and disturbs BCSCs’ niche [55]. Bevacizumab, an anti-VEGF blocking antibody, is an approved drug targeting the VEGF/VEGFR system. In contrast, sunitinib, pazopanib, and sorafenib are VEGFR-2 pathway inhibitors (Fig. 3). VEGF induced angiogenesis through mutual interactions between cell membrane transporter ABCG2 and tumor hypoxic environment led to increased BCSC stemness. Experimentally, Quinacrine has been shown to suppress VEGF-associated angiogenesis by inhibiting the interaction between ABCG2 and tumor hypoxic environment [56]. A very potent and highly selective small molecule inhibitor against the VEGFR family, Fruquintinib (HMPL-013), is currently in phase II clinical studies. (Fig. 3) [8]
Targeting HIF (hypoxia inducible factor)
Another remarkable feature of the tumor microenvironment is hypoxia, controlled by highly inductive transcription factors, hypoxia-inducible factor-1 (HIF-1), and hypoxia-inducible factor-2 (HIF-2). In this regard, for effective control of tumor metastasis, targeting HIF may improve chemo and radiotherapy outcomes [57]. In BC, the primary tumor growth was reduced with a reduction in the dissemination of cancer to the lungs by targeted inhibition of HIF via RNA interference or digoxin treatment of BrCa cells, which leads to the down-regulation of the expression of L1 cell adhesion molecule (L1CAM) and angiopoietin-like 4 (ANGPTL4) (Fig. 3) [8]. A recent study has evaluated the promising role of a compound 3-O-α-L Arabinosyl oleanolic acid in suppressing the growth of BCSCs by directly targeting hypoxia-inducible factors; HIF-1α and HIF-2α, facilitating BCSCs apoptosis by down-regulating them [58]. Moreover, in BC, hypoxia has been shown to induce BCSCs phenotype via HIF-dependent and ALBH5-driven m(6)-demethylation of NANOG mRNA (Fig. 3) [59].
Acidic extracellular pH
As reported in recent decades, most malignant tumors cherish a reduced extracellular pH. These peculiar characteristics of the tumor microenvironment contributed to the increased tumor cell metabolic activity and reduced tumor vascular perfusion [60, 61]. This extracellular acidic pH confers a careful advantage to tumor cells over normal cells and may promote drug resistance. From the management point of view, several stratagems have been considered, such as using delivery of drugs having specificity for acidic environments, use of systemic buffers, sodium bicarbonate, and using pH regulatory pathway inhibitors like carbonic anhydrase IX (CAIX) (Fig. 3) [62]. One way of improving drug delivery procedures is to entrap the drugs into silica matrices like doxorubicin and camptothecin (Fig. 3) [63]. Likewise, a systemic buffer consisting of sodium bicarbonate can be utilized to alkalinize the microenvironment [8].
Furthermore, one of the remarkable features of the TAT peptide, an arginine-rich peptide, is that it can quickly transport various types of small drug molecules, both in vivo and in vitro, into mammalian cells (Fig. 3). Attempts have been made to conjugate biotin with TAT, a pH-sensitive cargo. Under slightly acidic pH, this cargo reveals biotin. In a report by Lee et al., micelle was conjugated with TAT that hides TAT during circulation but facilitates the internalization process when exposed at a slightly acidic extracellular pH [64], resulting in a rapid increase in concentration of doxorubicin in the cytosol. This innovative cargo has been assessed in tumor xenografts of BC MCF-7 cells, where a noticeable decrease in tumor size has been observed [18].
Targeting CXCR1/2 receptors
Dysregulation of interleukins, such as interleukin-8 (IL-8) in malignant BC, is well recognized. Targeting CXCR1/2 signaling has shown considerable success in in vivo BC models [14]. The literature evidence suggests that IL-8 (CXCR1/2 ligand) is a critical extraneous regulator of BCSC activity. Nonetheless, considering IL-8 alone may confer little benefits, probably due to the co-regulation of CXCR1/2 agonists (CXCL2/GRO-β, CXCL1/GRO-α, CXCL5, and CXCL3/GRO-γ) along with IL-8 [65]. This can reasonably be controlled by blocking the CXCR1/2 axis. Furthermore, non-competitive antagonists of CXCR1 and CXCR2, orally active small molecules, such as SCH527123, repertaxin, and SCH479833 have shown anticancer effects in xenograft models of BC, whereas SCH563705 demonstrated improved efficacy at inhibiting primary human BCSC activity by binding to CXCR1 and CXCR2 (Fig. 3) [66, 67]. Despite the proven effectiveness of CXCR1/2 inhibitors in preclinical settings, they are still in the early stages of drug development. However, the only CXCR1/2 inhibitor, i.e., repertaxin, has been through clinical testing. In advanced BC patients, clinical trials are underway to estimate the combined effects of repertaxin and docetaxel chemotherapy for safety and clinical efficacy (Fig. 3) [68]. Thus, combining CXCR1/2 inhibitors with either current chemotherapy/endocrine therapy or HER2-targeted therapies might confer a more effective strategy to eradicate both the BCSC and non-BCSC populations that may lead to improved treatment outcomes involving both adjuvant and advanced clinical settings [14].
Targeting BCSCs via miRNA expression
MicroRNAs (miRs) are small, non-regulatory RNAs that affect various biological processes, such as self-renewal, cell division, and differentiation. These miRNAs maintain normal cell functions and involve a wide range of signaling networks that may convert stem cells into cancer stem cells. They can influence the cells either as oncogenes or suppressors of tumor growth by differentially regulating the key properties of BCSCs, such as cell cycle exit, survival, differentiation, migration, invasion, and endothelial-mesenchymal transition—all that promote progression and metastasis [69]. These oncogenic miRNAs can be targeted using anti-sense oligonucleotide inhibition. In this context, miR-21 is frequently up-regulated in various cancer stem cells, and the knocking down of miR-21 prevents cell migration, proliferation, and tumor growth in BC (Fig. 3) [70]. Additionally, the miRNA-200 family is known to be a chief regulator of BCSC function and development. In this regard, mi-RNA200c markedly suppressed the clonogenicity of BCSCs via BMI1 (protooncogene, polycomb zinc finger) [71] and miRNA200b by directly acting on Suz-12 (Suz12 polycomb repressive complex 2 subunit), both affecting BCSCs growth (Fig. 3) [72].
Lately, miRNA106b-25 has been shown to down-regulate SMAD family member-7 (SMAD7) protein that activates the TGF-β pathway in BC tumors [73]. miRNA-487a has been shown to trigger TGF-β mediated EMT, invasion, and migration by decreasing MAGI-2 expression (Fig. 3) [74]. Cao et al. demonstrated that miRNA-4469 negatively controls the expression of CDK-3 (cyclin-dependent kinase 3) in BC metastasis (Fig. 3). In contrast, increased expression of CDK3 inhibits tumor progression of breast tumor cells by inhibiting the Wnt/β-catenin pathway (Fig. 3) [75]. Kong et al. demonstrated that the up-regulation of miRNA27a resulted in the up-regulation of β-catenin and Wnt proteins, which triggered the Wnt/β-catenin cascade by inhibiting sFRP1 thereby activating migration and invasion of breast tumor [76]. More importantly, decreased expression of miRNA-134 enhanced tumorigenesis of breast cancer via up-regulation of KRas [77]. Furthermore, re-gaining the expression of miRNA 34a-5p has been shown to reduce the stemness of BCSCs by facilitating doxorubicin influx achieved through attenuation of ABCC1 expression in MDA-MB-231 cell lines [78]. Likewise, reduced expression of miRNA-29a inhibits the proliferation cell cycle and increases apoptosis, probably by controlling the NF-κB signaling pathway (Fig. 3) [79].
Targeting BCSCs by inducing apoptosis
Apoptosis is a uniquely regulated mode of cell death involving multifaceted signaling mechanisms. An escape from this regulatory process is a prerequisite for the cells to initiate cancer, and among others, the pro-apoptotic approach is the most common strategy in cancer therapy [8]. Due to its promising outlook in clinical settings, several compounds have been examined to target innate and extraneous apoptotic pathways.
One strategy is to activate the death receptors, trimeric human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), and CD95, which leads to caspase-8 activation. The use of TRAIL in combination with other anti-tumor mediators has shown encouraging results in eliminating cancer stem cells. Thus, TRAIL, combined with cisplatin, has decreased the triple-negative breast cancer (TNBC) stem cells by blocking Wnt signaling and promoting apoptosis [8]. A study by Jasim et al. showed the apoptosis-inducing activity of a phytochemical Corosolic acid (pentacyclic triterpenoid) in triple-negative breast cancer cells MADA-MB-231, demonstrating their anti-proliferative ability in MCF-7 cell lines, associated with significant inhibition of Jak/Stat signaling cascades [80]. Another signaling pathway that affects the control of apoptosis pathways in BCSCs is the NF-κB transcription factor and its downstream targets. Numerous reports suggest that NF-κB inhibits apoptosis and induces cell proliferation, inflammation, tumor progression, angiogenesis, and metastasis [81].
The small molecules, parthenolide, pyrrolidine dithiocarbamate, and its analog diethyldithiocarbamate by inhibiting NF-κB signaling, preferentially target breast cancer stem cells—through a more targeted approach is required to increase the specificity and proficiency of these agents in targeting cancer stem cells without affecting on normal tissue stem cells [8] [82].
Furthermore, salinomycin, isolated from Streptomyces albus, a polyether ionophore antibiotic, has been shown to reduce BCSCs by more than 100-fold compared to the conventional chemotherapeutic agent, paclitaxel [83]. Salinomycin induces pro-apoptotic effects in chemo-resistant tumor cells expressing higher levels of p-glycoprotein and Bcl-2 [84]. However, due to the potential toxicity of salinomycin exposure, either by ingestion or inhalation, the real-world therapeutic value of this remarkable compound has been compromised.
Another chemical component from medicinal herb ginseng called ginsenoside F2 demonstrated marked suppression of BCSC proliferation via innate apoptotic signals utilizing protective autophagy via phosphorylation of p53—a tumor suppressor. These effects of ginsenoside F2 were even more pronounced than the well-recognized phytochemicals, including quercetin, tangeretin, nobiletin, and baicalein in MCF-7 cells and BCSCs—dose-dependent decrease in the viability of BCSC after 24 h, while the phytochemicals represented a poor suppressive response toward BCSC proliferation [15]. More recently, a study evaluated the therapeutic potential of a candidate drug compound, Pitavastatin, against a triple-negative breast cancer phenotype. Pitavastatin exposure to 4T1 and BT549 cells significantly reduced stem cell-related properties through induction of mitochondria-mediated apoptosis. Furthermore, dysregulation of the STAT survival pathway, as well as down-regulation of survivin, Cyclin D, and vimentin, was observed, leading to suppression of stem-like properties of BCSCs as well as a significant reduction in angiogenesis and tumor growth [85].
Targeting BCSCs by inducing differentiation
Traditional chemotherapeutic agents target and manage differentiated cells without affecting cancer stem cells. This compels the researchers to utilize differentiation agents or target pathways that support cancer stem cell differentiation rather than self-renewal. Numerous differentiation agents, such as retinoic acid and its analogs (ATRA), miRNAs, tyrosine-kinase, histone deacetylase inhibitors, and signaling pathway inhibitors, have been proposed in this context.
A study was conducted to assess the anticancerous properties of Empagliflozin (EMPA) in breast cancer stem cells. Researchers found that EMPA acts as an enhancer of miR-128-3p, thereby causing the knockdown of its down-regulatory genes PRM2 and SP1, which are responsible for breast cancer stemness. Hence, EMPA was proved to be a potential therapeutic in suppressing tumor growth by inducing the differentiation of breast cancer stem cells [86]. Studies have shown that a lipophilic derivative of vitamin A, i.e., retinoic acid (RA), is a well-known inducer of stem cell proliferation and differentiation [87] [88]. The carboxylic form of vitamin A, also termed all-trans-retinoic acid, tretinoin upon treatment to BCSCs leads to their differentiation with subsequent reduction in invasion, migration, and increased sensitivity toward chemotherapy [89]. Another pivotal regulator of stem cell differentiation, i.e., let-7, is lowered in BCSCs, yet ectopic delivery of let-7 to BCSCs can hinder the proliferation of stem cells, maintenance, and sphere formation [90]. A promising work by Pham et al. showed that the knockdown of CD44 resulted in the loss of stemness of BCSCs into differentiated cells, resulting in increased susceptibility of BCSCs toward chemotherapy and radiation therapy [91].
The strategies to induce BCSC differentiation are not specific and may affect normal or stem cells. Nonetheless, more targeted delivery of the agents that cause differentiation would be relatively safer than other therapeutic options in BC treatment.
Targeting HER2 signaling
Human epidermal growth factor receptor 2 (HER2) positive BC subtype exhibited aggressive tumor growth. The most common effectors of HER2 signaling include the mitogen-activated protein kinase (MAPK) pathway, phosphoinositide-3-kinase (PI3K)/Akt signaling, and protein kinase C activation. With three significant and pivotal ways engaged, HER2 signaling is inherently involved in cell proliferation, survival, adhesion, migration, differentiation, and apoptosis [92].
Besides, an FDA-approved CDK-4/6 inhibitor, palbociclib, either alone or in combination with trastuzumab, has been shown to inhibit the growth of HER2+ cell lines [92]. Likewise, immune checkpoint inhibitors, cytotoxic T lymphocytes associated protein 4 (CTLA4), and programmed death 1 (PD1) have been associated with the poor overall survival of HER2+ BC. Furthermore, a strong association between IL-6 serum levels and poor clinical outcomes in HER2+ BC patients has been observed—probably the inflammatory feedback loops regulating BCSCs, involving STAT3/Akt/NF-κB signaling pathways, may contribute to trastuzumab resistance in HER2+ BC [93].
Diet mediated targeting
Dietary sources from vegetables and fruits are considered central for BC risk reduction. A study by Montales et al. demonstrated that soy isoflavone genistein (GEN), a metabolite of blueberry (BB) polyphenols, can efficiently weaken in vitro formation of mammosphere at physiologically apposite doses, suggesting that these compounds are most likely to repress cancer stem cells self-renewal and expansion in vivo [94].
Another alkaloid, berberine, extracted from Berberidaceae, Coptis chinensis, and Hydrastis Canadensis, when developed as liposomes, has been shown to modify the mitochondrial proteins and resistant membrane of BCSCs, effective for the prevention and treatment of BC relapse. The berberine liposomes could cross the membranes of BCSCs by gathering in the mitochondria and inhibiting ABC transporters (ABCG2, ABCC3, ABCC2, ABCC1) [12]. Furthermore, data suggested that these liposomes induce pro-apoptotic BAX protein and block anti-apoptotic BCL-2 protein, which causes the mitochondrial permeability transition pores to open, activation of caspase-9/caspase-3 and discharge of cytochrome c—all leading to the death of BCSCs owing to grave cytotoxic insult and induction of apoptosis [12].
Moreover, phytochemical compounds in vegetables from the Brassicaceae family, more than 350 plant species, such as cauliflower, broccoli, and cabbage, have shown documented potential in reducing the risk of developing cancer, including BC [95, 96]. A dietary component of broccoli, sulforaphane, has been shown to prevent and induce the proliferation and apoptosis, respectively, of BC cells, possibly via regulating many molecules, such as BCL-2 family proteins, p21, cyclins, cyclin-dependent kinases, and caspases [97]. Another study reported that sulforaphane caused a driven decrease in the growth of endothelial cells (EC) and pericytes by affecting intracellular signaling between ECs and pericytes, possibly through blocking of angiogenic factor, vascular endothelial growth factor (VEGF), in pericytes, thereby causing the activation of hypoxia-inducible factor 1 (HIF-1) and down-regulation of prolyl hydroxylase domain-containing protein 1 and 2 (PHD1/2) [98]. Sulforaphane also down-regulates the Wnt/β-catenin pathway in BC cells, as evident by a considerable reduction of 85% and 77% in β-catenin and cyclin D1 proteins, respectively, in MCF-7 cells [41]. It is further suggested that the chemo-preventive effects of sulforaphane are mainly achieved via blocking effects, possibly through inhibition of phase 1 metabolic enzymes that transform procarcinogens to carcinogens and mediating phase 2 metabolic enzymes that enable the expulsion of carcinogens [99].
Several other dietary compounds that oppose cancer stem cell self-renewal include curcumin, quercetin, and epigallocatechin-gallate [41]. The blocking of Wnt signaling by polyphenols obtained from the diet, such as piperine, has been shown to affect the mammosphere formation and decrease the proportion of ALDH1+ cells [100].
Use of dendritic cell vaccination
Among the widely adopted cancer immune therapy approaches, cancer vaccine, adoptive T cell therapy, and checkpoint inhibition are currently being practiced to treat BC. In this context, specialized antigen-presenting cells termed dendritic cells (DCs) originating from the bone marrow precursors possess high endocytic activity and low T cell activation in immature form, yet on maturation, they can sturdily activate T cell via cell–cell contact or by inducing cytokines production [101]. In BC patients, DCs exhibit intrinsic abnormalities affecting several vital processes, such as the inability to efficiently present tumor antigens to T cells, decreased antigen processing, reduced co-stimulator expression, decreased interleukin-12 and 9 production, and reduced migration [102]. It has been proposed that DC vaccination could be a suitable approach to prevent disease recurrence in BC in combination with radiation and chemotherapy. DCs can successfully present BCSC-acquired antigens to other WBCs, such as T and B cells, and stimulate these WBCs to attack tumor cells or BCSCs and retard tumor progression and invasion [91]. Recently, Ni et al. developed nanoparticles of cancer cell membranes coated with calcium carbonate that produce in situ tumor associate antigens (TAAs). They encapsulated low-dose doxorubicin hydrochloride (DOX) to activate immunogenic cell death, along with chlorins (Ce6), generally used photosensitizer, for efficient photodynamic therapy via the production of reactive oxygen species (ROS), thereby eliciting TAAs population and DC recruitment [103].
Use of oncolytic viruses
Another anticancer approach is viral cancer therapy, where oncolytic viruses increase their progeny in cancer cells and kill them, possibly through membrane fusion mechanisms or attaching to cell surface receptors, without damaging healthy cells [104]. The anti-tumor effects have been attributed to various means, including selective replication and following the lysis of cancer cells, modulation of apoptotic pathways, insertion of therapeutic genes into the viral genome, production of cytotoxic proteins, sensitization of cancer cells toward chemo/radiation therapy and triggering host immune responses [105]. Moreover, several different viruses evaluated in clinical trials have confirmed their safety in clinical settings, including reovirus, herpes simplex virus, measle virus, vesicular stomatitis virus, adenovirus, and vaccinia virus [106]. The methods used to administer oncolytic viruses include intra-arterial, intraperitoneal, intravenous and intratumoral delivery.
A study by Marcato et al. demonstrated that the intratumoral injection of reovirus reduced tumor growth and eliminated BCSCs in a BC mouse model of solid tumor xenografts [107]. Another report showed that BCSCs, having more significant ALDH1 activity, were infected with the vaccinia virus, which enhanced cytotoxicity and increased virus-mediated lysis of BCSCs [108]. Likewise, in Phase II clinical trials, data revealed that reoviruses could alter ras signaling in BC, affecting the BCSC population [90]. It is pertinent to mention that reovirus does not cause human disease, yet it destroys explicitly BCSCs. In vivo, experiments had shown that after treatment with reoviruses, there was a considerable decrease in CD44+/CD24-/low population and the tumor mass. Besides, employing in vitro settings, when ALDH+BCSCs were infected by reovirus, they activated apoptotic pathways that led to cell death [15].
Due to their preference for epithelial tissue, adenoviruses may be necessary to target BCSCs. In this context, an engineered adenovirus carrying CD40L has been shown to significantly reduce CD40+ breast cancer cells, while ensuing in vivo data showed a marked reduction in MDA-MB-231 cells in xenograft mice model—probably due to cell cycle arrest and apoptosis in virus-infected cells [109]. Arming viruses with transgenes that target BCSCs is another area of immense interest. In this regard, Zhang et al. reported that delivery of recombinant adenovirus having the human interferon gene (IFN-con1) significantly regressed tumor growth in the human BC xenograft model [110]. Additionally, TNBC 4T1 was treated with an oncolytic vaccinia virus containing chemokine receptor 4 (CXCR4) antagonist, which inhibited tumor growth with a marked decrease in angiogenesis [111]. Similarly, invasive BC cells were infected with adenovirus-containing receptors for TGF-type II, significantly reducing tumor size [90].
With a plethora of benefits of oncolytic viral therapy for BC come the drawbacks as well. The notable disadvantages included potential toxicity to non-targeted cells, lower efficacy in serum, and poor epidemiological control. For example, pre-treatment with cyclophosphamide completely circumvented the immune-moderated viral annihilation and increased the effectiveness of the oncolytic cure. However, the treatment with cyclophosphamide may result in immune suppression—posing considerable challenges to this cancer therapy method. Another pivotal factor affecting the efficacy is the route of administration. Due to hepatic sequestration, virus delivery via intravenous route is not suitable. Besides, limited entry of viruses into tumors may present another daunting challenge to the delivery systems. Adenoviral therapy may cause adverse effects, such as hepatotoxicity and systemic toxicity. Consideration must be given to the proper epidemiological control while utilizing the viruses for clinical purposes—though attenuated viral strains are superior. Thus, oncolytic viral therapy poses a significant challenge. Therefore, challenges to both efficacy and safety must be addressed [90].
Nanobiotechnological approaches
Most of the approaches mentioned above have specific characteristics that limit their effectiveness in vivo, such as poor solubility, instability, undesirable bio-distribution, off-target effects, circulation half-life, targeted and adequate delivery, and low therapeutic indices [112]. Nano-medicine has recast our approach to drug delivery by improving the therapeutic agents’ selective targeting of tumor tissue and cells while minimizing the toxicity to normal cells [113]. They offer BCSCs-specific therapeutics because of their inherent properties, such as solubility enhancement effects, high drug loading capacity, site-specific delivery, controlled release mechanisms, and minimal premature drug release [114].
BCSCs targeted therapy
The BCSCs were targeted to deliver the drugs employing a variety of nanocarriers such as polymeric nanoparticles, metal nanoparticles, polymeric micelles, liposomes, and carbon nanotubes with promising results [115]. One profitable strategy to overcome the selection bias toward the delivery systems for specific cancer cells is to assess the biological functionalization of nanocarriers [116]. In this regard, the delivery systems linked with specific agents with the ability to recognize and bind to definite surface markers of BCSCs are summarized in Table 1.
A study by Gener et al. demonstrated the development of CD44 functionalized and paclitaxel (PTX) loaded micelles that enhanced the internalization of PTX in BCSCs [117]. Similarly, CD44+ cancer cell lines and BCSCs were targeted by gemcitabine derivative-loaded multi-functionalized iron oxide magnetic nanoparticles that suppressed CD44+ cancer cells [116]. In another attempt, hyaluronic acid (HA)-based self-assembling nano-systems for siRNA delivery were developed to target CD44 receptors, efficiently delivering siRNA and silencing the gene in drug-resistant CD44 expressing tumor models [118]. Furthermore, polymeric nanoparticles coated with chitosan and loaded with doxorubicin (DTX) have been designed to target the CD44 receptors on breast cancer stem cells (BCSCs). Compared to the drug’s effectiveness when administered freely, this targeted approach has enhanced the efficacy of eliminating BCSCs by nearly sixfold with the loaded DTX [119]. More recently, Gaio et al. showed target destruction of CD44 over expressing BCSCs by co-delivery of photosensitizer meso-tetraphenyl chlorine disulfonate (TPSC2a) and DTX employing HA-coated polymeric nanoparticles (HA-NPs) [120]. Another recent report demonstrated targeting of TNBC via CD44 mediated apoptosis by developing nano-micelles, hyaluronic acid decorated oligomer containing styrene maleic anhydride (SMA) and D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) encapsulated in curcumin analog curcumin-difluorinated [121, 122].
In recent years, for BCSCs’ targeted drug delivery, various nanocarriers, such as polymeric micelles, nanoparticles, liposomes, inorganic nanoparticles, and nanogels, have been explored (Table 2). These delivery systems have demonstrated marked improvements in the stability of drugs and empowered the controlled release of higher concentrations of the medications to BCSCs and the tumor [112]. The various nanocarrier categories introduced and assessed for targeted delivery of multicomponent drug cargos to BCSCs are summarized in Table 2. Multiple types of nano-medicines employed for drug-targeted distribution to BCSCs are further described below under separate sub-headings (Fig. 4).
Liposomes
Liposomes, amphiphilic phospholipid bilayers, are colloidal nanocarriers and can load hydrophilic and lipophilic drugs. Moreover, they are considered classical nanocarriers for anti-BCSC treatment due to their impulsive characteristics, which include biocompatibility, ease of surface modifications, and prolonged blood circulation time [123]. In line with this, daunorubicin plus quinacrine liposomes for mitochondrial targeting were developed to prevent disease recurrence, possibly due to BCSCs—MCF-7 cancer cells. Data suggested that liposomes targeting the mitochondria increased the uptake of drugs into mitochondria, initiated pro-apoptotic BAX protein, opened permeability transition pores, reduced mitochondrial membrane potential, translocated cytochrome c, and triggered pro-apoptotic signals, caspase 9 and 3, thereby causing apoptosis of MCF-7 cancer cells [124]. More recently, Yu et al. tested glucosamine-labeled liposomes coupled with carboplatin and paclitaxel for their therapeutic potential against breast cancer stem cells. They showed a remarkable synergistic effect with apparent tumor reduction in animal models by directly targeting hypoxic niches. Hence, they proved to act as an ideal treatment approach for breast cancer [125]. Nguyen et al. developed a liposomal nano-complex by functionalizing folic acid (FA) molecules on liposomes encapsulating both DOX and gold nanorods to have the combination of chemo and photothermal therapy that markedly reduced the growth of tumor in vivo in breast tumor-bearing mouse model [126]. Another study claimed that the cytotoxic activity of folate-targeted liposomal bleomycin (FL-BLM) was meaningfully higher than free folate BLM and only BLM in MCF-7 cells [127]. Likewise, anacardic acid-functionalized liposomes (DTX-AA-PEG-liposomes) loaded with DTX have selective distribution; higher uptake by cells showed apoptosis of MCF-7 cells and reduced toxicity compared to free DTX [128]. Recently, cell membranes of red blood cells from rabbits combined with self-assembling AS1411 DNA aptamer were employed to prepare α-Gal liposomes. These surface functionalized α-Gal liposomes can recognize anti-Gal antibodies and nucleolin expressed by cancer cells to activate immune system attack. This resulted in the lysis of tumor cells, MCF-7, by AS1411 modified α-Gal liposomes [129]. A report by Kim et al. showed that cross-linked multilamellar liposome vesicles (cMLVs) co-delivered salinomycin and DOX targeting BCSCs—which resulted in improved suppression of BCSCs in vitro and in vivo (Fig. 4) [130]. Besides, using cationic lipid-conjugated estrogenic derivative (ESC8) in combination with dexamethasone (dex) associated liposomes, named DXE liposome. Conjugated delivery of Genudin and P-glycoprotein in the form of liposome nanoparticles showed promising therapeutic effects against BCSCs in terms of enhanced anti-proliferative ability, and regulation of genes associated with BCSC survival, including p53, Cyclin D, ABCB1 and Bax [131]. The neuropilin-1 (NRP-1) gene-targeted shRNA was co-delivered with DEX and DEX-NRP-1 for enhanced tumor regressing effects. In a mouse-bearing ANV-1 xenograft model, DXE-NRP-1 significantly reduced tumor volume compared to DXE-control (Fig. 4) [132].
Polymeric micelles
These colloidal particles have both hydrophobic and hydrophilic components—providing a platform to improve the target efficiency of several medications (Fig. 4). Polymeric micelles are considered popular drug carriers in anticancer treatment due to their size, circulation time, and uniformity [123].
A study by Zhang et al. developed PEG-b-PCL, octreotide modified, polymeric micelles loaded with PTX (Oct-M-PTX) and with SAL (Oct-M-SAL) employing thin film hydration method to target BC cells overexpressing somatostatin receptors specifically. They showed that Oct-M-PTX showed potent inhibition against MCF-7 cells compared to M-PTX but demonstrated synergistic effects in combination with M-SAL—similar synergism observed in the in vivo settings [133]. Furthermore, both DOX and thioridazine (THZ) were loaded into micelle, collected from a blend of acid-functionalized poly(carbonate) poly(ethylene glycol) di-block co-polymer (PEG-PUC) having inhibitory solid effects on sorted side population CSCs obtained from MCF-7 and BT-474 cancer cells, and on BT-474 xenograft mouse model (Fig. 4) [134]. Another study reported the synthesis of anti-CD44 functionalized PLGA-co-PEG polymeric micelles loaded with PTX against breast cancer cell lines with increased sensitivity of PTX toward cancer cells [117]. Polymeric micelles were developed for drug-resistant recurrent breast tumors by loading epirubicin (Epi) and staurosporine (STS) to treat recurrent breast tumors. Data suggested that Epi/STS-loaded micelles showed potent anticancer effects against both naïve orthotopic and recurrent Epi-resistant tumors (Fig. 4) [135]. More recently, Xiang and co-workers developed a PTX-binding polymeric micelle with a core having thiourea group attached to PTX from poly (ethylene glycol)-block-dendritic polylysine that demonstrated improved tumor uptake and proficient in vivo therapeutic effects in both subcutaneous and orthotropic human BC xenografts [136]. Likewise, another recent study investigated the nano-encapsulation of curcumin (CUR) within polymeric micelle of pristine and glucosylated poly(ethylene-oxide)-poly(propylene-oxide) co-block polymers and their targeted in vitro and in vivo effects in BC model. CUR encapsulation increased intracellular accumulation and cytotoxicity in 4T1 cells compared to free drugs and intratumoral accumulation in 4T1 implanted BALB/c mice representing stage IV human BC animal model [137].
Nanoparticles
Nanoparticles (NPs) have tremendous potential to deliver drugs at the target site and are widely used for the treatment of cancer (Fig. 4). NPs confer several advantages, such as good stability, high encapsulation, and the ability to encapsulate both hydrophobic and hydrophilic drugs [138]. Nonetheless, some likely risks are associated with their use, such as easy access to membranes and body parts, which may interact with cells unfavorably and cause intrinsic toxicity (Table 2) [138].
To induce BCSC differentiation, Sun and co-workers synthesized PEG-b-PLA nanoparticles by encapsulating all-trans-retinoic acid (ATRA) and DOX for co-delivery, which resulted in reduced tumor initiation ability with increased sensitivity to DOX both in vitro and in vivo in reducing tumor growth compared to NPDOX and NPATRA (Fig. 4) [139]. Moreover, PTX encapsulated in nanostructured lipid carriers (75 nm) demonstrated significant anticancer activity against MDA-MB-231 and MCF-7 cells [140]. Besides, the co-encapsulation method has been used to deliver quercetin and vincristine in a PEGylated liposome to treat trastuzumab and hormone-sensitive BC. Data demonstrated that co-encapsulation enhanced synergism, controlled drug release, and protracted drug circulation in plasma, leading to effective growth inhibition of JMIT-1 cells [141]. In another study, employing in vitro settings, co-delivery of siRNA, both siHIF1 (hypoxia-inducible factor) and siVEGF (vascular endothelial growth factors), was achieved using chitosan-coated liposomal NPs (LNPs), which significantly reduced the proliferation of MDA-MB-435 and MCF-7 BC cells (Fig. 4) [142]. Li et al. reported that treatment of MDA-MB-231 cells with nanoparticles loaded with low-dose decitabine (DAC) in combination with DOX-NPs reduced the proportion of BCSCs [143]. In another approach, Jin et al. synthesized cyclic arginine-glycine-aspartic acid peptide-decorated polymeric NPs with photosensitizer (2-methoxy-5-(2-ethyl-hexyloxy)-1,4-phenylenevinylene) with minimal cytotoxic potential but could preferentially destroy MDA-MB-231 cells expressing αvβ3 integrin—a subtype of TNBC cells [144]. In another study on TNBC, bortezomib encapsulated nanoparticles (NPBTZ) efficiently deliver the drug to BCSCs, affecting their stemness (Fig. 4) [145]. Nanoparticle-induced autophagy using a combination of chloroquine, DOX, and docetaxel (DTXL) resulted in persistent and effective control of tumor growth by eliminating BCSCs in the orthotropic tumor murine model [146].
More lately, co-encapsulation of salinomycin (SAL) and DOX in PLGA/TGPS (tocopherol polyethylene glycol 1000 succinate) nanoparticles, 1:1 molar ratio, showed synergistic effects against MCF-7 and MCF-MS breast cancer cells with prolong circulation time and in vivo synergism over 24 h [147]. Another study reported PTX-loaded iron oxide (PTX-FA-NP) NPs for targeted breast cancer therapy. PTX-FA-NP demonstrated uniform size distribution and high drug loading capacity, while FA conjugation leads to higher drug uptake by cancer cells—enhanced cytotoxicity compared to free PTX [148]. Likewise, dual-loaded, DTX and vorinostat (VRS) lipid polymer hybrid nanoparticles were developed for co-delivery, which resulted in a synergistic effect on cancer cell apoptosis with a more significant reduction in MDA-MB-231 and MCF-7 cancer cells [149]. In a combination approach, noscapine-loaded PLGA-NPs were used with DOX HCl to assess their anticancer activity alone or in combination in 4T1 cells and mice models. Data suggested that Nos-NPs in combination with DOX synergistically inhibited 4T1 cancer cells and tumor progression in vitro and in vivo, respectively, more efficiently than Nos-NPs and DOX alone [150]. Lately, combination nano-therapy was employed using a hybrid nanoparticle-based co-delivery platform combining PTX and verteporfin (VP-NP) to target TNBC patient-derived xenograft tumors and CSCs. Data revealed accumulation of VP-NPs in tumors and retarded tumor growth in TNBC patient-derived xenografts—attributed to inhibition of NF-κB, Wnt, and YAP pathways known to have a crucial role in cancer growth, CSC development, and tumorigenesis [151]. Using lovastatin (L) loaded Janus camptothecin-floxuridine conjugate (CF) nano-capsules (NCs), LCF-NCs, innovative ternary cocktail chemotherapy was used to suppress tumor growth of TNBCs by inhibiting the growth and metastasis of BCSCs, in vitro, and in tumor-bearing mice, in vivo [152]. In the CSCs targeted approach, salinomycin prodrug NPs possessing vitamin E-based redox sensitivity and hyaluronic acid-coated TS-NPs (HTS-NPs) were synthesized to deliver PTX for CSCs targeted combination therapy. In 3D tumor spheroids culture, both TS and HTS NPs demonstrated synergistic anticancer effects with PTX, attributed to improved intracellular drug delivery [153]. Zinc sulfide nanoparticles have been shown to inhibit the metastasis of MCF-7 stem cells in a dose-dependent manner by inhibiting the epithelial-mesenchymal transition process [154]. More recently, a study evaluated the therapeutic potential of Quinacrine-gold hybrid nanoparticles against patient-derived BCSCs and in xenograft animal models. The use of targeted nanoparticles in conjunction with radiation therapy has effectively reduced angiogenesis in the tumor microenvironment, leading to reduced tumor growth and metastasis [155]. Another study utilized a novel nano-approach based on siProminin2-loaded iron oxide hydroxide nanoparticles coated with hyaluronic acid to induce ferroptosis in targeted BCSCs. These pH-sensitive nanoparticles specifically inhibited tumor growth in an acidic tumor microenvironment through significant down-regulation of GPX4, thus acting as potential candidates for targeting BCSCs [156].
Nanogels
Nanogels, one of the systemic drug delivery carriers, are hydrogels with three-dimensional (3D) adjustable porous structures with a particle size range between 20 and 250 nm (Table 2). Numerous studies have investigated the application of nanogels in the treatment of breast cancer, leveraging their capabilities. Due to the higher affinity of BC cells toward hyaluronic acid (HA), investigators have developed cholesteryl-hyaluronic acid (CHA) nanogel-drug conjugates, especially for drug-resistant and CD44-expressing BCSCs (Fig. 4). These nanogels tend to internalize via endocytosis mediated through CD44 receptors. They can interact with the cancer cell membrane, possibly owing to functionalization with HA coating and increased uptake of salinomycin (SLM) nanoparticles, 1.5 folds, by the cells [112]. Moreover, curcumin-loaded gum arabic aldehyde gelatin (GA Ald-Gel) nanogels were synthesized having enhanced cellular uptake, drug release, and improved cytotoxicity in MCF-7 breast cancer cells compared to bare curcumin [157]. Likewise, chitosan-based nanogels were made by loading 10-hydroxy-camptothecin (HCPT) to form CS/HCPT. After 48 h of exposure to 4T1 breast cancer cells, CS/HCPT demonstrated enhanced cytotoxicity and apoptosis compared to free HCPT, attributed to enhanced drug internalization and release (Fig. 4) [158].
Nano-micelles
Nano-micelles, colloidal constructs, are composed of amphiphilic nanomers having small hydrophobic heads and long hydrophilic tails (Table 2). The BCSCs expressed higher levels of vasoactive intestinal peptide (VIP) receptors than most cancer cells, making VIP a potentially active target. A stabilized novel curcumin phospholipid nano-micelles (C-SSM) surface conjugated with VIP was successfully delivered to retard the growth of BCSCs by tumor-spheres formation assay (Fig. 4) [159]. To treat the resistant BC, PTX hybrid nano-micelles were prepared using dequalinium (DQA) and polymer soluplus d-α-tocopheryl polyethylene glycol 1000 succinate (TGPS1000). The formed nano-micelles had improved cellular uptake and co-localization with mitochondria, which resulted in the induction of pro-apoptotic proteins cytochrome C, BAX, and apoptotic enzymes caspase9 and 3, along with the blocking of anti-apoptotic proteins BCL2 and MCL-1 [160]. In another approach, C-DVM (CREKA, doxorubicin, vinorelbine micelles) dual-acting nano-micelles were developed having high stability, functionalized with fibronectin targeting CREKA peptide encapsulating vinorelbine (NVB) and DOX, which effectively delivered the drugs to 4T1 BC cells and disrupted microtubules, ultimately inhibiting the development of metastatic foci, 90%, with reduced invasion of metastasis (Fig. 4) [161].
Exosomes
The exosomes, nanovesicles of approximately 30–100 nm in diameter, secreted by all viable cells involving endosomal pathways, are known to play a pivotal role in cell-to-cell communications. Exosomes can be exploited as RNA carriers, as evidenced by the proficient delivery of microRNA (miRNA) to epidermal growth factor receptor (EGFR)-expressing breast cancer cells. A recent experimental study proved that MDA-MB-231 triple-negative breast cancer cell lines, when treated with exosomes loaded with a non-coding RNA (7SK), significantly decreased cellular proliferation, invasion, and migration. Efficient reduction in the expression of EMT-related markers, reduced tumor formation, and increased apoptotic activity was observed [162]. Being natural carriers of miRNA, having acceptable toxicity and biocompatibility profiles support their application in drug delivery systems [163]. In this context, IL-4 triggered macrophages, and BC cells were co-cultured, which resulted in the release of IL-4 specific miRNA, miRNA223, by the exosomes via activated macrophages in co-cultivated MDA-MB-231 and SKBR3 cells. After treating IL-4-triggered macrophages with miRNA 223 anti-sense oligonucleotide, the invasiveness of the co-cultured BC cells decreased significantly [164].
Immunotherapy
Immune therapy was first utilized in lung cancer and melanoma, particularly the checkpoint inhibitors. Early immune therapy studies in BC were not promising, suggesting that BC is not highly immunogenic [165]. A recent in vitro (MD231 cell lines), in vivo (mouse models), and ex vivo (patient-specific organoids) experimental study accessing the potential of immunotherapy against triple-negative breast cancer was conducted. It demonstrated the efficient role of Mesothelin-targeted CAR-natural killer cells in visibly reducing tumor growth by directly targeting and killing TNBC cells, acting as a promising therapeutic approach for treating breast cancer [166]. However, in the early trial, 1b KEYNOTE-012, the checkpoint inhibitor, pembrolizumab monotherapy, was evaluated in metastatic TNBC. Data revealed an 18.5% overall response rate in 27 patients, though it was not very promising [167]. In the phase I clinical trial on advanced metastatic BC patients, refractory to standard care therapy received avelumab, which resulted in an overall response rate of 3% and 5.2% in patients with TNBC [168]. In a phase 3 trial, compared to placebo in patients with untreated metastatic TNBC, the overall median survival improved in patients with PD-L1 positive tumors—the median overall survival was 25.5 months versus 15.5 months [169]. Furthermore, a phase II KEYNOTE-086 study assessed second-line agents in metastatic TNBC, i.e., pembrolizumab. Data revealed that in TNBC patients, the median progression-free survival (PFS) and overall survival (OS) were 2 and 9 months, respectively, with manageable side effect profiles [170]. In another trial, four cycles of pembrolizumab every three weeks were added to neo-adjuvant therapy, carboplatin, and paclitaxel, or placebo every three weeks plus carboplatin and paclitaxel. The groups received four additional cycles of placebo or pembrolizumab and epirubicin-cyclophosphamide or doxorubicin-cyclophosphamide. After a median follow-up of 15.5 months, compared to the placebo-chemotherapy group (11.8%), only 7.4% of patients in pembrolizumab had disease progression [171].
Gene therapy
Aberrant gene expression is a hallmark of many cancers, including BC. In this context, Lang et al. used gene therapy to induce apoptosis of BCSCs, which not only improved the effects of chemotherapy but also reduced the chances of relapse. The mutant form of the proapoptotic gene BIK was introduced in BCSCs, resulting in the reduction of BCSCs population by blocking the activity of MCL-1, BCL-2, BCL-xL, a family of proteins vital for tumor growth and therapy resistance [172]. More recently, TRAIL expressing plasmid along with siRNAs, silencing BCL-2 like 12 (BCL2L12) or superoxide dismutase 1 (SOD1), co-delivered using low molecular weight poly-ethylenimine (PEI) resulted in significant cell death in different BC cells and retarded the genesis of tumor in BC xenograft mice model [173]. Likewise, in another approach, CRISPR mediated activation of tumor suppressor genes, cysteine-rich 61/connective tissue growth factor/nephroblastoma overexpressed 6 (CCN6, WISP3) and mammary serine protease inhibitor (MASPIN/SERPINB5) were observed in a mouse model of BC. Data suggested that in vitro activation of tumor suppressor genes caused loss of tumorigenic properties in BC cells and reduced tumor growth in MCF-7 xenograft in BALB/c mice [174]. Recently, in efforts to target breast cancer stem cells (BCSCs), various approaches have been explored, including the utilization of constructs such as Pseudomonas exotoxin, CXCR1, which provides specificity for BCSCs, and 5ʹ UTR of the essential fibroblast growth factor (bFGF), which offers specificity at the translational level. BC cell lines were transfected with the constructs encoding luciferase PE38 under CMV/CXCR1 promoter, with or without bFGF 5ʹ UTR. This strategy enhanced BCSC specificity, resulting in cell death in tumorigenic cell lines while sparing normal cells. [175]. Similarly, the polio-like kinase 1 gene was targeted using siRNA that efficiently killed BCSCs obtained from MDA-MB-123 cells—probably by inhibiting the TGF-β signalling pathway [176].
Recent approvals by the federal drug agency (FDA)
Here, we report the most recently approved drugs by the Federal Drug Agency (FDA), USA, in 2019 and 2020 for the prevention and treatment of BC. In 2019, the FDA approved only two drugs: Alpelisib (Piqray) and fam-trastuzumab-deruxtecan-nxki (Enhertu). Alpelisib was approved on May 24, 2019, marketed by Novartis, to treat HER2-positive metastatic BC patients and those having a mutant form of the PIK3CA gene, while Fam-trastuzumab-deruxtecan-nxki, HER2-directed antibody, and topoisomerase conjugate, was approved on December 20, 2019, and marketed by Daiichi Sankyo, Japan, for the treatment of unresectable, HER2 positive BC patients with prior anti-HER2 therapy in metastatic settings [177]. In 2020, the FDA approved only three drugs for the prevention and treatment of breast cancer. These include Tucatinib (Tukysa) by Seattle Genetics, USA, Sacituzumab-govitecan-hziy (Trodelvy) by Immunomedics, USA, and Fluoroestradiol-F18 by Zionexa US Corporation, USA. Tucatinib, approved on April 17, 2020, acts as an anti-HER2 agent and inhibits MAPK and AKT signaling for the treatment of HER2-positive breast cancer patients. Sacituzumab-govitecan-hziy targets trophoblast cell surface antigen-2 and is a topoisomerase inhibitor drug conjugate approved for pretreated TNBC patients. Fluoroestradiol-F18 is an imaging agent used in positron emission tomography (PET) to detect estrogen-positive breast cancer patients [178].
Conclusion
Increasingly yet impressive, shreds of evidence indicate that tumor-initiating cells have the self-renewal capacity differentiating potential that may confer tumor relapse, metastasis, heterogeneity, and drug resistance. The literature evidence here distinctly advocates significant advancements in identifying, isolating, and characterizing BCSCs. All the presented targets of BCSCs, ranging from tumor cell surface receptors, ABC transporters, signaling pathways, nano-medicine approaches, microenvironment, immunotherapy, and gene therapy, might offer reasonable benefits to the patients by targeting the BCSCs population. However, we deduced from the literature that, in most cases, combination therapy approaches could provide an edge over monotherapy by overcoming the selection pressure of BCSC-resistant clones, which came into existence as a result of certain monotherapy. Nevertheless, therapy must give broad coverage to target not only BCSCs but also the non-stem-like cells owing to the plasticity of BCSCs to target differentiated progenitors and tumors in bulk. However, the field is still in its infancy and requires extensive research to produce viable products. Significant challenges include drug selectivity and efficacy, reduced toxicity to normal cells, reduction in adverse drug reactions, and delivery of concentrated drugs at the target site.
Data availability
Not applicable for this review.
References
Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Organization, W.H. Preventing Cancer. 2020 [cited 2020 18-11-2020]; Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019.
Zhou J, et al. Stem cells and cellular origins of breast cancer: updates in the rationale, controversies, and therapeutic implications. Front Oncol. 2019;9:820–820.
Elbaiomy MA, et al. Clinical impact of breast cancer stem cells in metastatic breast cancer patients. J Oncol. 2020;2020:2561726.
Bianchini G, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–90.
Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–13.
Tudoran OM, Balacescu O, Berindan-Neagoe I. Breast cancer stem-like cells: clinical implications and therapeutic strategies. Clujul Medical. 2016;89(2):193.
Dragu DL, et al. Therapies targeting cancer stem cells: current trends and future challenges. World J Stem Cells. 2015;7(9):1185.
Al-Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8.
Shipitsin M, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11(3):259–73.
Kai K, et al. Breast cancer stem cells. Breast Cancer. 2010;17(2):80–5.
Ma X, et al. Modulation of drug-resistant membrane and apoptosis proteins of breast cancer stem cells by targeting berberine liposomes. Biomaterials. 2013;34(18):4452–65.
Tam WL, et al. Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell. 2013;24(3):347–64.
Singh JK, et al. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013;15(4):210.
Gangopadhyay S, et al. Breast cancer stem cells: a novel therapeutic target. Clin Breast Cancer. 2013;13(1):7–15.
Glumac PM, LeBeau AM. The role of CD133 in cancer: a concise review. Clin Transl Med. 2018;7(1):18.
Vikram R, et al. Tumorigenic and metastatic role of CD44(-/low)/CD24(-/low) cells in luminal breast cancer. Cancers. 2020;12(5):1239.
Chen K, Huang Y-H, Chen J-L. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin. 2013;34(6):732.
Muntimadugu E, et al. CD44 targeted chemotherapy for co-eradication of breast cancer stem cells and cancer cells using polymeric nanoparticles of salinomycin and paclitaxel. Colloids Surf B Biointerfaces. 2016;143:532–46.
Daquan Chen GW, Song W, Zhang Q. Novel CD44 receptor targeting multifunctional “nano-eggs” based on double pH-sensitive nanoparticles for co-delivery of curcumin and paclitaxel to cancer cells and cancer stem cells. J Nanopart Res. 2015;17:10.
Najjar MK, Manore SG, Regua AT, Lo HW. Antibody-drug conjugates for the treatment of HER2-positive breast cancer. Genes (Basel). 2022;13:2065.
Luksik AS, Yazigi E, Shah P, Jackson CM. CAR T cell therapy in glioblastoma: overcoming challenges related to antigen expression. Cancers (Basel). 2023;15:1414.
Yanze Sun XY, Wang X, et al. Bispecific antibodies in cancer therapy: Target selection and regulatory requirements. Acta Pharm Sin B. 2023;13:3583–97.
Choi YH, Yu AM. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des. 2014;20(5):793–807.
Yue-Li Sun AP, Kumar P, Chen Z-S. Role of ABC transporters in cancer chemotherapy. Chin J Cancer. 2012;31(2):51.
Chuthapisith S, et al. Breast cancer chemoresistance: emerging importance of cancer stem cells. Surg Oncol. 2010;19(1):27–32.
Shivhare S, Das A. Cell density modulates chemoresistance in breast cancer cells through differential expression of ABC transporters. Mol Biol Rep. 2023;50(1):215–25.
Cui J, et al. Targeting ABCA12-controlled ceramide homeostasis inhibits breast cancer stem cell function and chemoresistance. Sci Adv. 2023;9(48):1891.
Wu CP, Ohnuma S, Ambudkar SV. Discovering natural product modulators to overcome multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12(4):609–20.
Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10(2):86–103.
Mao L. NOTCH mutations: multiple faces in human malignancies. Cancer Prev Res (Philadelphia, Pa). 2015;8:259–61.
Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther. 2014;141(2):140–9.
Aithal MG, Rajeswari N. Role of Notch signalling pathway in cancer and its association with DNA methylation. J Genet. 2013;92(3):667–75.
Harrison H, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Can Res. 2010;70(2):709–18.
Hermawan A, et al. Bioinformatics and in vitro studies reveal the importance of p53, PPARG and notch signaling pathway in inhibition of breast cancer stem cells by hesperetin. Adv Pharm Bull. 2021;11(2):351.
Cejuela M, Martin-Castillo B, Menendez JA, Pernas S. Metformin and breast cancer: Where are we now? Int J Mol Sci. 2022;23(5):2705.
Rossini M, Martini F, Torreggiani E, Fortini F, Aquila G, Sega FV, Patergnani S, Pinton P, Maniscalco P, Cavallesco G, Rizzo P. Metformin induces apoptosis and inhibits notch1 in malignant pleural mesothelioma cells. Front Cell Dev Biol. 2021;8:534499.
Chia YH, Ma CX. Hedgehog pathway inhibitors: potential applications in breast cancer. Curr Breast Cancer Rep. 2011;3:15–23.
Tsao A-N, et al. Dinaciclib inhibits the stemness of two subtypes of human breast cancer cells by targeting the FoxM1 and Hedgehog signaling pathway. Oncol Rep. 2022;47(5):1–12.
Zhang Y, et al. Discovery of YH677 as a cancer stemness inhibitor that suppresses triple-negative breast cancer growth and metastasis by regulating the TGFbeta signaling pathway. Cancer Lett. 2023;560:216142.
Li Y, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16(9):2580–90.
Moynagh PN. The NF-κB pathway. J Cell Sci. 2005;118(20):4589–92.
Marquardt JU, et al. Curcumin effectively inhibits oncogenic NF-κB signaling and restrains stemness features in liver cancer. J Hepatol. 2015;63(3):661–9.
Zhou J, et al. NF-κB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat. 2008;111(3):419–27.
Schreck R, et al. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175(5):1181–94.
MacKenzie CJ, et al. Enhancement of lipopolysaccharide-stimulated JNK activity in rat aortic smooth muscle cells by pharmacological and adenovirus-mediated inhibition of inhibitory kappa B kinase signalling. Br J Pharmacol. 2003;139(5):1041–9.
Clayton S, Mousa SA. Therapeutics formulated to target cancer stem cells: Is it in our future? Cancer Cell Int. 2011;11:7.
Gargini R, et al. Cancer stem cell-like phenotype and survival are coordinately regulated by Akt/FoxO/Bim pathway. Stem Cells. 2015;33(3):646–60.
Korkaya H, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7(6):e1000121.
Ock CW, Kim GD. Dioscin decreases breast cancer stem-like cell proliferation via cell cycle arrest by modulating p38 mitogen-activated protein kinase and akt/mtor signaling pathways. J Cancer Prev. 2021;26(3):183.
Huang CC, et al. KLF4 and PCNA identify stages of tumor initiation in a conditional model of cutaneous squamous epithelial neoplasia. Cancer Biol Ther. 2005;4(12):1401–8.
Yu F, et al. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene. 2011;30(18):2161.
Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–48.
Welschinger R, et al. Plerixafor (AMD3100) induces prolonged mobilization of acute lymphoblastic leukemia cells and increases the proportion of cycling cells in the blood in mice. Exp Hematol. 2012;41(3):293–302.
Prager GW, et al. Angiogenesis in cancer: Anti-VEGF escape mechanisms. Transl Lung Cancer Res. 2012;1(1):14–25.
Das B, et al. Quinacrine inhibits HIF-1α/VEGF-A mediated angiogenesis by disrupting the interaction between cMET and ABCG2 in patient-derived breast cancer stem cells. Phytomedicine. 2023;117:154914.
Zhao J, et al. The role of hypoxia-inducible factor-2 in digestive system cancers. Cell Death Dis. 2015;6(1):e1600.
Muthoni DK, et al. Identification of 3-O-α-l-arabinosyl oleanolic acid, a triterpenoid saponin, as a new breast cancer stem cell growth inhibitor. Nat Prod Res. 2022;36(11):2923–6.
Zhang C, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A. 2016;113(14):E2047–56.
Chen M, et al. Extracellular pH is a biomarker enabling detection of breast cancer and liver cancer using CEST MRI. Oncotarget. 2017;8(28):45759–67.
Wojtkowiak JW, et al. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm. 2011;8(6):2032–8.
Chamie K, et al. Carbonic anhydrase-IX score is a novel biomarker that predicts recurrence and survival for high-risk, nonmetastatic renal cell carcinoma: data from the phase III ARISER clinical trial. Urol Oncol. 2015;33(5):204.e25-33.
Xu Z, et al. Preparation of a camptothecin prodrug with glutathione-responsive disulfide linker for anticancer drug delivery. Chem Asian J. 2014;9:199–205.
Lee ES, et al. Super pH-sensitive multifunctional polymeric micelle for tumor pH(e) specific TAT exposure and multidrug resistance. J Controll Release : Off J Controll Release Soc. 2008;129(3):228–36.
Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem. 1996;271(34):20545–50.
Ginestier C, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120(2):485–97.
Singh JK, et al. Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clin Cancer Res. 2013;19(3):643–56.
Schott AF, et al. Phase Ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER-2-negative metastatic breast cancer. Clin Cancer Res. 2017;23(18):5358–65.
Garg M. MicroRNAs, stem cells and cancer stem cells. World J Stem cells. 2012;4(7):62–70.
Yan LX, et al. Knockdown of miR-21 in human breast cancer cell lines inhibits proliferation, in vitro migration and in vivo tumor growth. Breast Cancer Res. 2011;13(1):R2.
Shimono Y, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138(3):592–603.
Iliopoulos D, et al. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39(5):761–72.
Gong C, et al. MiR-106b expression determines the proliferation paradox of TGF-β in breast cancer cells. Oncogene. 2015;34(1):84–93.
Juric et al., (2016) Ribociclib (LEE011) and letrozole in estrogen receptor-positive (ER+), HER2-negative (HERR ) advanced breast cancer (aBC): Phase Ib safety, preliminary efficacy and molecular analysis. 34, 568–568.
Cao T, et al. CDK3, target of miR-4469, suppresses breast cancer metastasis via inhibiting Wnt/β-catenin pathway. Oncotarget. 2017;8(49):84917–27.
Kong L-Y, et al. In vivo and in vitro effects of microRNA-27a on proliferation, migration and invasion of breast cancer cells through targeting of SFRP1 gene via Wnt/β-catenin signaling pathway. Oncotarget. 2017;8(9):15507–19.
Su X, et al. MicroRNA-134 targets KRAS to suppress breast cancer cell proliferation, migration and invasion. Oncol Lett. 2017;13(3):1932–8.
Yahya SM, et al. Restoring microRNA-34a overcomes acquired drug resistance and disease progression in human breast cancer cell lines via suppressing the ABCC1 gene. Breast Cancer Res Treat. 2023;204(1):133–49.
Zhao Y, et al. miR-29a suppresses MCF-7 cell growth by downregulating tumor necrosis factor receptor 1. Tumor Biol. 2017;39(2):1010428317692264.
Jasim SA, et al. An in vitro investigation of the apoptosis-inducing activity of corosolic acid in breast cancer cells. Iran J Basic Med Sci. 2023;26(4):453–60.
Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–62.
Zhou J, et al. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat. 2008;111(3):419–27.
Gupta PB, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–59.
Fuchs D, et al. Salinomycin induces apoptosis and overcomes apoptosis resistance in human cancer cells. Biochem Biophys Res Commun. 2009;390(3):743–9.
Ko D, et al. Anti-metastatic potential of pitavastatin in triple-negative breast cancer via targeting breast cancer stem-like properties and STAT3 signaling. Cancer Res. 2023;83(7):5800–5800.
Nalla LV, Khairnar A. Empagliflozin mediated miR-128-3p upregulation promotes differentiation of hypoxic cancer stem-like cells in breast cancer. Eur J Pharmacol. 2023;943:175565.
Yan Y, et al. All-trans retinoic acids induce differentiation and sensitize a radioresistant breast cancer cells to chemotherapy. BMC Complement Altern Med. 2016;16(1):113.
Jin X, Jin X, Kim H. Cancer stem cells and differentiation therapy. Tumor Biol. 2017;39(10):1010428317729933.
Yan Y, et al. All-trans retinoic acids induce differentiation and sensitize a radioresistant breast cancer cells to chemotherapy. BMC Complement Altern Med. 2016;16:113.
Patel SA, et al. Challenges in the development of future treatments for breast cancer stem cells. Breast cancer: Targ Ther. 2010;2:1.
Van Pham P, et al. Targeting breast cancer stem cells by dendritic cell vaccination in humanized mice with breast tumor: preliminary results. Onco Targets Ther. 2016;9:4441.
Shah D, Osipo C. Cancer stem cells and HER2 positive breast cancer: The story so far. Genes Dis. 2016;3(2):114–23.
Korkaya H, et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47(4):570–84.
Montales MTE, et al. Repression of mammosphere formation of human breast cancer cells by soy isoflavone genistein and blueberry polyphenolic acids suggests diet-mediated targeting of cancer stem-like/progenitor cells. Carcinogenesis. 2012;33(3):652–60.
Higdon JV, et al. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224–36.
Ambrosone CB, et al. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134(5):1134–8.
Li Y, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res: Off J Am Assoc Cancer Res. 2010;16(9):2580–90.
Wang Y, et al. Differential effects of sulforaphane in regulation of angiogenesis in a co-culture model of endothelial cells and pericytes. Oncol Rep. 2017;37(5):2905–12.
Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269(2):291–304.
Zhang M, et al. Cancer stem cells as a potential therapeutic target in breast cancer. Stem Cell Investig. 2014. https://doi.org/10.3978/j.issn.2306-9759.2014.06.01.
Berzofsky JA, et al. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Investig. 2004;113(11):1515–25.
Satthaporn S, et al. Dendritic cells are dysfunctional in patients with operable breast cancer. Cancer Immunol Immunother. 2004;53(6):510–8.
Ni J, et al. Dendritic cell vaccine for the effective immunotherapy of breast cancer. Biomed Pharmacother. 2020;126:110046.
Choi AH, et al. From benchtop to bedside: a review of oncolytic virotherapy. Biomedicines. 2016;4(3):18.
Russell SJ, Peng K-W, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–70.
Rowan K. Oncolytic viruses move forward in clinical trials. JNCI J Natl Cancer Inst. 2010;102(9):590–5.
Marcato P, et al. Oncolytic reovirus effectively targets breast cancer stem cells. Mol Ther. 2009;17(6):972–9.
Wang H, et al. Oncolytic vaccinia virus GLV-1h68 strain shows enhanced replication in human breast cancer stem-like cells in comparison to breast cancer cells. J Transl Med. 2012;10:167.
Gomes EM, et al. Antitumor activity of an oncolytic adenoviral-CD40 ligand (CD154) transgene construct in human breast cancer cells. Clin Cancer Res. 2009;15(4):1317–25.
Zhang JF, et al. Treatment of a human breast cancer xenograft with an adenovirus vector containing an interferon gene results in rapid regression due to viral oncolysis and gene therapy. Proc Natl Acad Sci USA. 1996;93(9):4513–8.
Gil M, et al. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proc Natl Acad Sci U S A. 2013;110(14):E1291–300.
He L, et al. Nanomedicine-mediated therapies to target breast cancer stem cells. Front Pharmacol. 2016;7:313–313.
Gao Y, et al. Nanotechnology-based intelligent drug design for cancer metastasis treatment. Biotechnol Adv. 2014;32(4):761–77.
Estanqueiro M, et al. Nanotechnological carriers for cancer chemotherapy: the state of the art. Colloids Surf B Biointerfaces. 2015;126:631–48.
Shen S, Xia JX, Wang J. Nanomedicine-mediated cancer stem cell therapy. Biomaterials. 2016;74:1–18.
Aires A, et al. Multifunctionalized iron oxide nanoparticles for selective drug delivery to CD44-positive cancer cells. Nanotechnology. 2016;27(6):065103.
Gener P, et al. Fluorescent CSC models evidence that targeted nanomedicines improve treatment sensitivity of breast and colon cancer stem cells. Nanomedicine. 2015;11(8):1883–92.
Ganesh S, et al. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34(13):3489–502.
Rao W, et al. Chitosan-decorated doxorubicin-encapsulated nanoparticle targets and eliminates tumor reinitiating cancer stem-like cells. ACS Nano. 2015;9(6):5725–40.
Gaio E, et al. CD44 targeting mediated by polymeric nanoparticles and combination of chlorine TPCS(2a)-PDT and docetaxel-chemotherapy for efficient killing of breast differentiated and stem cancer cells in vitro. Cancers. 2020;12(2):278.
Wang Z, et al. CD44 directed nanomicellar payload delivery platform for selective anticancer effect and tumor specific imaging of triple negative breast cancer. Nanomed Nanotechnol Biol Med. 2018;14(4):1441–54.
Zhang X, et al. Dual-responsive nanoparticles based on chitosan for enhanced breast cancer therapy. Carbohydr Polym. 2019;221:84–93.
Jeong K, et al. Development of highly efficient nanocarrier-mediated delivery approaches for cancer therapy. Cancer Lett. 2016;374(1):31–43.
Zhang L, et al. Mitochondrial targeting liposomes incorporating daunorubicin and quinacrine for treatment of relapsed breast cancer arising from cancer stem cells. Biomaterials. 2012;33(2):565–82.
Yu LY, et al. Glucose transporter 1-mediated transcytosis of glucosamine-labeled liposomal ceramide targets hypoxia niches and cancer stem cells to enhance therapeutic efficacy. ACS Nano. 2023;17(14):13158–75.
Nguyen VD, et al. Folate receptor-targeted liposomal nanocomplex for effective synergistic photothermal-chemotherapy of breast cancer in vivo. Colloids Surf B Biointerfaces. 2019;173:539–48.
Chiani M, et al. Folic acid conjugated nanoliposomes as promising carriers for targeted delivery of bleomycin. Artif Cells Nanomed Biotechnol. 2018;46(4):757–63.
Kushwah V, et al. Improved antitumor efficacy and reduced toxicity of docetaxel using anacardic acid functionalized stealth liposomes. Colloids Surf B Biointerfaces. 2018;172:213–23.
Hong S, et al. Aptamer-integrated α-Gal liposomes as bispecific agents to trigger immune response for killing tumor cells. J Biomed Mater Res A. 2019;107(6):1176–83.
Kim YJ, et al. Co-eradication of breast cancer cells and cancer stem cells by cross-linked multilamellar liposomes enhances tumor treatment. Mol Pharm. 2015;12(8):2811–22.
Rana MS, et al. A new liposomal nanocarrier for co-delivery of gedunin and p-glycoprotein siRNA to target breast cancer stem cells. Nat Prod Res. 2022;36(24):6389–92.
Ahmad A, et al. Development of liposomal formulation for delivering anticancer drug to breast cancer stem-cell-like cells and its pharmacokinetics in an animal model. Mol Pharm. 2016;13(3):1081–8.
Zhang Y, et al. The eradication of breast cancer and cancer stem cells using octreotide modified paclitaxel active targeting micelles and salinomycin passive targeting micelles. Biomaterials. 2012;33(2):679–91.
Ke XY, et al. Co-delivery of thioridazine and doxorubicin using polymeric micelles for targeting both cancer cells and cancer stem cells. Biomaterials. 2014;35(3):1096–108.
Zhang J, et al. Effective treatment of drug resistant recurrent breast tumors harboring cancer stem-like cells by staurosporine/epirubicin co-loaded polymeric micelles. J Control Release. 2017;264:127–35.
Xiang J, et al. Synthesis and evaluation of a paclitaxel-binding polymeric micelle for efficient breast cancer therapy. Sci China Life Sci. 2018;61(4):436–47.
Lecot et al., Glucosylated polymeric micelles actively target a breast cancer model. n/a(n/a), 2000010
Amreddy N, et al. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv Cancer Res. 2018;137:115–70.
Sun R, et al. Co-delivery of all-trans-retinoic acid and doxorubicin for cancer therapy with synergistic inhibition of cancer stem cells. Biomaterials. 2015;37:405–14.
de Sousa Marcial SP, Carneiro G, Leite EA. Lipid-based nanoparticles as drug delivery system for paclitaxel in breast cancer treatment. J Nanoparticle Res. 2017;19(10):340.
Wong MY, Chiu GN. Liposome formulation of co-encapsulated vincristine and quercetin enhanced antitumor activity in a trastuzumab-insensitive breast tumor xenograft model. Nanomedicine. 2011;7(6):834–40.
Şalva E, et al. The enhancement of gene silencing efficiency with chitosan-coated liposome formulations of siRNAs targeting HIF-1α and VEGF. Int J Pharm. 2015;478(1):147–54.
Li SY, et al. Combination therapy with epigenetic-targeted and chemotherapeutic drugs delivered by nanoparticles to enhance the chemotherapy response and overcome resistance by breast cancer stem cells. J Control Release. 2015;205:7–14.
Jin G, et al. Theranostics of triple-negative breast cancer based on conjugated polymer nanoparticles. ACS Appl Mater Interfaces. 2018;10(13):10634–46.
Shen S, et al. Delivery of bortezomib with nanoparticles for basal-like triple-negative breast cancer therapy. J Control Release. 2015;208:14–24.
Sun R, et al. Nanoparticle-facilitated autophagy inhibition promotes the efficacy of chemotherapeutics against breast cancer stem cells. Biomaterials. 2016;103:44–55.
Gao J, et al. Co-delivery of docetaxel and salinomycin to target both breast cancer cells and stem cells by PLGA/TPGS nanoparticles. Int J Nanomed. 2019;14:9199–216.
Jeon M, et al. Paclitaxel-loaded iron oxide nanoparticles for targeted breast cancer therapy. Adv Ther. 2019;2(12):1900081.
Tran T, et al. Synergistic therapeutic strategy of dual drug-loaded lipid polymer hybrid nanoparticles for breast cancer treatment. Indian J Pharm Sci. 2019;81:474–82.
Esnaashari SS, et al. A combinational approach towards treatment of breast cancer: an analysis of noscapine-loaded polymeric nanoparticles and doxorubicin. AAPS PharmSciTech. 2020;21(5):166.
Sulaiman A, et al. Co-targeting bulk tumor and CSCs in clinically translatable tnbc patient-derived xenografts via combination nanotherapy. Mol Cancer Ther. 2019;18(10):1755.
Zhang N, et al. Loading lovastatin into camptothecin-floxuridine conjugate nanocapsules for enhancing anti-metastatic efficacy of cocktail chemotherapy on triple-negative breast cancer. ACS Appl Mater Interfaces. 2018;10(35):29385–97.
Liang DS, et al. Vitamin E-based redox-sensitive salinomycin prodrug-nanosystem with paclitaxel loaded for cancer targeted and combined chemotherapy. Colloids Surf B Biointerfaces. 2018;172:506–16.
Tran TA, et al. Inhibitory effect of zinc sulfide nanoparticles towards breast cancer stem cell migration and invasion. J Biomed Nanotechnol. 2016;12(2):329–36.
Dash SR, et al. Near-infrared enhances antiangiogenic potentiality of quinacrine-gold hybrid nanoparticles in breast cancer stem cells via deregulation of HSP-70/TGF-beta. Nanomedicine (Lond). 2023;18(1):19–33.
Wu K, et al. An iron oxyhydroxide-based nanosystem sensitizes ferroptosis by a “Three-Pronged” strategy in breast cancer stem cells. Acta Biomater. 2023;160:281–96.
Sarika PR, Nirmala RJ. Curcumin loaded gum arabic aldehyde-gelatin nanogels for breast cancer therapy. Mater Sci Eng, C. 2016;65:331–7.
Guo H, et al. Chitosan-based nanogel enhances chemotherapeutic efficacy of 10-hydroxycamptothecin against human breast cancer cells. Int J Polym Sci. 2019;2019:1914976.
Gülçür E, et al. Curcumin in VIP-targeted sterically stabilized phospholipid nanomicelles: a novel therapeutic approach for breast cancer and breast cancer stem cells. Drug Deliv Transl Res. 2013;3(6):562–74. https://doi.org/10.1007/s13346-013-0167-6.
Dian L-H, et al. Fabrication of paclitaxel hybrid nanomicelles to treat resistant breast cancer via oral administration. Int J Nanomed. 2018;13:719–31.
Gong Z, et al. Fibronectin-targeted dual-acting micelles for combination therapy of metastatic breast cancer. Signal Transduct Target Ther. 2020;5(1):12.
Farhadi S, et al. Exosomal delivery of 7SK long non-coding RNA suppresses viability, proliferation, aggressiveness and tumorigenicity in triple negative breast cancer cells. Life Sci. 2023;322:121646.
Ohno S-I, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–91.
Yang M, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10(1):117.
Mina LA, et al. Immunotherapy for the treatment of breast cancer: emerging new data. Breast cancer (Dove Medical Press). 2019;11:321–8.
Yang M, et al. Mesothelin-targeted CAR-NK cells derived from induced pluripotent stem cells have a high efficacy in killing triple-negative breast cancer cells as shown in several preclinical models. J Immunother. 2023;46(8):285–94.
Nanda R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460–7.
Dirix LY, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor study. Breast Cancer Res Treat. 2018;167(3):671–86.
Schmid P, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21.
Adams S, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397–404.
Schmid P, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382(9):810–21.
Lang J-Y, et al. BikDD eliminates breast cancer initiating cells and synergizes with lapatinib for breast cancer treatment. Cancer Cell. 2011;20(3):341–56.
Thapa B, et al. Breathing new life into TRAIL for breast cancer therapy: co-delivery of pTRAIL and complementary siRNAs using lipopolymers. Hum Gene Ther. 2019;30(12):1531–46.
Kretzmann JA, et al. Tumor suppression by targeted intravenous non-viral CRISPRa using dendritic polymers. Chem Sci. 2019;10(33):7718–27.
Moradian C, Rahbarizadeh F. Targeted toxin gene therapy of breast cancer stem cells using CXCR1 Promoter and bFGF 5’UTR. Onco Targets Ther. 2019;12:8809–20.
Zuo ZQ, et al. Promoting tumor penetration of nanoparticles for cancer stem cell therapy by TGF-beta signaling pathway inhibition. Biomaterials. 2016;82:48–59.
Agency FD. New Drug Approvals for 2019. 2019 [cited 2020 18-11-2020]; Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019.
Agency FD, Novel drug approvals for 2020. 2020 [cited 2020 18-11-2020]; Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020.
Acknowledgements
None
Funding
This work received no funding.
Author information
Authors and Affiliations
Contributions
First two authors contributed equally to this work. KA and MN wrote the manuscript and conceived the idea, edited the manuscript and worked on nano-technology approaches, gene therapy and immunotherapy and diet mediate therapy. FM provided in-depth information about the signaling pathways and made that figure, and edited the manuscript; MIQ and MI contributed in providing valuable information on surface receptors and transporters, revised the manuscript, and tabulated the studies on nanocarriers; MFR and FKH edited the manuscript and wrote tumor microenvironment and miRNA-based approaches; SAH prepared figures for the manuscript and edited the manuscript; HS supervised the work, edited the manuscript and tabulated the results on surface receptor targeting, and provided literature searches. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, K., Nabeel, M., Mohsin, F. et al. Recent developments in targeting breast cancer stem cells (BCSCs): a descriptive review of therapeutic strategies and emerging therapies. Med Oncol 41, 112 (2024). https://doi.org/10.1007/s12032-024-02347-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02347-z


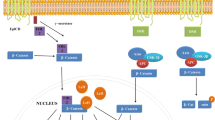
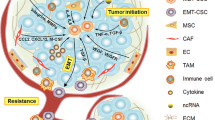


 )
)
 ) by various therapeutic options
) by various therapeutic options
 )
)