Abstract
The use of bortezomib in the clinic has significantly improved outcomes for patients with multiple myeloma (MM), even those harboring high-risk cytogenetic abnormalities or those classified in the high-risk category according to the International Staging System (ISS). In this study, we analyzed the association between immunophenotyping on myeloma cells and the clinical outcomes of patients who received bortezomib-based regimens as first-line therapy. Immunophenotypic analysis before bortezomib therapy was performed by flow cytometry, and whether the immunophenotyping results influenced the clinical outcomes of the patients was investigated. Seventy-four newly diagnosed patients with MM were included in this study. We found that the expression of MPC-1 significantly predicted the time to next therapy (TNT), with a longer TNT in the MPC-1 positive group (p = 0.005), whereas it did not affect overall survival (OS; p = 0.773). In addition, we found that CD45-positivity was associated with shorter TNT (p = 0.0432). Following ISS assessment at treatment initiation, patients who were classified as stage I showed a slightly longer OS compared to those at stage II or III; however, these results were not significant (p = 0.0987). Furthermore, multivariate analysis revealed the prognostic significance of MPC-1 expression, as MPC-1-negativity was associated with a worse TNT. The combination of MPC-1 and CD45 status more sensibly predicted the TNT for bortezomib therapy. Our results demonstrate the clinical importance of immunophenotyping on myeloma cells to determine patient prognoses in this era of novel therapeutic agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma (MM), a hematologic malignancy characterized by the accumulation of mutated plasma cells in the bone marrow, affects various organs such as bones and kidneys. This disease is heterogeneous, with great diversity in its cytogenetic and molecular characteristics in each individual, and difficult to cure [1,2,3]. It has been recognized that different subpopulations of malignant cells exist at diagnosis and persist during anti-MM therapy [4]; they are considered alternatively replaced over the clinical course and are a cause of recurrence and drug resistance [3,4,5]. Therefore, MM treatment strategies for each patient need to be optimized, taking into account the characteristics of the abnormal plasma cells that persist in the bone marrow.
Whereas MM cannot yet be cured, the recent development of novel agents including proteasome inhibitors (bortezomib, carfilzomib, and ixazomib), immunomodulatory drugs (IMiDs; thalidomide, lenalidomide, and pomalidomide), and monoclonal antibodies (daratumumab and elotuzumab) has led to dramatic improvements in outcomes for patients with MM [6,7,8]. Bortezomib, a first-in-class proteasome inhibitor, has become available in 2006 for refractory or relapsed MM in Japan, and nowadays, it is available for newly diagnosed MM and is widely used in the clinic. However, a subset of patients is refractory to bortezomib therapy; in these patients, MM progresses quickly [8]. Importantly, clinical evidence regarding the identification of predictors of treatment response or prognosis with respect to bortezomib therapy has rarely been described. Recent studies have demonstrated that in the era of chemotherapy and autologous hematopoietic stem cell transplantation (ASCT), bortezomib can overcome adverse prognostic factors such as the cytogenetic abnormalities t(4;14), t(14;16), or 17p deletion [9, 10]. The prognostic impact of serum beta-2 microglobulin and serum albumin levels in the blood, as defined by the International Staging System (ISS), are also reduced in the era of novel agents [11]. Therefore, the identification of reliable predictive factors of bortezomib therapy is necessary.
Mature plasma cell 1 (MPC-1) is a surface antigen that is expressed in mature myeloma cells and normal plasma cells but not in immature myeloma cells [12, 13]. In the era of novel agents, we recently showed that patients with MM who were MPC-1-negative at diagnosis showed significantly shorter survival durations than those who were MPC-1-positive [14]. Myeloma cell maturity seems to be closely associated with the expression levels of X-box binding protein 1 (XBP1), which is required for the unfolded protein response [15,16,17]; accordingly, we hypothesized that myeloma cell maturity, as assessed based on MPC-1 expression, might predict the efficacy of bortezomib therapy. In this study, we comprehensively investigated the clinical impact of myeloma cell immunophenotyping (including MPC-1 expression) with respect to treatment outcomes for patients with newly diagnosed MM, to provide new insights into MM therapy in the context of treatment optimization.
Patients and methods
Patients
We conducted a retrospective review of patient data in our institution. This study included patients with symptomatic MM who were treated with a bortezomib-based regimen, including bortezomib plus dexamethasone (VD), bortezomib, cyclophosphamide, and dexamethasone (VCD), and bortezomib, melphalan, and prednisolone (VMP) as first-line therapy. Patients who were administered any immunomodulatory drug (e.g., thalidomide or lenalidomide) in combination with bortezomib were excluded from this analysis. A single course of high-dose dexamethasone therapy prior to bortezomib administration was allowed. The study was approved by the Research Ethics Board of Nihon University Itabashi Hospital (Tokyo, Japan; identifier, RK-180710-28; approved in July 2018), and the study was conducted in accordance with the Declaration of Helsinki.
Flow cytometry
The immunophenotyping of bone marrow samples before initial treatment was performed at Bio Medical Laboratories, Inc. (Tokyo, Japan), as described previously [14, 18]. Briefly, nuclear cells collected from bone marrow samples were stained with monoclonal antibodies against CD13, CD19, CD20, CD38, CD45, CD49e, CD56, CD138, and MPC-1 surface antigens and their values were analyzed by flow cytometry (CELLQuest, version 3.3; Becton-Dickinson, San Jose, CA, USA) with a minimum acquisition of 20,000 events. The CD38bright/side scatterlow population represented the plasma cell fraction. The samples were considered positive when ≥ 20% of the myeloma cells expressed a specific antigen.
Statistical analysis
The time to next therapy (TNT) was defined as the period between the date of the beginning of bortezomib treatment and the date of the initiation of subsequent therapy, any cause of death, or last follow-up. Patients who underwent ASCT were censored on the day of transplantation for the assessment of TNT. The overall survival (OS) was defined as the period between the date of the beginning of the bortezomib treatment and the date of any cause of death or a last follow-up. The Kaplan–Meier method was used to estimate TNT and OS and the log-rank test was used to compare differences between groups. Factors that affected the outcomes of patients with MM were analyzed by multivariate Cox proportional hazard regression models. To consider the optimal cut-off point of MPC-1 expression, the 95% confidence interval was calculated. A p value < 0.05 was considered significant. The statistical analyses were performed using the JMP version 11.0 software (SAS Institute, Cary, NC, USA).
Results
Patient characteristics and treatment modalities
Of the patients diagnosed with symptomatic MM, 82 were treated with bortezomib-based regimen between January 2009 and December 2018. Among them, 74 were available for immunophenotyping, and the characteristics of these patients are presented in Table 1. This study included 41 men and 33 women, and the median age at diagnosis was 66 years (range 39–87 years). Following ISS assessments at treatment initiation, 20, 16, 33, and 5 patients were classified as being at stages I, II, III, and missing, respectively. For the VD treatment regimen, bortezomib was administered at a dose of 1.3 mg/m2 on days 1, 4, 8, and 11 every 3 weeks or on days 1, 8, 15, and 22 every 5 weeks, either intravenously or subcutaneously in combination with 20 mg/body of oral dexamethasone on the day of bortezomib administration; however, doses of dexamethasone were adjusted according to the physician’s decision or patient’s background. The VCD regimen for transplant-eligible patients and the VMP regimen for transplant-ineligible patients have been described previously [19, 20]. Ten patients received ASCT following bortezomib-based regimen and two after subsequent therapy.
Association between immunophenotyping results and prognosis
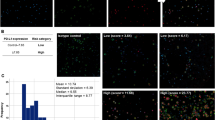
Of the 74 patients analyzed, CD13, CD45, CD49e, CD56, and MPC-1 were positively expressed in 4 (5%), 29 (39%), 5 (7%), 48 (65%), and 63 (85%) patients, respectively. The Kaplan–Meier curves for TNT and OS according to MPC-1 status are shown in Fig. 1a, b. The expression of MPC-1 was significantly related to TNT, with a median TNT of 13 months in the MPC-1-positive group and 7 months in the MPC-1-negative group (p = 0.005, Fig. 1a). However, the OS did not differ between the groups stratified according to MPC-1 status (p = 0.773, Fig. 1b). Further, CD45 positivity adversely affected TNT (p = 0.0432, Fig. 1c) but not OS (p = 0.706, Fig. 1d). Other immunophenotyping parameters did not affect TNT or OS.
Prognostic factors other than surface antigen expression
Following ISS assessment at treatment initiation, the median TNT for the patients at stage I, II, or III was 12, 13, and 14 months, respectively, and therefore almost the same among the three groups (p = 0.9747, Fig. 2a). Additionally, patients who were classified as ISS stage I had longer OS than the others; however, these data were not statistically significant (p = 0.0987, Fig. 2b). Patient age and sex did not affect TNT or OS.
Univariate and multivariate analyses
Further analysis revealed the impact of immunophenotyping data and other factors on the TNT. The results of univariate and multivariate analyses regarding TNT are presented in Table 2. Univariate analysis confirmed the prognostic significance of MPC-1 expression; specifically, MPC-1 negativity was associated with a worse TNT. Additionally, multivariate analysis showed that both MPC-1 negativity and CD45 positivity were independent adverse prognostic factors for TNT. Furthermore, stratification according to MPC-1 and CD45 expression significantly predicted the TNT in our cohort (Fig. 3). Thus, combined MPC-1 and CD45 expression status could sensibly predict response to bortezomib therapy.
Discussion
In this study, we comprehensively investigated the association between immunophenotype and prognosis in patients with MM who were initially treated with a bortezomib-based regimen. Our study showed that MPC-1 and CD45 status are possible determinants of the efficacy of bortezomib therapy, as concomitant MPC-1 positivity and CD45 negativity were closely associated with longer TNT.
The mechanism of action of bortezomib is to augment the unfolded protein response through proteasome inhibition, leading to myeloma cell apoptosis [21]. This response is known to be regulated by the transcription factor XBP-1, which is predominantly expressed in mature plasma cells [16, 17, 21, 22]. Therefore, it is logical that the expression status of MPC-1, a marker of mature plasma cells, could sensibly predict the response to bortezomib therapy.
In this study, immunophenotyping data before subsequent therapy were available for 21 patients. In the refractory setting or after relapse, seven of 21 patients showed reduction (> 20%) in the expression levels of MPC-1 on myeloma cells, suggesting that the persistence of myeloma cells with an immature phenotype is one of the underlying mechanisms that confers bortezomib resistance or disease relapse. However, MPC-1 status did not affect OS in the current study. One possible explanation for this discrepancy is that treatment subsequent to bortezomib, such as the administration of lenalidomide, pomalidomide, and other antibody agents, contributed to modification of survival rates. Although it has been shown that MPC-1-negative myeloma cells are resistant to thalidomide therapy [23], subsequent lenalidomide-containing regimens seemed to be effective even for those without MPC-1 expression. Therefore, we are now investigating the factors that could affect outcomes for patients treated with lenalidomide, including immunophenotypes, cytogenetics, and the dynamics of the immune system after lenalidomide administration.
Previous studies have shown higher treatment efficacy and tolerability of IMiDs in combination with antibody agents such as elotuzumab or daratumumab [24, 25]. Along with significant changes in the treatment of MM, the outcome of patients with this disease has continuously been improving even after the introduction of novel agents [26]. We propose that the early use of IMiDs (e.g., VD plus lenalidomide as initial therapy or lenalidomide in combination with antibody agents in the refractory or relapsed setting) is recommended particularly in patients with MM showing MPC-1 negativity.
The prognostic significance of CD45 expression has been reported previously. Consistently with our results, CD45 positivity has been identified as an adverse prognostic factor for both TNT and OS in patients diagnosed in the era of novel agents [18, 27]. However, it is of interest that opposite results are obtained in patients treated with chemotherapy and ASCT, as CD45 negativity adversely affected OS at both initial diagnosis and relapse [28]. In our study population, doublet VD treatment was the preferred regimen and alkylating agents were rarely combined except for in the treatment of transplant-eligible patients. Based on results from other studies and ours, bortezomib might be less effective in CD45-positive plasma cells and the use of alkylating agents might be recommended for patients presenting with this phenotype.
According to previous study results derived from patient data in the era of novel agents, the prognostic impact of ISS seems to be reduced [29,30,31]. In particular, the difference in survival between patients at ISS stage II and III appears to be trivial [29,30,31]. In the current study, although patients categorized into ISS stage I showed relatively favorable OS compared to other groups, the TNT was similar among all groups, suggesting the ISS is not predictive of bortezomib therapy efficacy.
This study has some limitations. For example, immunophenotyping of bone marrow samples was not performed for all patients initially treated with bortezomib; this could result in the underestimation of the impact of possible prognostic factors. The small number of patients included in this study and its retrospective design might have also negatively affected our conclusions. In addition, whether the use of IMiDs or ASCT reduces the impact of MPC-1 expression on prognosis is not clear. The treatment regimens and administration schedules were not uniform among patients enrolled in this study. Some patients who responded well to bortezomib therapy received maintenance therapy with the drug, which might lead to the overestimation of TNT in these cases. Because cytogenetic classification by fluorescence in situ hybridization was unavailable in most cases, the revised ISS, a prognostic factor established in the era of novel agents [32], was not evaluated in this study.
In conclusion, we demonstrated the significance of immunophenotyping for predicting the prognosis of patients treated with bortezomib. Thus, the continuous evaluation of immunophenotyping results during myeloma therapy might be useful to optimize treatment strategies for each patient.
References
Pawlyn C, Davies FE. Toward personalized treatment in multiple myeloma based on molecular characteristics. Blood. 2019;133:660–75.
Corre J, Munshi N, Avet-Loiseau H. Genetics of multiple myeloma: another heterogeneity level? Blood. 2015;125:1870–6.
Bianchi G, Munshi NC. Pathogenesis beyond the cancer clone(s) in multiple myeloma. Blood. 2015;125:3049–58.
Manier S, Salem KZ, Park J, Landau DA, Getz G, Ghobrial IM. Genomic complexity of multiple myeloma and its clinical implications. Nat Rev Clin Oncol. 2017;14:100–13.
Corre J, Cleynen A, Robiou du Pont S, Buisson L, Bolli N, et al. Multiple myeloma clonal evolution in homogeneously treated patients. Leukemia. 2018;32:2636–47.
Moreau P. How I treat myeloma with new agents. Blood. 2017;130:1507–13.
Chim CS, Kumar SK, Orlowski RZ, Cook G, Richardson PG, Gertz MA, et al. Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond. Leukemia. 2018;32:252–62.
Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A, Harousseau JL. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120:947–59.
Liu J, Yang H, Liang X, Wang Y, Hou J, Liu Y, et al. Meta-analysis of the efficacy of treatments for newly diagnosed and relapsed/refractory multiple myeloma with del(17p). Oncotarget. 2017;8:62435–44.
Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955–62.
Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, et al. International Staging System for multiple myeloma. J Clin Oncol. 2005;23:3412–20.
Harada H, Kawano MM, Huang N, Harada Y, Iwato K, Tanabe O, et al. Phenotypic difference of normal plasma cells from mature myeloma cells. Blood. 1993;81:2658–63.
Huang N, Kawano MM, Harada H, Harada Y, Sakai A, Kuramoto A, Niwa O. Heterogeneous expression of a novel MPC-1 antigen on myeloma cells: possible involvement of MPC-1 antigen in the adhesion of mature myeloma cells to bone marrow stromal cells. Blood. 1993;82:3721–9.
Iriyama N, Miura K, Hatta Y, Uchino Y, Kurita D, Takahashi H, et al. Plasma cell maturity as a predictor of prognosis in multiple myeloma. Med Oncol. 2016;33:87.
Lin K-I, Tunyaplin C, Calame K. Transcriptional regulatory cascades controlling plasma cell differentiation. Immunol Rev. 2003;194:19–28.
Iwakoshi NN, Lee A-H, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4:321–9.
Bagratuni T, Wu P, GonzalezdeCastro D, Davenport EL, Dickens NJ, Walker BA, et al. XBP1 s levels are implicated in the biology and outcome of myeloma mediating different clinical outcomes to thalidomide-based treatments. Blood. 2010;116:250–3.
Iriyama N, Miura K, Hatta Y, Kobayashi S, Uchino Y, Kurita D, et al. Clinical effect of immunophenotyping on the prognosis of multiple myeloma patients treated with bortezomib. Oncol Lett. 2017;13:3803–8.
Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Hentz J, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23:1337–41.
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–17.
Ri M. Endoplasmic-reticulum stress pathway-associated mechanisms of action of proteasome inhibitors in multiple myeloma. Int J Hematol. 2016;104:273–80.
Leung-Hagesteijn C, Erdmann N, Cheung G, Keats JJ, Stewart AK, Reece DE, et al. Xbp1 s-negative tumor B cells and pre-plasmablasts mediate therapeutic proteasome inhibitor resistance in multiple myeloma. Cancer Cell. 2013;24:289–304.
Kuroda Y, Sakai A, Okikawa Y, Munemasa S, Katayama Y, Hyodo H, et al. The maturation of myeloma cells correlates with sensitivity to chemotherapeutic agents. Int J Hematol. 2005;81:335–41.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–31.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31.
Costa LJ, Brill IK, Omel J, Godby K, Kumar SK, Brown EE. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Adv. 2017;1:282–7.
Gonsalves WI, Timm MM, Rajkumar SV, Morice WG, Dispenzieri A, Buadi FK, et al. The prognostic significance of CD45 expression by clonal bone marrow plasma cells in patients with newly diagnosed multiple myeloma. Leuk Res. 2016;44:32–9.
Moreau P, Robillard N, Avet-Loiseau H, Pineau D, Morineau N, Milpied N, et al. Patients with CD45 negative multiple myeloma receiving high-dose therapy have a shorter survival than those with CD45 positive multiple myeloma. Haematologica. 2004;89:547–51.
Kuroda J, Shimura Y, Ohta K, Tanaka H, Shibayama H, Kosugi S, et al. Limited value of the International Staging System for predicting long-term outcome of transplant-ineligible, newly diagnosed, symptomatic multiple myeloma in the era of novel agents. Int J Hematol. 2014;99:441–9.
Tan D, Kim K, Kim JS, Eom HS, Teoh G, Ong KH, et al. The impact of upfront versus sequential use of bortezomib among patients with newly diagnosed multiple myeloma (MM): a joint analysis of the Singapore MM Study Group and the Korean MM Working Party for the Asian myeloma network. Leuk Res. 2013;37:1070–6.
Maltezas D, Dimopoulos MA, Katodritou I, Repousis P, Pouli A, Terpos E, et al. Re-evaluation of prognostic markers including staging, serum free light chains or their ratio and serum lactate dehydrogenase in multiple myeloma patients receiving novel agents. Hematol Oncol. 2013;31:356–62.
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised International Staging System for multiple myeloma: a Report From International Myeloma Working Group. J Clin Oncol. 2015;33:2863–9.
Acknowledgements
We thank Ms. Sachiyo Mazume for collecting the data necessary to make this study possible.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
NI received honoraria and speaker fees from Bristol-Myers Squibb, Celgene K.K., Takeda Pharma Co., Ltd., and Ono Parma Co., Ltd. KM received honoraria and speaker fees from Celgene K.K., Takeda Pharma Co., Ltd., and Ono Parma Co., Ltd. HT, MN, and TH reports honoraria and speaker fees from Bristol-Myers Squibb. YH received speaker fees and honoraria from Celgene K.K., Janssen Pharmaceutical K.K., Bristol-Myers Squibb, Takeda Pharma Co., Ltd., and Ono Parma Co., Ltd. MT received honoraria from Janssen Pharmaceutical K.K., Bristol-Myers Squibb, Takeda Pharma Co., Ltd., and Ono Parma Co., Ltd., supports for extension lectures from Bristol-Myers Squibb, and scholarship funds from Takeda Pharma Co., Ltd. and Ono Parma Co., Ltd. In addition, MT is part of an international clinical trial evaluating an investigational drug that was developed by Bristol-Myers Squibb for the treatment of Sjögren’s syndrome and has cooperative research with Celgene K.K. The remaining co-authors declare no competing financial interests.
Ethical approval
All procedures were performed in accordance with the ethical standards of the Institutional Research Committee of the Nihon University Itabashi Hospital and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was not required due to retrospective nature of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kurihara, K., Iriyama, N., Miura, K. et al. MPC-1 expression in myeloma cells is associated with the efficacy of bortezomib therapy. Med Oncol 36, 75 (2019). https://doi.org/10.1007/s12032-019-1298-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-019-1298-5