Abstract
Pancreatic neuroendocrine tumors (pNETs) are rare, slow-growing cancers. Optimal treatment of advanced pNETs is unclear. The aim of this study was to examine treatment patterns and preferences among an academic tertiary medical center and community-based oncology practices. Retrospective chart review was performed for patients with newly diagnosed locally advanced, metastatic, or unresectable pNET diagnosed between January 2010 and December 2013 at an academic tertiary cancer center [University of California, San Francisco (UCSF)] or a large network of community oncology practices [Altos Solutions’ OncoEMR database (ALTOS)]. Fifty-four eligible patients (N UCSF = 23; N ALTOS = 31) were identified. Median time to treatment initiation was 1.1 months; median follow-up time was 22.9 months. UCSF patients underwent more lines of therapy than ALTOS patients despite similar follow-up times. UCSF tended toward more invasive treatment than ALTOS. The median time to treatment discontinuation was statistically significantly shorter for patients on chemotherapy than on targeted therapy in the combined UCSF and ALTOS groups (chemotherapy = 2.1 months vs. targeted = 18.6 months, p < 0.001). Treatment patterns and duration for newly diagnosed advanced pNETs vary widely both within and between different practice settings. Further studies are warranted to investigate the significant difference in duration of targeted therapy compared to chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic neuroendocrine tumors (pNETs) account for approximately 1% of all pancreatic neoplasms diagnosed each year [1, 2]. Typically well-differentiated and exhibiting indolent behavior, their prevalence is relatively high (10% of all pancreatic cancer patients). They can present with a wide array of clinical features, from incidentally discovered mass on imaging to classic hormone-mediated syndromes (e.g., due to insulin or gastrin excess) [3]. In many cases, the disease is metastatic at diagnosis [1, 4]. Interestingly, the precise timing and role of therapy for advanced disease varies widely from patient to patient. In the absence of resectable disease, many experts suggest delaying therapy until evidence of clinical or radiographic progression [5]. This, coupled with the dramatically changing treatment landscape of pNETs over the past 5 years, has led to a lack of consensus regarding the optimal treatment sequence for this disease [6]. Surgical resection of all sites of disease is typically the first line of treatment in pNETs when feasible [6, 7]. However, the optimal treatment for unresectable disease is unclear, and considerable confusion surrounds how best to sequence therapeutic options [5, 6]. An array of management options is now available for metastatic disease, including somatostatin analogs (SSAs), targeted agents (everolimus, sunitinib), chemotherapy, and liver-directed therapy [2, 8, 9]. Sunitinib (an oral inhibitor of vascular endothelial growth factor signaling) and everolimus (an inhibitor of mTOR pathway activity) were both approved for use in the USA in 2011 based their ability to delay progression in patients with pNETs [7, 10]. Outside the USA, peptide receptor radionuclide therapy has gained significant traction [11, 12]. In this study, we aimed to evaluate treatment patterns in the era of targeted therapy among patients with newly diagnosed advanced pNETs in both academic and community practice settings.
Materials and methods
This study was conducted in compliance with the Health Insurance Portability and Accountability Act (HIPAA) and conducted under institutional review board (IRB) approval, as a minimal risk study. A retrospective chart review [via the electronic medical record (EMR)] was undertaken at the University of California, San Francisco (UCSF) (a tertiary care academic medical center) and a large network of 33 community oncology practices with locations throughout 36 states in the USA (ALTOS). Designed as a pilot study, the target sample size was based on feasibility (N = 50 per center). The main objective of the study was to assess real-world treatment patterns among patients with advanced pNETs.
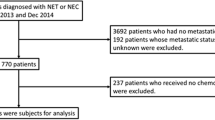
Patients with newly diagnosed advanced (i.e., unresectable, locally advanced, or metastatic) pNETs between January 1, 2010, and December 31, 2013, were included, with the index date being the date of diagnosis. The observation period started with the date of diagnosis of advanced pNET to August 12, 2014, at ALTOS and March 17, 2015, at UCSF, or the date of patient death. Visualization of study schema is seen in Online Resource 1. Eligible patients were 18 years or older at the time of diagnosis. Patients categorized with poorly differentiated or high-grade (G3) tumors (mitotic rate > 10/10 HPF or Ki-67 > 20%), small or large cell carcinomas, or mixed histology tumors were excluded. Tumor differentiation was determined using Ki-67 proliferation index, mitotic rate, or a combination of both. In cases where tumor grade and/or differentiation was not specified in the chart and was unable to be confirmed using Ki-67 proliferation index, pathologic grade or differentiation, or mitotic rate, eligibility determination was made by nurse assessment of other elements in the pathology report as well as physicians’ notes. Patients had to be actively treated and/or followed with surveillance for at least 6 months after the diagnosis date, with at least two visits in a 12-month period.
Data abstraction from EMR included basic demographic information (age, sex, race, and ethnicity), diagnostic characteristics (histology, histologic grade, tumor functionality, mitotic rate, and Ki-67 proliferation index), comorbidity data, imaging, lines of therapy, clinic visits, surgeries, and survival data (if applicable). The baseline period for patient characteristics was established at the time of diagnosis of advanced pNET. Determination of lines of therapy was based on the date of treatment initiation, and it was noted that some lines of therapy could have occurred concurrently.
Descriptive statistics were used to examine demographic trends, treatment patterns and decisions, metastatic data, and overall survival among UCSF and ALTOS patients. The Wilcoxon rank sum test was used to compare continuous variables. Chi-square tests (and Fisher’s exact tests, as appropriate) were used for comparison of categorical variables. A p value of 0.05 was used to define statistical significance. All analyses were performed using SAS release 9.3 or newer (SAS Institute, Inc., Cary, NC) and R 3.0.2.
Results
A total of 54 eligible patients were identified between UCSF and ALTOS out of 159 charts screened. Patients deemed ineligible upon screening at UCSF were excluded because metastatic disease had been diagnosed before January 1, 2010, a high-grade tumor and/or ineligible tumor type (e.g., adenocarcinoma or mixed histology) was present, and/or the criteria for the minimum number of clinic visits during the observation period were not met. Detailed information about screen failures was not available for ALTOS patients.
Overall patient demographics were examined (Table 1), and no significant differences in the age and gender composition of the patient populations of UCSF and ALTOS were observed. The overall mean age (±SD) at diagnosis of advanced pNET was 60.6 ± 15.8 years. For UCSF and ALTOS separately, the mean age was 56.6 ± 14.6 and 63.5 ± 16.2 years, respectively (p = 0.05).
Gender composition tended toward male patients 61.1% overall, and individually at UCSF and ALTOS (60.9 and 61.3%, respectively; p = 0.98). The distribution of race/ethnicity was generally very similar between the two groups. Median follow-up time was 22.9 months overall (24.1 and 22.4 months at UCSF and ALTOS, respectively; p = 0.41). Patient mortality in the follow-up period was 20.4% overall (13.0 and 25.8% at UCSF and ALTOS, respectively; p = 0.42).
Diagnostic characteristics were examined as well (Table 1). A functional tumor was identified in 20.4% of pNETs (26.1 and 16.1% at UCSF and ALTOS, respectively; p = 0.57). Overall, 37.0% of pNETs in the study were non-functional (56.5 and 22.6% at UCSF and ALTOS, respectively; p = 0.01). Functional status was unknown in 42.6% of patients overall (17.4 and 61.3% at UCSF and ALTOS, respectively; p < 0.01). There were 46.3% of pNETs that were well differentiated (73.9 and 25.8% for UCSF and ALTOS, respectively; p < 0.01). Information regarding histologic differentiation was missing in 42.6% of cases, the majority of which were ALTOS patients (71.0% compared to 4.3% at UCSF; p < 0.01). Grade was largely unknown, missing in 48.1% of cases (80.6% ALTOS and 4.3% UCSF; p < 0.01), although more UCSF patients had Grade 1 than ALTOS patients (52.2 vs. 12.9%; p < 0.01). Ki-67 proliferation index was missing in 61.1% of cases (87.1% ALTOS and 26.1% UCSF; p < 0.01). Mitotic rate was missing in 64.8% cases (87.1% ALTOS and 34.8% UCSF cases; p < 0.01).
Additionally, 74.1% of patients were found to have metastatic disease overall (Table 2), 87.0% of UCSF patients and 64.5% of ALTOS patients, respectively (p = 0.06). Liver was the most common site of metastasis (UCSF 60.9% and ALTOS 51.6%; p = 0.50). A statistically significant difference was noted in the proportion of lymph node metastases (UCSF 39.1% and ALTOS 9.7%; p = 0.02). Mean age in years of patients with metastatic disease was 58.6 ± 15.0 overall and 54.0 ± 13.3 and 63.1 ± 15.6 for UCSF and ALTOS, respectively (p = 0.04).
Treatment patterns were examined (Table 3) with some differences in trends identified between sites. As a group, 83.3% of all patients underwent at least one line of therapy, with a larger proportion (91.3%) of UCSF patients undergoing therapy compared with ALTOS (77.4%; p = 0.18). The same pattern was observed when examining the number of patients receiving at least two and three lines of therapy. Overall, 38.9% of patients underwent two or more lines of therapy (Fig. 1a, b), 60.9% at UCSF compared to 22.6% at ALTOS (p < 0.01). Treatments were categorized as: (1) chemotherapy, (2) liver-directed therapy (bland embolization, chemoembolization, or selective internal radiation therapy), (3) surgery (including resection, ablation, or a combination), (4) targeted therapy (e.g., everolimus, sunitinib), or (5) SSA.
a ALTOS treatment flowchart. b UCSF treatment flowchart. Note: A small minority of patients went on to have additional lines of therapy (up to seven). Other chemotherapy includes capecitabine, carboplatin, cisplatin, cladribine, etoposide, gemcitabine, irinotecan, oxaliplatin, docetaxel, and paclitaxel. EBRT electronic beam radiation therapy
Differences were noted in the most common treatments employed in each setting. At UCSF, the most common treatments were SSA (52.2 vs. 45.2% in ALTOS; p = 0.61), surgery (47.8 vs. 6.5% in ALTOS; p < 0.01), and chemotherapy (26.1 vs. 19.4% in ALTOS; p = 0.56). In the ALTOS group, on top of SSA and chemotherapy, everolimus was one of the most common forms of treatment (22.6 vs. 21.7% in UCSF; p = 1.00). Median time to the first-line treatment initiation from advanced pNET diagnosis date was 1.1 months, 1.1 and 1.2 months at UCSF and ALTOS, respectively (p = 0.34). Overall, median time to treatment discontinuation (Fig. 2) for the first- or second-line therapy, starting at that specific line of therapy, was statistically significantly (p < 0.01) shorter for patients on chemotherapy (2.1 months) versus patients on targeted therapy (18.6 months). Importantly, in both settings, a wide range of therapeutic sequences was observed (Fig. 1a, b; Table 4).
Reasons for initiation and discontinuation of treatment were also examined (Table 4). Chemotherapy and targeted therapy were separated into two distinct categories; however, it is noted that patients could have received both, in separate lines of therapy. At ALTOS, there were 24 instances of chemotherapy, with the reasons for initiation being largely unknown at 70.8% (compared to 12.5% at UCSF; p = 0.01), followed by radiographic progression of disease in 12.5% (compared to 0.0% at UCSF; p = 0.82). The main reasons for chemotherapy discontinuation at ALTOS were disease progression (29.4 vs. 28.6% at UCSF; p = 1.00), unknown (23.5 vs. 0.0% at UCSF; p = 0.32), or treatment completion (17.6 vs. 14.3% at UCSF; p = 1.00). At UCSF, there were eight instances of chemotherapy initiation, with the most common reason for initiation being that it was recommended in treatment guidelines (87.5 vs. 0.0% at ALTOS; p < 0.01). The most common reason for chemotherapy discontinuation at UCSF was treatment toxicity/intolerance (42.9 vs. 5.9% at ALTOS; p = 0.21) followed by disease progression. Regarding targeted therapy, 12 instances were recorded at ALTOS with the reasons for initiation unknown in 100% (compared to 22.2% at UCSF; p < 0.01). Among these, the most common reasons for targeted therapy discontinuation were treatment toxicity/intolerance (40.0 vs. 100.0% at UCSF; p = 0.06), death (20.0 vs. 0.0% at UCSF; p = 0.46), and disease progression (20.0 vs. 0.0% at UCSF; p = 0.46). At UCSF, nine instances of targeted therapy were recorded, with 77.8% being initiated because it was recommended in treatment guidelines (compared to 0.0% at ALTOS; p < 0.01). The most prevalent reason for targeted therapy discontinuation at UCSF was treatment toxicity/intolerance.
Discussion
This study aimed to examine overall treatment patterns in the management of advanced pNET through retrospective EMR and medical chart review in academic and community-based settings. This analysis was undertaken, recognizing that a wide range of approaches are routinely used to treat patients with advanced pNETs and that the optimal therapeutic sequence remains unknown [5, 13, 14].
A wide variety of interventions were used to treat pNET in both settings. SSA, everolimus, and chemotherapy were used with approximately the same frequency at UCSF and in ALTOS. However, in the academic setting as compared to ALTOS, patients received more lines of therapy and were more likely to undergo surgery (47.8 vs. 6.5%; p < 0.01) and/or liver-directed therapy (21.7 vs. 3.2%; p = 0.09). At UCSF, 34.8% of patients received at least three lines of therapy, compared to 12.9% in ALTOS (p = 0.12). In terms of the first-line therapy, surgery was the most common intervention at UCSF (38.1 vs. 8.3% at ALTOS; p = 0.04); at ALTOS, use of SSA was most common (45.8 vs. 23.8% at UCSF; p = 0.22).
Several factors could account for the apparent differences in choice of the first-line therapy, sequence, and number of therapies. Liver resection is commonly reserved for patients in whom at least 90% of the tumor bulk can be removed and with less than 50% bilobar liver involvement [15]. As a result, location and extent of tumor influence choice of therapy, along with age and comorbidities. It is possible that the UCSF patient population was more physically fit (i.e., able to tolerate invasive therapies) and/or enriched with patents with resectable metastases as compared to the ALTOS population. A higher rate of liver-dominant disease could also have contributed to the relatively large proportion of patients undergoing liver-directed therapy of some type at UCSF. Similarly, the availability of expertise in liver resection, ablation, and/or embolization may have influenced the choice of therapy at UCSF relative to the community setting. The trend toward a higher number of lines of therapy in the tertiary care setting could stem from UCSF patients harboring less indolent disease, having access to more lines of therapy, and/or more aggressive management by their providers, relative to ALTOS.
It is acknowledged that there were limitations in our methodology. Considering the short follow-up period, it is very likely that most patients had not yet undergone all lines of therapy; thus, this study predominantly provides insight into the choice of the initial lines of therapy. The expected median overall survival in patients with advanced pNET is more than 5 years, and this is supported by the fact that 75% of patients were alive in each group at the end of the observation period (median follow-up only about 23 months) [13, 16, 17]. Furthermore, it is possible that all treatments received at other institutions were not captured (e.g., ALTOS patients treated at tertiary care centers). Additionally, patients at ALTOS tended to be older and with higher mortality rate during follow-up and therefore may not have been able to undergo as many lines of therapy as patients at UCSF or remain in treatment as long. As a result, conclusions regarding differing patterns of care between settings must be made with caution. Similarly, it is worth noting that a significant amount of data was missing, particularly from the ALTOS data (e.g., 61% of patients harbored tumors with an unknown functional status, and the reasons for treatment initiation were missing in most cases). Despite the presence of a classification system that is based on proliferation and mitotic rate, grade was not specified in 80% of ALTOS cases, and 58% of these patients were missing information on grade, differentiation, Ki-67, and mitotic rate. However, these patients were still included in the study based on information provided in physician notes and/or pathology reports, suggesting that the patients were, in fact, eligible for the study (e.g., records with terms like “low grade,” “rare mitotic figures,” and/or mention of a lack of high-grade features). The data suggest that incomplete information in the EMR and other charts can be a major limitation in studying pNETs in some healthcare systems, especially if qualitative clinical assessments may not be available. Our data also suggest that changes to the histopathologic classification system for pNETs are adopted very slowly in practice. The current classification system was published in 2010, yet it does not appear to be routinely used in all practices [18].
Of interest, while everolimus was used as the first-line therapy in 8.9% of patients (12.5% of ALTOS patients and 4.8% of UCSF patients), sunitinib was rarely used in either setting (4.2% at ALTOS and 0.0% at UCSF). It is possible that the drug was simply being reserved for later lines of therapy. However, assuming both drugs were equally available, the data suggest that everolimus is the preferred targeted agent in both settings (UCSF and ALTOS). The basis for this is uncertain (e.g., unclear if perceived by patients or providers to be more efficacious, better tolerated or both).
Treatment patterns differed widely both between and within a tertiary care cancer center and a network of 33 community oncology practices. Despite similar demographics and median follow-up time between institutions, aside from SSAs, approaches to therapy showed a trend toward more surgical management and liver-directed therapy in the academic center setting, and an emphasis on targeted therapy and chemotherapy in the community-based setting. Prospective studies are needed to more completely examine factors affecting choice of therapy. Importantly, insufficient tumor information in the EMR could be a major limitation in studies analyzing the management of pNET in some healthcare systems.
References
Capozzi M, Caterina I, De Divitis C, et al. Everolimus and pancreatic neuroendocrine tumors (PNETs): activity, resistance and how to overcome it. Int J Surg. 2005;21(Suppl 1):S89–94.
Cloyd JM, Poultsides GA. Non-functional neuroendocrine tumors of the pancreas: advances in diagnosis and management. World J Gastroenterol. 2015;21(32):9512–25.
Martin-Perez E, Capdevila J, Castellano D, et al. Prognostic factors and long-term outcome of pancreatic neuroendocrine neoplasms: Ki-67 index shows a greater impact on survival than disease stage. The large experience of the Spanish National Tumor Registry (RGETNE). Neuroendocrinology. 2013;98:156–68.
Centzone DC, Cinardi N, Giannone G. Surgical resection for neuroendocrine tumors of the pancreas: a fourteen years single institutional observation. Eur Rev Med Pharmacol Sci. 2004;18(Suppl 2):32–5.
Kulke MH, Shah MH, Benson AB 3rd, et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Cancer Netw. 2015;13(1):78–108.
Berardi R, Rinaldi S, Torniai M, et al. Gastrointestinal neuroendocrine tumors: searching the optimal treatment strategy—a literature review. Crit Rev Oncol Hematol. 2016;98:264–74.
Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–13.
Deutsch GB, Lee JH, Bilchik AJ. Long-term survival with long acting somatostatin analogues plus aggressive cytoreductive surgery in patients with metastatic neuroendocrine carcinoma. J Am Coll Surg. 2015;221(1):26–36.
Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121(4):589–97.
Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–23.
Van Essen M, Krenning EP, De Jong M, Valkema R, Kwekkeboom DJ. Peptide receptor radionuclide therapy with radiolabelled somatostatin analogues in patients with somatostatin receptor positive tumours. Acta Oncol. 2007;46(6):723–34.
Campana D, Capurso G, Partelli S, et al. Radiolabelled somatostatin analogue treatment in gastroenteropancreatic neuroendocrine tumours: factors associated with response and suggestions for therapeutic sequence. Eur J Nucl Med Mol Imaging. 2013;40:1197–205.
Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–72.
Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42(4):557–77.
Folkert IW, Hernandez P, Roses RE. Multidisciplinary management of nonfunctional neuroendocrine tumors of the pancreas. World J Gastroenterol. 2016;22(11):3105–16.
Strosberg JR, Cheema A, Weber J, et al. Prognostic validity of a novel American Joint Committee on Cancer Staging Classification for pancreatic neuroendocrine tumors. J Clin Oncol. 2011;29(22):3044–9.
Ter-Minassian M, Brooks NV, Brais LK, et al. Association of progression-free survival with overall survival (OS) in patients (pts) with neuroendocrine tumor (NET) treated with somatostatin analogs. J Clin Oncol. 2015; 33(suppl; abstr 4090).
Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press; 2010.
Strosberg JR, Nasir A, Hodul P, Kyols L. Biology and treatment of metastatic gastrointestinal neuroendocrine tumors. Gastrointest Cancer Res. 2008;2:113–25.
Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–12.
Acknowledgements
This study was supported by Novartis Pharmaceutical Corporations, East Hanover, New Jersey, USA. LH, MSD, and FV are employees of Analysis Group, Inc., a consulting company that has received research funding from Novartis. AT is a former employee of Analysis Group, Inc. EB is an employee of the University of California, San Francisco (UCSF) Helen Diller Family (HDF) Comprehensive Cancer Center (CCC) which received research funding from Novartis to support this work. EB has served as a consultant for Novartis, Lexicon, and Ipsen (uncompensated). MH is a former employee of UCSF HDF CCC. MN is an employee of Novartis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Approval was obtained from the ethics committee (institutional review board) at the University of California, San Francisco (UCSF). All procedures were performed in accordance with the ethical standards of the UCSF Institutional Review Board and the 1964 Helsinki declaration and its later amendments. No prospective studies with human participants or animals were performed by any of the authors for this article.
Informed consent
Informed consent was waived for individual participants included in the study given the retrospective nature of this work and in accordance with UCSF Institutional Review Board standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Herring, M., Huynh, L., Duh, M.S. et al. Real-world treatment patterns in advanced pancreatic neuroendocrine tumors in the era of targeted therapy: perspectives from an academic tertiary center and community oncology practices. Med Oncol 34, 88 (2017). https://doi.org/10.1007/s12032-017-0927-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-0927-0