Abstract
Purpose
Peptide receptor radionuclide therapy (PRRT) is a relatively new treatment modality for patients with unresectable or metastatic gastroenteropancreatic neuroendocrine tumours (GEP NETs). The aim of this study was to determine the time to progression of patients treated with PRRT and to identify the prognostic factors related to treatment response.
Methods
Patients with sporadic GEP NETs prospectively treated with PRRT were retrospectively analysed. The primary end point was progression-free survival (PFS).
Results
A total of 69 patients (37 men and 32 women; 45 with pancreatic and 24 with gastrointestinal lesion; 22 NET G1 and 41 NET G2) were treated with 90Y or 177Lu. The objective response rate was 27.5 % (partial response, PR), while 50.7 % had stable disease and 23.2 % had progressive disease. Significant differences in PFS were observed in relationship to the stage of the disease (44 months for stage III, 23 months for stage IV), the evidence of a PR 6 months after the end of the PRRT (39 months in patients with a PR, 22 months in patients without a PR) and previous transarterial chemoembolization (TACE, yes 13 months vs no 31 months). Stage IV, NET G2 and previous TACE were found to be significant factors for tumour progression at multivariate analysis.
Conclusion
Low tumour burden and a low proliferation index represent independent prognostic factors for long PFS, while previous chemoembolization techniques represent independent prognostic factors for early tumour progression and shorter PFS. Our data suggest that chemoembolization techniques to reduce the hepatic tumour burden should be avoided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine tumours (NETs) are rare neoplasms, having an incidence of 5.25/100,000 age-adjusted for the 2004 US standard population [1]. They are considered indolent tumours, due to slow growth and a relatively good prognosis. Overexpression of somatostatin receptor subtypes (mainly sst2) on the membrane of the NET cells [2, 3] justified the use of somatostatin analogue treatment and peptide receptor radionuclide therapy (PRRT). When metastasized, treatment with somatostatin analogues reduced hormonal overproduction and achieved symptomatic relief in most cases, but it was rarely successful in terms of tumour size reduction [4–6]. However, it has recently been shown that long-acting somatostatin analogues significantly lengthen the time to tumour progression as compared to a placebo in patients with functionally active and inactive metastatic midgut NETs [7].
The use of radiolabelled somatostatin analogues is a relatively new treatment modality for patients with unresectable or metastatic gastroenteropancreatic (GEP) NETs. The most extensively studied radiopeptides for PRRT, derived from phase I-II trials, are 90Y-DOTATOC and 177Lu-DOTATATE [8–14]. Despite the differences found in the various available studies, complete, partial and minor response was registered in up to 46 % of the patients. A clear survival benefit was also reported after the use of either 90Y-DOTATOC [11, 14] or 177Lu-DOTATATE [12].
The aim of this study was to determine the time to progression of patients treated with radiolabelled somatostatin analogues and to identify the prognostic factors related to treatment response.
Materials and methods
Study design and patients
The study design consisted of a multicentre, retrospective analysis of prospective institutional databases. The study included all consecutive patients with GEP NET treated with PRRT from November 1999 to September 2010 who were followed at the participating centres (i.e. Verona, Negrar, Rome and Bologna).
The inclusion criteria were: (1) a histologically confirmed diagnosis of sporadic GEP NET, (2) measurable (according to the RECIST criteria) and advanced disease not suitable for radical surgery or residual disease after debulking surgery, (3) positive 111In-DTPA-octreotide (OctreoScan®) scintigraphy or positron emission tomography (PET) with 68Ga-DOTANOC/TOC, (4) PRRT treatment with either 177Lu-DOTATATE or 90Y-DOTATOC and (5) radiological assessment every 6 months (±1 month) during the follow-up until disease progression.
At baseline evaluation, all patients underwent a clinical evaluation, routine haematology, liver and kidney function tests, a computed tomography scan (CT) and/or magnetic resonance imaging (MRI) and somatostatin receptor scintigraphy (or PET/CT with 68Ga-DOTANOC/TOC). In order to undergo the PRRT, all patients gave their informed written consent, and the therapy was approved by the local Ethics Committee. Routine haematology, liver and kidney function tests were performed before each cycle of therapy as well as at follow-up visits. For renal protection purposes, all patients were treated with amino acids before and after the injection of the tracers [8]. The intratherapeutic biodistribution of the radiopeptide was assessed using planar whole-body imaging after each cycle of therapy.
The CT scan (or MRI) was repeated 4/6 months after the end of the therapy and every 6 months (±1 month) until disease progression according to RECIST criteria (unless clinical conditions required shorter intervals). Somatostatin receptor scintigraphy or PET/CT with 68Ga-DOTANOC/TOC was repeated yearly.
Clinical response and toxicity of the PRRT after each cycle of therapy was assessed. After PRRT, the clinical response was considered to be positive when there was a greater than 50 % reduction of diarrhoea and/or flushing in carcinoid syndrome, diarrhoea in Verner-Morrison syndrome, and diarrhoea and abdominal pain in Zollinger-Ellison syndrome. The toxicity was evaluated using the Common Terminology Criteria for Adverse Events v3.0 (CTCAE).
Data analysis
All data were prospectively collected at the centre where the patient had been enrolled. A unique computerized data sheet was created, and data regarding demographic, clinical and pathological features were retrospectively analysed. The histological specimens were examined by an experienced pathologist at each centre. When required, additional centralized revision of the tumour specimens was performed. The tumours were classified according to the 2010 WHO classification [15] and the novel tumour node metastasis (TNM) classification/G grading system [16, 17]. The Ki-67 proliferation index was expressed as a percentage based on the count of Ki-67-positive cells in 2,000 tumour cells in areas of the highest immunostaining using the MIB1 antibody (DBA, Milan, Italy). The tumours were measured and scored according to the RECIST criteria [18]. Progression-free survival (PFS) was defined as the interval between the beginning of the therapy and the time of progression of disease (PD). PFS was measured using the Kaplan-Meier method, and the results were compared using the log-rank test. Analysis of the predictive risk factors for PD was carried out by univariate and multivariate analysis using the Cox proportional hazards method. Risk factors were expressed as hazard ratios (HR) [95 % confidence interval (CI)]. The multivariate model was constructed using the forward stepwise method after including all variables. All analyses carried out for risk factors are listed in the tables. The distribution of the continuous variables was reported as median and interquartile range (IQR, 25th to 75th percentiles). The comparison between the subgroups was carried out using Pearson’s chi-square test (Fisher’s exact test was used when necessary) or the Mann–Whitney U test for continuous variables. The p value was considered significant when less than 0.05. The statistical analysis was carried out using dedicated software (SPSS version 19.0, SPSS Inc.).
Results
Study population
One hundred and thirty-one patients with GEP NETs were enrolled at the participating centres. Of these, 62 patients (47.3 %) were excluded, 11 because the radiological assessment during follow-up was not available, 25 because the PRRT had not been concluded at the time of the data collection and 26 because they were re-treated with supplementary doses of 90Y or 177Lu before assessment of disease progression. Thus, a total of 69 patients were included in the final analysis. The characteristics of all 69 patients are listed in Table 1. There were 37 men and 32 women with a median age of 57.6 years (IQR 50.0–65.5 years). In 45 of the 69 patients (65.2 %), the primary lesion was located in the pancreas, whereas in 24 (34.8 %) it was in the gastrointestinal (GI) tract (21 in the ileum, 1 in the duodenum, 1 in the colon and 1 in the appendix). According to the 2010 WHO classification, 22 patients (31.9 %) had a NET G1 and 41 (59.4 %) a NET G2. In 6 of the 69 patients, histological revision was not possible due to the scarcity of the tissue samples.
Fifteen (21.7 %) patients had functioning tumours (ten carcinoid syndrome, three Zollinger-Ellison syndrome, one Verner-Morrison syndrome and one symptomatic hypercalcaemia). At the beginning of the PRRT, 35 patients (50.7 %) had PD, while the remaining 34 patients had stable disease (SD, 19 naïve patients and 15 who had undergone previous treatment: 15 with somatostatin analogues, 2 with chemoembolization and 3 with chemotherapy).
Treatment with radiolabelled somatostatin analogue
Forty-nine (71.0 %) patients were treated with 90Y-DOTATOC, while 20 (29.0 %) were treated with 177Lu-DOTATATE. For the 90Y-DOTATOC group, the median number of therapy cycles was 4 (IQR 4–5) and the median time to therapy was 7 months (IQR 6–9 months). The median cumulative dose of radiolabelled somatostatin analogue administered was 10.3 GBq (IQR 8.8–11.8 GBq) with a median dose per cycle of 2.2 GBq (IQR 2.0–2.6 GBq).
For the 177Lu-DOTATATE group, the median number of therapy cycles was 4 (IQR 4–5) and the median time to therapy was 9 months (IQR 6.25–11.75 months). The median cumulative dose of radiolabelled somatostatin analogue administered was 25.2 GBq (IQR 19.0–27.2 GBq) with a median dose per cycle of 5.3 GBq (IQR 3.9–7.2 GBq).
Efficacy
At the first check-up after the last cycle of PRRT, 19 patients (27.5 %) had a partial response (PR, 31.1 % of those with pancreatic lesions and 20.8 % of those with GI tumours, p = 0.363), 50.7 % had SD (40.0 % of those with pancreatic lesions and 66.7 % of those with GI tumours, p = 0.070) and 23.2 % had PD (28.9 % of those with pancreatic lesions and 12.5 % of those with GI tumours, p = 0.124). In the 15 patients with functioning tumours, PRRT led to a subjective improvement of symptoms in 13 patients (86.7 %) (10 with carcinoid syndrome, 2 with Zollinger-Ellison syndrome and 1 with Verner-Morrison syndrome); 2 (13.3 %) did not have any clinical benefit.
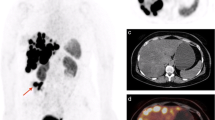
The potential prognostic factors for predicting a PR or PD at 6 months after therapy are reported in Table 1. The significant factors correlated with PD were baseline tumour progression and previous treatment with transarterial chemoembolization (TACE). Regarding TACE, 9/12 (75 %) patients had a progressive disease after PRRT. In six patients, the progression after TACE was in and around the chemoembolized lesion(s), whilst in three patients progression was both around the chemoembolized lesion(s) and in other segments of the liver (Fig. 1a, b). The only significant factor correlated with a PR was the use of 177Lu-DOTATATE as a radiolabelled somatostatin analogue.
Predictors for tumour progression
At univariate analysis, the variables considered as risk factors for tumour progression after PRRT are summarized in Table 2. The major risk factor for tumour progression was the absence of tumour response at the first check-up after the last cycle of PRRT (HR 4.021, p = 0.008). Stage IV of the disease and previous TACE represent the other risk factors for tumour progression. The absence of tumour response after PRRT, histological evidence of NET G2 and previous TACE were also confirmed at multivariate analysis (Table 3, model A). If, at multivariate analysis, the factors known only after PRRT (type of radiopeptides and the absence of tumour response at the first check-up) were excluded, stage IV and previous TACE were found to be significant for tumour progression (Table 3, model B).
Progression-free survival
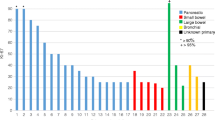
Overall, median PFS was 28 months (Fig. 2). Significant differences in PFS were observed in relationship to the stage of the disease (44 months for stage III, 23 months for stage IV, p = 0.009), the evidence of a PR 6 months after the end of the PRRT (39 months in patients with a PR, 22 months in patients without a PR, p = 0.004) and previous TACE (yes 13 months vs no 31 months, p = 0.002, Fig. 3). No statistical difference was found according to tumour differentiation (median 35 months for NET G1 and 23 months for NET G2, p = 0.077), primary site (pancreas vs GI: 23 vs 31 months, p = 0.285), baseline tumour progression (PD vs SD: 21 vs 34 months, p = 0.130) and type of radiolabelled somatostatin analogue used for therapy (90Y 23 months, 177Lu 35 months, p = 0.055).
Toxicity
In the 69 patients, nausea and vomiting within 24 h after treatment, due to the amino acid infusion, occurred in 11.6 % of the patients. In one case, acute diarrhoea grade 2 was observed. Haematological toxicity, grades 1 and 2, occurred in 29 patients (42.0 %), while grade 3 occurred in 1 case (1.4 %). Regarding renal toxicity, grade 1 occurred in seven cases (10.1 %).
Discussion
In clinical practice, treatment with radiolabelled somatostatin analogues has already been recognized as a promising tool in the management of patients with unresectable or metastasized NETs. Kwekkeboom et al. in 2008 and Imhof et al. in 2011 [12, 14] reported high tumour response rates and a long PFS for 177Lu and 90Y, respectively. However, these studies investigated the significant factors predicting disease-specific survival, but predictors for tumour response were not evaluated. The present study was aimed at detecting possible predictors of tumour response to treatment in a large and relatively homogeneous series of advanced GEP NETs treated with PRRT. Moreover, efficacy in terms of objective response rate and PFS were also evaluated.
The principal results of this study confirmed the important role of PRRT in the management of patients with locally advanced or metastatic NETs. In particular, the treatment with radiolabelled somatostatin analogues showed more efficacy in the presence of low tumour burden (stage III) and a low proliferation index (G1). Our study demonstrated the negative role of previous treatment with TACE in terms of objective response and PFS.
The central role of PRRT in GEP NETs is highlighted by the high objective response rate as well as a long PFS; a PR was observed in 28.8 % of the patients, while none had a complete response. Acknowledging the inherent problems of inter-study comparisons, our results are better than those reported in studies regarding chemotherapy in GEP NETs, which are usually less than 20 % [19–21], or targeted therapies, such as sunitinib (9.3 %) [22] and everolimus (5 %) [23]. These data suggest a potentially strong role of PRRT in the reduction of the disease burden also in the setting of neoadjuvant therapy.
Regarding time to progression, PRRT has a very long PFS (28 months) [11, 12] when compared to that reported for chemotherapy (median PFS less than 18 months) [20, 21] or other targeted therapies, such as sunitinib and everolimus (11.4 and 11.0 months, respectively) [22, 23].
The differences in terms of PFS were less evident if we considered only patients with progressive disease at the start of the PRRT (21 months). Interestingly, we did not find significant differences in median PFS according to the primary site of the tumours (GI vs pancreatic origins: 31 vs 23 months). Regarding pancreatic NETs, in our series, PRRT showed longer PFS when compared to that reported in a recent paper by Panzuto et al. in which the median PFS for metastatic pancreatic NETs, treated or not treated with antitumoural therapy (such as PRRT, somatostatin analogues and/or chemotherapy), was 15 and 7 months, respectively [24]. On the other hand, PRRT in GI NETs has a median PFS similar to that reported by Panzuto et al. (31 vs 36 months) in a large retrospective analysis of metastatic jejunoileal NETs [25].
Assessments of the factors which can identify patients who may benefit from PRRT demonstrate the role of tumour burden and the proliferation index. Tumour burden was a well-known significant predictor of disease-specific survival [12, 14]. In these studies, a significant correlation between survival and the extent of liver involvement was reported. In the present study the independent role of the stage of the disease in PFS after PRRT was documented. These data confirmed that PRRT is more efficacious in patients with limited disease and suggest it be initiated as early as possible.
An expected risk factor for tumour progression was the degree of the tumour proliferation index according to the WHO classification (NET G2 vs NET G1, HR 3.481, p = 0.003 at multivariate analysis). In general, it is well known that Ki-67 is a major prognostic factor for NETs [26–28]. However, our data confirmed the role of tumour differentiation and Ki-67 as crucial therapeutic prognostic factors for response to PRRT in NETs as well.
However, the more relevant clinical result of the present study was that previous TACE was an independent risk factor for disease progression. This evidence has never previously been reported and may be due to the fact that embolization impairs the possibility of the radiopeptide reaching the ischaemic area. This finding might have an important implication in the algorithm and the sequence of therapeutic options. In fact, it is well known, as was also confirmed in the present study, that PRRT is more efficacious in the presence of minor tumour burden [12, 14]. In order to reach this end, it has been suggested that cytoreductive surgery and/or ablative therapies [i.e. TACE and radiofrequency thermal ablation (RFTA)] should precede PRRT courses. Our data suggested that chemoembolization techniques to reduce the hepatic tumour burden should be avoided.
This study might have some potential biases due to the retrospective evaluation and a certain degree of heterogeneity of the population enrolled. However, this population includes: (1) only GEP NETs (no lung or other sites) and (2) patients with advanced disease without re-treatment with supplementary doses. However, the heterogeneity of the population (i.e. pancreas vs gastroenteric, 90Y vs 177Lu, stage III vs stage IV, previous therapy) might represent, at the same time, a strength of the study, since it allows a better understanding of potential factors which might affect PRRT results. Univariable and multivariable analyses have been applied in order to reduce these potential biases due to the heterogeneity.
In conclusion, treatment with radiolabelled somatostatin analogues is an important therapeutic option in the management of patients with unresectable or metastasized NETs, allowing a high objective response rate and long PFS. Low tumour burden and a low proliferation index represent independent prognostic factors for long PFS, while previous TACE represents an independent prognostic factor for both early tumour progression and shorter PFS. These data confirmed the fact that PRRT should be performed discerningly in the sequence of therapeutic options and suggests that the pre-procedural use of TACE for reducing the hepatic tumour burden should be avoided. Prospective and comparative studies are necessary to confirm these data.
References
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–72.
Reubi JC, Laissue JA. Multiple actions of somatostatin in neoplastic disease. Trends Pharmacol Sci 1995;16:110–5.
Reubi JC, Waser B, Liu Q, Laissue JA, Schonbrunn A. Subcellular distribution of somatostatin sst2A receptors in human tumors of the nervous and neuroendocrine systems: membranous versus intracellular location. J Clin Endocrinol Metab 2000;85:3882–91.
Arnold R, Benning R, Neuhaus C, Rolwage M, Trautmann ME. Gastroenteropancreatic endocrine tumours: effect of Sandostatin on tumour growth. The German Sandostatin Study Group. Digestion 1993;54 Suppl 1:72–5.
Janson ET, Oberg K. Long-term management of the carcinoid syndrome. Treatment with octreotide alone and in combination with alpha-interferon. Acta Oncol 1993;32:225–9.
Ducreux M, Ruszniewski P, Chayvialle JA, Blumberg J, Cloarec D, Michel H, et al. The antitumoral effect of the long-acting somatostatin analog lanreotide in neuroendocrine tumors. Am J Gastroenterol 2000;95:3276–81.
Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27:4656–63.
Bodei L, Cremonesi M, Zoboli S, Grana C, Bartolomei M, Rocca P, et al. Receptor-mediated radionuclide therapy with 90Y-DOTATOC in association with amino acid infusion: a phase I study. Eur J Nucl Med Mol Imaging 2003;30:207–16.
Waldherr C, Pless M, Maecke HR, Haldemann A, Mueller-Brand J. The clinical value of [90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol 2001;12:941–5.
Waldherr C, Pless M, Maecke HR, Schumacher T, Crazzolara A, Nitzsche EU, et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq (90)Y-DOTATOC. J Nucl Med 2002;43:610–6.
Valkema R, Pauwels S, Kvols LK, Barone R, Jamar F, Bakker WH, et al. Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0, Tyr3]octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med 2006;36:147–56.
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0, Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008;26:2124–30.
Kwekkeboom DJ, de Herder WW, van Eijck CH, Kam BL, van Essen M, Teunissen JJ, et al. Peptide receptor radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med 2010;40:78–88.
Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol 2011;29:2416–23.
Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC; 2010.
Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes JM, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2007;451:757–62.
Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2006;449:395–401.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16.
Bajetta E, Catena L, Procopio G, De Dosso S, Bichisao E, Ferrari L, et al. Are capecitabine and oxaliplatin (XELOX) suitable treatments for progressing low-grade and high-grade neuroendocrine tumours? Cancer Chemother Pharmacol 2007;59:637–42.
Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG, Eastern Cooperative Oncology Group. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol 2005;23:4897–904.
Kouvaraki MA, Ajani JA, Hoff P, Wolff R, Evans DB, Lozano R, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 2004;22:4762–71.
Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501–13.
Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514–23.
Panzuto F, Boninsegna L, Fazio N, Campana D, Pia Brizzi M, Capurso G, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol 2011;29:2372–7.
Panzuto F, Campana D, Fazio N, Brizzi MP, Boninsegna L, Nori F, et al.: Risk factors for disease progression in advanced jejunoileal neuroendocrine tumors. Neuroendocrinology 2012;96:32–40.
Bettini R, Boninsegna L, Mantovani W, Capelli P, Bassi C, Pederzoli P, et al. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann Oncol 2008;19:903–8.
Panzuto F, Nasoni S, Falconi M, Corleto VD, Capurso G, Cassetta S, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer 2005;12:1083–92.
Scarpa A, Mantovani W, Capelli P, Beghelli S, Boninsegna L, Bettini R, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol 2010;23:824–33.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Gianfranco Delle Fave and Massimo Falconi share the senior authorship.
Rights and permissions
About this article
Cite this article
Campana, D., Capurso, G., Partelli, S. et al. Radiolabelled somatostatin analogue treatment in gastroenteropancreatic neuroendocrine tumours: factors associated with response and suggestions for therapeutic sequence. Eur J Nucl Med Mol Imaging 40, 1197–1205 (2013). https://doi.org/10.1007/s00259-013-2402-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2402-2