Abstract
To evaluate the efficacy and safety of a combined regimen of bendamustine (B) and rituximab (R) in Japanese patients with relapsed/refractory (r/r) indolent B-cell non-Hodgkin lymphomas (B-NHLs) and mantle cell lymphoma (MCL). Patients aged 20–79 years with pathologically confirmed B-NHLs or MCL, which were r/r after 1–2 R-containing regimens, were included in this study. The BR regimen consisted of B (90 mg/m2) for two consecutive days and R (375 mg/m2) on day 1, 2, or 3. The course was repeated every 4 weeks for up to four cycles. Fifty-three patients were enrolled in this study and analyzed. The diagnosis included follicular lymphoma (FL) (77 %), mucosa-associated lymphoid tissue lymphoma (13 %) and others (10 %). Forty-seven (90 %) patients completed four cycles of treatment as per schedule. Best overall response rate (ORR) and complete response rate (CRR) was 94 and 71 %, respectively (for FL, ORR 95 % and CRR 80 %). The treatment was well tolerated and the primary toxicity was myelosuppression; the incidence of grade 3/4 leukopenia and neutropenia were 42 and 40 %, respectively. There were no grade 5 toxicities. The BR regimen is safe in Japanese patients with r/r indolent B-NHLs and MCL, and is effective for those with r/r indolent B-NHLs. For the evaluation of late toxicity, especially infection, longer follow-up of this cohort is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indolent B-cell non-Hodgkin lymphomas (B-NHLs) and mantle cell lymphoma (MCL) are slowly but steadily progressing lymphoma. Although the combination of chemotherapy with rituximab has significantly improved the response rate as well as survival [1], most patients still continue to relapse and become refractory to currently available treatment, such as cladribine [2], fludarabine [3], immunoradiotherapy [4], and high-dose chemotherapy [5]. Therefore, the effective treatment for relapsed or refractory indolent B-NHLs and MCL is eagerly needed.
Bendamustine is one of the promising drugs in this setting because of its low cross-resistance feature and its safer toxicity profiles, and the results of several phase II studies assessing the efficacy and toxicity of bendamustine for refractory and/or relapsed indolent B-NHL and/or MCL have been reported [6–9]. 120 mg/m2 of bendamustine monotherapy has reported to be effective and safe for relapsed or refractory indolent B-NHL and/or MCL in Caucasian [6] and Japanese [7, 10]. However, severe myelotoxicity would be the risk in this dosage. In vitro experiments with CD20-positive lymphoma cell lines demonstrated synergy between bendamustine and rituximab [11], and two clinical studies showed that rituximab-combined bendamustine regimen, in which lower dosage of bendamustine were applied, is effective and safe for relapsed and/or refractory B-NHL and MCL in Caucasian [8]. Based upon these data, reduced dose of bendamustine to 90 mg/m2 when combined with rituximab is currently recommended as an optimal dosage in international consensus panel of bendamustine treatment for follicular/low-grade NHL [12]. It also describes that in the relapsed/refractory setting, 4–6 cycles can be delivered as tolerated [12]. However, the optimal dose and treatment cycles of the combination with bendamustine plus rituximab (BR) have not been fully elucidated in Japanese population. Therefore, we designed and conducted this prospective study to investigate the efficacy and safety of BR regimen for relapsed or refractory indolent B-NHLs and MCL in Japanese patients.
Patients and methods
Study design and objective
The aim of this multicenter, open-label, single-arm, phase II clinical study is to determine the efficacy and safety of combination chemotherapy with BR in Japanese patients with relapsed or refractory indolent B-NHLs and MCL. Primary endpoint of this study was the best overall response rate (ORR) up to the end of chemotherapy. Secondary endpoint included safety, complete response rate (CRR), progression-free survival (PFS), and overall survival (OS). This study was approved by the institutional review board of each participating center and institutional review board-approved consent form was obtained before participating in this study from all patients.
Eligibility
Patients age ≥20 and <80 years with a ECOG performance status of 0–2 were eligible for this study. Patients must have previously treated, histologically documented CD20-positive indolent B-NHLs or MCL that failed to respond to, or relapsed after, a maximum of two prior chemotherapy regimens. The regimens must contain rituximab. Confirmation of CD20 positivity and histology at relapse or at the time of enrollment was not mandatory if those had been previously confirmed. Patients were required to have bi-dimensionally measurable disease with at least one lesion measuring ≥1.5 cm in a single dimension. Patients were also required to have adequate blood cell counts (absolute neutrophil count ≥1,500 cells/μL and platelet count ≥100,000 cells/μL, and Hb ≥9.0 g/dL), adequate renal function (≤1.5× the upper limit of the normal range; ULN for creatinine) and hepatic function (≤2.5× ULN for AST, ALT, and ALP and ≤1.5× ULN for total bilirubin), and expected survival was more than 3 months. ECG was required to be normal or minor abnormalities not requiring specific treatment.
Patients were excluded if they were pregnant or lactating, or had psychiatric diseases that may affect the study results. Patients were also excluded if they had moderate or severe comorbidity, malignancies of disease-free period less than 5 years other than their primary lymphomas, CNS involvement, lymphoma cells less than 25,000/μL in the peripheral blood, previous treatment with bendamustine. At the time of enrollment, patients with histological transformation diagnosed histologically or clinically [13, 14] were excluded. Previous history of hepatitis B virus infection was not excluded if HBsAg remained negative and patients were to be adequately monitored for viral reactivation according to the currently available guidelines [15, 16].
Treatment
Baseline evaluation included medical history and physical examination, complete blood count, serum electrolytes and blood chemistry, bone marrow examination, and tumor staging using contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). BR regimen consisted of bendamustine (90 mg/m2) for two consecutive days and rituximab (375 mg/m2) on either day 1, 2, or 3 (Fig. 1). Any one of these four combinations could be selected according to the clinical convenience of each institution. The course was repeated every 4 weeks for 4 cycles. If grade 3 or 4 non-hematologic or grade 4 hematologic toxicity, especially 7 or more days of neutropenia or 3 or more days of febrile neutropenia, occurred according to the Common Terminology Criteria for Adverse Events (CTCAE) (version 4.0), the dose of bendamustine was reduced to 60 mg/m2 in the subsequent cycle. If the similar severity of toxicity occurred at the reduced dose, study treatment was discontinued. Primary prophylactic use of granulocyte colony-stimulating factor (G-CSF) was discouraged; however, treatment was allowed for prolonged neutropenia. If toxicities remained at ≥grade 2 in neutropenia and thrombocytopenia, ≥grade 3 in the other toxicities except for fatigue, nausea, vomiting, anorexia, hyponatremia, and lymphopenia, the next cycle was postponed. If recovery was not evident within 2 weeks of a scheduled treatment, the study treatment was discontinued. The decision of whether the patients continued to be treated or followed without any further treatments until progression was made by each investigator.
Assessment criteria and follow-up
Response was assessed by including enhanced CT or MRI between the day 5 and day 28 of the second cycle and the day 5 and day 36 of the fourth cycle or the end of study treatment in case of discontinuation. Overall response were classified into complete response (CR), unconfirmed complete response (CRu), partial response (PR), stable disease (SD), or progression disease (PD) according to International Working Group Response Criteria for NHL [17], using the same imaging method (CT or MRI) used to establish baseline tumor measurements. Then progression was monitored by each investigator including with enhanced CT or MRI every 6 months for 3 years and every year for 2 years thereafter. PFS was defined as the time from the first dose of study drug to the first documentation of disease progression or death. The case which received allogeneic-hematopoietic stem cell transplantation (HSCT) as a subsequent treatment was censored on the day of transplantation. OS was defined as the time from the first day of BR therapy to the date of death of any causes. Laboratory assessments were performed at baseline and just prior to the each cycle. The severity of adverse events was determined and recorded using CTCAE version 4.0. Data for adverse events were collected until 15 weeks after the end of study treatment or until the initiation of subsequent treatment. Second malignancy was defined as any malignancies observed after induction of BR treatment.
Statistical methods
We hypothesized that treatment with BR regimen in our population would produce an ORR ≥75 %. On the basis of prior studies indicating an ORR of 91–75 % after single-agent rituximab, a sample size of 40 patients was planned to yield more than 80 % power (using an overall, two-sided, 5 % significance level) to detect an increase of 20 % in ORR after treatment with BR. ORR was calculated as the number of patients achieving a best response of CR, CRu, or PR divided by the number of patients treated with at least one dose of bendamustine or rituximab. CRR was calculated as the number of patients achieving a best response of CR or CRu divided by the number of patients treated with at least one dose of bendamustine or rituximab. A two-sided 95 % exact CI for ORR was calculated using the binominal distribution. The Kaplan–Meier method was used to estimate median PFS, and two-sided 95 % CI were calculated using the Brookmeyer–Crowley nonparametric method. Treatment delay was defined as that the start of next cycle was beyond 31 days since the start day in the current. Absolute dose intensity of bendamustine (mg/m2/week) was calculated for each patient as the sum of doses administered divided by the number of weeks in the treatment period. Relative dose intensity for each agent (%) was then calculated as the dose intensity was divided by the weekly intended dose and then multiplied by 100.
Results
Patients disposition and characteristics
Fifty-three patients were enrolled into this study at Keio University Hospital and ten affiliated hospitals in Japan from April 2011 to March 2013. Data were fixed on 31th July in 2013 with the median follow-up period of 14 (6.9–21) months. One patient with follicular lymphoma (FL) in the small intestine was excluded as it had no measureable disease after enrollment. Patient characteristics are shown in Table 1. Median age was 67 years (range 59–71). ECOG performance status (PS) was 0 in 47 (90 %) patients and 1 in 5 (10 %). Hepatitis B antibody was positive in ten (19 %). Sixteen patients (31 %) underwent re-biopsy at relapse or at the time of study enrollment to confirm CD20 positivity and histology without transformation. The diagnosis included 40 FL (77 %), 7 mucosa-associated lymphoid tissue lymphoma (MALT) (13 %), 3 MCL (6 %), and one lympho-plasmacytic lymphoma (LPL) (2 %), and one indolent B-cell lymphoma not otherwise specified (2 %). Forty patients had relapsed diseases and 11 had refractory diseases. Thirty-nine patients (75 %) were in Stage III/IV at the study entry and 16 (31 %) had bone marrow involvement. Number of prior treatment regimens was one in 32 (62 %) patients and two in 20 (38 %). The first treatment included rituximab plus chemotherapy in 36 (69 %), rituximab alone in 14 (27 %), and other regimens in 2 (4 %), (melphalan and prednisolone and CHOP one each). The second regimen included 11 rituximab monotherapy (55 %), 8 rituximab plus chemotherapy (40 %), and one fludarabine monotherapy (5 %). Prior radiotherapy was given in 5 patients (10 %). Median time from prior treatment to the first cycle of BR was 15 (8.0–34) months and the duration was less than 3 months in five (10 %).
Efficacy
ORR as the best response was 94 %, including 40 % CR, 31 % CRu, 23 % PR and 6 % SD (Table 2). In 40 patients with follicular lymphoma, ORR was 95 % including 45 % CR, 35 % CRu, 15 % PR, and 5 % SD. Seven patients with MALT showed ORR of 100 % including 43 % CR, 14 % CRu, and 43 % PR. Three patients with MCL exhibited an ORR of 100 %, including 33 % CRu, and 67 % PR. The patients with LPL and the other indolent B-cell lymphoma exhibited PR and stable disease, respectively. Response in five patients, whose duration from the last prior treatment to the start of BR was less than 3 months, were one CRu in one, PR in 2, and SD in 2. The ORR and CRR in patients without MCL were 94 and 73 %, respectively.
Regarding the subsequent treatment after the completion of study treatment until progression, forty-three were closely observed without any additional treatments, eight continued to receive additional courses of BR up to 4 cycles (one cycle of BR in 1 case, two cycles in 5, three cycles in 1 and four cycles in 1). One patient underwent allogeneic-HSCT.
The patient with unclassified indolent B-cell lymphoma had disease progression after two cycles of BR in which the best response was SD, and discontinued study treatment as per protocol. One patient with FL obtained CR at the second cycle, but the response was judged as PD at the fourth cycle.
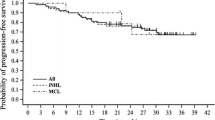
Median PFS was 17.95 months (95 % CI 15.91 to NA) and 17.75 months (95 % CI 14.66 to NA) for 48 patients with indolent B-NHLs and 39 patients with FL, respectively (Fig. 2). For 42 patients with indolent B-NHLs and 33 patients with FL without receiving subsequent treatment until progression median PFS had not yet been reached (95 % CI 14.53 to NA), and 17.75 months (95 % CI 11.05 to NA), respectively. In both analyses one patient who underwent allogeneic-HSCT was excluded. Only one patient died of disease progression with clinical decision of histological transformation during the follow-up period.
Kaplan–Meier curves of progression-free survival (PFS) in patients with indolent B-NHLs excluding one patient who underwent allogeneic-HSCT as subsequent treatment (n = 48) (thick line) and in those for follicular lymphoma excluding the one (n = 39) (thin line). B-NHLs B-cell non-Hodgkin lymphomas, HSCT hematopoietic stem-cell transplantation
Safety
Forty-seven patients (90 %) received four cycles of treatment. Of the total 202 patient cycles administered, cycles could not be started as per scheduled in 35 (17 %); however, in 97 % of cycles the delay was less than 14 days. The mean relative dose intensity for bendamustine was 93 %. Bedamustine was discontinued before completing four cycles in five cases because of hematological adverse events (n = 3), non-hematological adverse event (n = 1), and disease progression (n = 1).
The most frequently observed toxicities were mild nausea (48 %) and reversible myelosuppression (81 %); grade 3 or 4 neutropenia was observed in 21 cases (40 %), including eight (15 %) grade 4 toxicity (Table 3). Prophylactic anti-emetics were administered in almost all cases (98 %). G-CSF was administered in 20 cases (38 %). The rest of grade 3/4 toxicities were anemia (2 %), ileus/enteritis (2 %), renal (2 %) and hepatic (2 %) toxicity, hyponatoremia (2 %), nausea (2 %), rash (2 %) and venous thrombosis (2 %). There were no grade 5 toxicities (Tables 3, 4).
One patient with FL developed grade 4 neutropenia in the second cycle and did not receive further BR cycle because of insufficient recovery from the toxicity within 42 days after the day of commencing the third cycle. This patient achieved CR and it was maintained during 9.5 months of follow-up. One patient with FL developed grade 3 ileus/enteritis during the first and second cycles of BR, and its toxicity did not recover in 42 days after starting the second cycle, and the BR was discontinued. In this patient, BR was restarted after remaining progression-free for 13.5 months. One patient developed grade 3 renal damage during the first cycle. It recovered within 42 days, and the second cycle was started with reduced dose of bendamustine of 60 mg/m2, and the patient could complete four cycles without recurrence of the toxicity. This patient achieved PR and remains disease-free during 13-month observation. One patient developed grade 3 hepatic enzyme elevations in the second cycle and the toxicity grade decreased to grade 2, but the toxicity recurred after the third cycle and did not recover from grade 3. This case achieved CR which was maintained for 16.5 months during observation. One patient developed hyponatremia but could continue its cycles without any delay from the protocol without exacerbation of hyponatremia. One patient developed rash in the third cycle. It did not recover within 42 days and required the systemic administration of steroid. As the treatment for rash may have affected the efficacy evaluation of BR, this case was taken off from the study treatment. This case achieved PR and there was no progression during 4-month observation. One patient with FL developed venous thrombosis in her right leg during the second cycle which seemed to cause by lymphoma mass in its right iliac area. Anticoagulation was started and the toxicity recovered to grade 2 within 42 days and the case received the third cycle with reduced dose of bendamustine of 60 mg/m2. The patient completed 4 cycles of BR and received allogeneic-HSCT 7 months after remaining in PR.
Infection requiring hospitalization was observed in one patient (2 %). Trimethoprim–sulfamethoxazole or pentamidine for the prevention of pneumocystis pneumonia was given in 80 % of patients and acyclovir for the prophylaxis of herpes infection was given in 30 % of patients. No patients developed pneumocystis pneumonia and only one patient (2 %) developed herpes infection. No second malignancies were observed during the observation period.
Discussion
This is a non-GCP-based multi-institutional phase II study. Our study enrolled relapsed or refractory cases who had been administered one or two rituximab-containing regimens as prior treatment. We decided prior rituximab to be essential for accordance with practice in current treatment for indolent B-NHL and MCL patients in Japan. In the setting of relapsed or refractory B-NHLs and MCL, potentially advanced diseases, standard treatment regimen has not been established yet. In our study, thirteen patients (25 %) of Stage I/II localized disease were selected for systemic treatment of BR under investigator’s decision.
Robinson et al. reported the efficacy and safety of BR regimen for relapsed indolent B-NHLs and MCL [9]. Their study was designed to evaluate the best ORR and toxicities during up to 6 cycles of BR. Their treatment also included two more additional doses of rituximab both in pre- and post-BR. Patient characteristics; FLIPI score of FL patients, and number of prior regimens were basically similar to those of our study. However, there are some differences between two studies in addition to the difference in the treatment schedule. In Robinson’s study 44 % of patients were rituximab-naïve and did not include chemo-resistant patients. Histologic subtype of lymphoma included 15 % of small lymphocytic lymphoma and more MCL than our study probably reflecting racial differences. Our study included only 3 patients with MCL so that we could not evaluate the efficacy of BR in patients with MCL.
Reported ORR in Robinson’s study of 93 % is comparable to 94 % in our study, and CRR seemed to be slightly better in our study (73 vs 54 %) in indolent B-NHLs. This may be explained by the difference in the proportion of histologic subtype which likely achieves CR or CRu such as FL or MALT. The male/female ratio of patients may also affect the outcome as recent studies suggested that female had better outcomes when treated with rituximab than male [18, 19]. However, better CR rate did not seemed to be related to longer PFS, that is, median PFS in indolent B-NHLs was 17.95 months in our study, and the median PFS reported by Robinson et al. was 22.92 months (including MCL due to absence of specific data of PFS for MCL). PFS curve was roughly the same. Five months difference of median PFS between two studies appears to be more affected with median number of cycles of BR, 4 vs 6, than with male/female ratio. However, we cannot exclude that patients with MCL possessed prolonged PFS.
In our study, forty-seven patients (90 %) received scheduled cycles of treatment and the mean relative dose intensity for bendamustine was 93 %. Only one patient could not complete four cycles of BR because of hematological toxicity. The incidence of grade 3/4 neutropenia was observed in our study was almost the same as observed in Robinson’s study (40 vs 37 %). However, febrile neutropenia was not observed in our study and the differences might be explained by the positive prophylactic use of G-CSF. G-CSF was used in 38 % of patients in our study, but only 12 % in their study. Prior treatments including purine-analog (13 % in our study vs. 23 % in their study) may have also affected sustained severe neutropenia leading to febrile neutropenia. Frequency of severe infection was also low in our study, but Robinson reported six grade 3/4 infections (10 %). Frequent use of prophylactic trimethoprim–sulfamethoxazole and acyclovir together with G-CSF mentioned above in our study may contribute to infection rate of 2 % with grade 3/4 and 10 % with all grades. In addition, fewer cycle of BR suppressed immune function less than six cycles of BR, and may also explain the low infection rate. Although the comparison of different study population and the shorter follow-up limits to draw any firm conclusions, these results suggest that four cycles of BR are less toxic in patients with indolent B-NHLs and MCL, however, might be less effective in PFS than 6 cycles of BR in patients with indolent B-NHLs who had received a few prior treatments and had comorbid less than moderate severity. To clarify this, a randomized study comparing 4 cycles to 6 cycles of BR is warranted.
Although the efficacy and safety of bendamustine has not been fully evaluated in Japanese patients with indolent B-NHLs and MCL, Ohmachi et al. reported their experience with bendamustine monotherapy at a dose of 120 mg/m2 in the same population [7]. Their study evaluated best ORR and toxicities for up to 6 cycles of bendamustine monotherapy. Patient characteristics were similar to ours; however, their study included more refractory cases and more MCL and less MALT compared with our study.
The ORR and CRR are slightly better in our study than Ohmachi’s (ORR; 94 vs 90 %, CRR; 73 vs 66 %, respectively) in patients with indolent B-NHLs. This comparable CRR may be explained by sufficient dose of 90 mg/m2 of bendamustine with addition of rituximab in Japanese population. In subgroup analysis, they showed inferior response rate in the group received less than three cycles of bendamustine (71 % of ORR and 41 % of CRR), whose planned dose intensity of bendamustine fell behind ours (45 mg/m2/week). This suggests that at least 4 cycles of 90 mg/m2 of bendamustine plus rituximab are required for obtaining optimal response rate. Estimated PFS rate at 1 year was 79 % (95 % CI 61–89 %) in ours and 70 % in theirs. However, our median PFS was 17.95 months after median follow-up time of 14 months, and theirs had not reached after the comparable median follow-up of 13 months. Better CRR does not seem to be related to the maintained PFS. Considering the fact that estimated calculated median treatment duration is similar, 112 weeks in ours and 105 weeks in theirs, those results may show deeper response in 120 mg/m2 of bendamustine monotherapy by six cycles than 90 mg/m2 bendamustine with rituximab for four cycles.
However, 120 mg/m2 of bendamustine monotherapy would be more toxic than 90 mg/m2 bendamustine plus rituximab for indolent B-NHLs and MCL Japanese patients. Although the use of G-CSF seems comparable between two studies (G-CSF was used in 31 % in our study and 39 % in theirs), grade 3/4 neutropenia was observed in 72 % patients including 48 % grade 4 neutropenia. The incidence was much higher than those in our study. Febrile neutropenia was not observed in our study, but one in theirs. Grade 3/4 thrombocytopenia was not detected in our study but 16 % in theirs. Non-hematological toxicities were also more frequent in their study. Higher incidence of toxicities associated with their study compromised the intensity of monotherapy.
Median completed number of treatment cycles of their study was five, and 13 patients (22 %) needed dose reduction of bendamustine. The rate of dose reduction was twice as much as observed in our study (11 %). The mean relative dose intensity for bendamustine was 93 % in ours and 88 % in theirs. Forty-seven patients (90 %) achieved four cycles of BR in our study, however, 19 patients (28 %) required the early discontinuation of treatment because of adverse events mainly myelosuppression in Ohmachi’s. In contrast, the study treatment was discontinued in only 8 % of patients before completing four cycles from adverse events in our study.
Based upon the above comparison between our study and two other studies on the treatment of relapsed and/or refractory indolent B-NHLs and MCL with bendamustine, optimal use of bendamustine in this setting may be concluded as follows: In case achieving good response with significant toxicity after initial four cycles of BR, additional BR treatment cycles could not be recommended, and retreatment [20] strategy will be recommended at the time of disease recurrence or progression. In contrast, additional cycles of BR will be warranted for obtaining deeper response in cases with good response and less toxicity after four cycles of BR. We currently perform clinical study to assess the efficacy and toxicity of retreatment strategy in Japanese patients with indolent B-NHLs and MCL.
In conclusion, BR is safe treatment in Japanese patients with relapsed or refractory indolent B-NHLs and MCL and effective for those with relapsed or refractory indolent B-NHLs who had no more than two prior treatments containing rituximab and no moderate or severe comorbidity. Longer follow up of this cohort is clearly warranted to clarify the late toxicities of this treatment, especially late infectious complication and occurrence of secondary malignancies.
References
Schulz H, Bohlius JF, Trelle S, Skoetz N, Reiser M, Kober T, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2007;99:706–14.
Ogura M, Morishima Y, Kobayashi Y, Uike N, Sugai S, Chou T, Cladribine Study Group, et al. Durable response but prolonged cytopenia after cladribine treatment in relapsed patients with indolent non-Hodgkin’s lymphomas: results of a Japanese phase II study. Int J Hematol. 2004;80:267–77.
Tobinai K, Watanabe T, Ogura M, Morishima Y, Ogawa Y, Ishizawa K, et al. Phase II study of oral fludarabine phosphate in relapsed indolent B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2006;24:174–80.
Tobinai K, Watanabe T, Ogura M, Morishima Y, Hotta T, Ishizawa K, et al. Japanese phase II study of 90Y-ibritumomab tiuxetan in patients with relapsed or refractory indolent B-cell lymphoma. Cancer Sci. 2009;100:158–64.
Schouten HC, Qian W, Kvaloy S, Porcellini A, Hagberg H, Johnson HE, et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin’s lymphoma: results from the randomized European CUP trial. J Clin Oncol. 2003;21:3918–27.
Kahl BS, Bartlett NL, Leonard JP, Chen L, Ganjoo K, Williams ME, et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study. Cancer. 2010;116:106–14.
Ohmachi K, Ando K, Ogura M, Uchida T, Itoh K, Kubota N, Japanese Bendamustine Lymphoma Study Group, et al. Multicenter phase II study of bendamustine for relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci. 2010;101:2059–64.
Rummel MJ, Al-Batran SE, Kim SZ, Welslau M, Hecker R, Kofahl-Krause D, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:3383–9.
Robinson KS, Williams ME, van der Jagt RH, Cohen P, Herst JA, Tulpule A, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:4473–9.
Ogura M, Uchida T, Taniwaki M, Ando K, Watanabe T, Kasai M, Japanese Bendamustine Lymphoma Study Group, et al. Phase I and pharmacokinetic study of bendamustine hydrochloride in relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci. 2010;101:2054–8.
Chow KU, Sommerlad WD, Boehrer S, Schneider B, Seipelt G, Rummel MJ, et al. Anti-CD20 antibody (IDEC-C2B8, rituximab) enhances efficacy of cytotoxic drugs on neoplastic lymphocytes in vitro: role of cytokines, complement, and caspases. Haematologica. 2002;87:33–43.
Cheson BD, Wendtner CM, Pieper A, Dreyling M, Friedberg J, Hoelzer D, et al. Optimal use of bendamustine in chronic lymphocytic leukemia, non-Hodgkin lymphomas, and multiple myeloma: treatment recommendations from an international consensus panel. Clin Lymphoma Myeloma Leuk. 2010;10:21–7.
Bastion Y, Sebban C, Berger F, Felman P, Salles G, Dumontet C, et al. Incidence, predictive factors, and outcome of lymphoma transformation in follicular lymphoma patients. J Clin Oncol. 1997;15:1587–94.
Al-Tourah AJ, Gill KK, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:5165–9.
Tsubouchi H, Kumada H, Kiyosawa K, Mochida S, Sakaida I, Tanaka E, et al. Prevention of immunosuppressive therapy or chemotherapy-induced reactivation of hepatitis B virus infection—joint report of the Intractable Liver Diseases Study Group of Japan and the Japanese Study Group of the standard antiviral therapy for viral hepatitis. Kanzo. 2009;50:38–42.
Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology. JSH Guidelines for the Management of Hepatitis B Virus Infection. Hepatol Res. 2014;44(Suppl S1):1–58.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17:1244–53.
Jäger U, Fridrik M, Zeitlinger M, Heintel D, Hopfinger G, Burgstaller S, et al. Rituximab serum concentrations during immunochemotherapy of follicular lymphoma correlate with patient gender, bone marrow infiltration and clinical response. Haematologica. 2012;97:1431–8.
Riihijärvi S, Taskinen M, Jerkeman M, Leppä S. Male gender is an adverse prognostic factor in B-cell lymphoma patients treated with immunochemotherapy. Eur J Haematol. 2011;86:124–8.
Weide R, Feiten S, Friesenhahn V, Heymanns J, Kleboth K, Thomalla J, et al. Retreatment with bendamustine-containing regimens in patients with relapsed/refractory chronic lymphocytic leukemia and indolent B-cell lymphomas achieves high response rates and some long lasting remissions. Leuk Lymphoma. 2013;54:1640–6.
Acknowledgments
The authors thank to the patients who participated in this multicenter study, and to the following collaborators who have substantially contributed to data acquisition: Norihide Sato, Hideo Uchida, Takahide Kikuchi, Akihiro Yokoyama, Saigen Boku, Hiroshi Murakami, Taku Kikuchi, Eri Matsuki, Jo Ishizawa, Tomoki Ueda, and Takehiko Mori. We also thank the staffs at the Keio Center for Clinical Research for their technical assistance in data management.
Conflict of interest
Authors have no conflict of interests to declare. Dr. Shinichiro Okamoto received research fund from Eisai Co., Ltd. in 2010, not relevant to this work.
Author information
Authors and Affiliations
Consortia
Corresponding author
About this article
Cite this article
Matsumoto, K., Takayama, N., Aisa, Y. et al. A phase II study of bendamustine plus rituximab in Japanese patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma previously treated with rituximab: BRB study. Int J Hematol 101, 554–562 (2015). https://doi.org/10.1007/s12185-015-1767-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-015-1767-3