Abstract
Purpose
To evaluate the efficacy and tolerability of systematic treatment of unresectable advanced or metastatic gastric cancer (A/MGC) based on EOF5 regimen (the combination of epirubicin, oxaliplatin and 5-day continuous infusion of 5-fluorouracil).
Patients and methods
Twenty-six patients (18 males, 8 females; age range, 35–72 years) with histologically confirmed metastatic (n = 23) or unresectable advanced (n = 3) gastric adenocarcinoma with (n = 6) or without previous chemotherapy (n = 20) were consented to receive EOF5 (epirubicin 50 mg/m2 and oxaliplatin 130 mg/m2 on day 1, followed by continuous infusion of 5-fluorouracil 375–425 mg/m2 day−1 on day 1–5), and the treatment cycle was repeated every 3 weeks. Responses to treatment and toxicity were evaluated every 2 cycles.
Results
In the first-line treatment group of 20 patients, complete (CR) and partial (PR) remission were observed in two (10%) and six (30%) patients, respectively with an overall response rate of 40%). Eleven (55%) patients showed stable (SD) and one (5%) progressive disease (PD). One-year survival rate, time to progression (TTP) and median overall survival (OS) were 45%, 9.7 and 12.5 months, respectively. In the second-line treatment group of six patients, the numbers of CR, PR, SD and PD were 0, 1, 4 and 1, respectively. Symptomatic response rates were 88.2, 76.9, 89.5, and 88.9% for abdominal pain, distention, anorexia and weight loss. The mean Karnofsky performance status score was increased (P < 0.001) and maintained after two and four cycles treatment. The major adverse events were nausea/vomiting, oral mucositis, peripheral neuropathy, phlebitis, constipation and myelosuppression. CTC grade 3 or 4 hematologic toxicities included leucopenia (7.7%), neutropenia (15.4%), thrombocytopenia (19.2%), and anemia (3.8%). No treatment-related deaths were recorded.
Conclusions
EOF5 regimen shows good efficacy and an acceptable safety profile in A/MGC patients, and would be a suitable alternative regimen for this indication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the second most common fatal malignancy in the world, and more than 60% of these cases were diagnosed in the developing country (Parkin 1998). About 934,000 new cases were reported worldwide in 2002. Gastric cancer causes more than 700,000 death annually (Parkin et al. 2005), with Chinese accounting for about 380,000 of these new cases and approximately 300,000 death annually (Yang 2006). There has been little progress in the therapy for patients with advanced gastric cancer; only a few large randomized phase III trials have been conducted over the past decade (Waters et al. 1999; Ohtsu et al. 2003; Vanhoefer et al. 2000; Ross et al. 2002). The ECF regimen, combination of epirubicin, cisplatin and 21-day continuous infusion of 5-fluorouracil (5-FU), has shown an consistent efficacy in the treatment of advanced gastric cancer in several phase II and III trials (Waters et al. 1999; Ross et al. 2002; Webb et al. 1997). And now it is considered as a reference regimen for gastric cancer in Europe. However, significant treatment related toxicities were reported in patients receiving ECF, which prevents this protocol from becoming the standard treatment regimen. Oxaliplatin, a third generation platinum compound with the 1,2-diaminocyclohexane (DACH) carrier ligand, has a higher preclinical antitumoral potency than cisplatin in models such as HT29 colon cell line (Pendyala et al. 1993), and has demonstrated synergy with 5-FU in vitro, in vivo, and in the clinical setting in advanced colorectal cancer (Raymond et al. 1997; André et al. 1999). It presents a better toxicity profile than cisplatin, with the main and dose-limiting toxicity being only acute, cumulative short-term sensory peripheral neurotoxicity, resulting in acral paresthesia/dysesthesia, exacerbated by cold (Extra et al. 1990). Recently some studies used combination regimens of oxaliplatin, folinic acid, and continuous infusion 5-FU for about 44 h (e.g., FOLFOX4) to treat advanced or metastatic gastric cancer (A/MGC), and yielded response rates in the range of 38–43% and median overall survival times of approximately 10 months (range 9.6–11.2) (Louvet et al. 2002; De Vita et al. 2005; Lordick et al. 2005). These oxaliplatin containing regimens resulted in lower rates of grade 3–4 adverse events. Therefore, it is logical to modify ECF regimen, with oxaliplatin replacing cisplatin and a short-term FU infusion replacing 21-day FU infusion. The aim of this clinical trial was thus to evaluate the activity and tolerability of a combined regimen–EOF5, containing epirubicin 50 mg/m2 day−1, oxaliplatin 130 mg/m2 day−1 and 5-FU 375–425 mg/m2 day−1 on day 1–5 continuous infusion, repeated every 3 weeks, in patients with A/MGC.
Patients and methods
Patient eligibility
Patients were eligible for the trial if they met the following inclusion criteria: with histologically confirmed advanced or metastatic adenocarcinoma of the stomach, at least one measurable lesion (larger than 10 mm in diameter by spiral CT scan), Karnofsky performance status higher than 60 (equal to Eastern Cooperative Oncology Group performance scale 0–2) (Webb et al. 1997; Lordick et al. 2005; Rubin et al. 2001) and adequate hepatic, renal, heart, and hematologic functions (platelets > 80 × 109/L, neutrophil > 2.0 × 109/L, serum creatinine ≤ 1.5 mg/dL, total bilirubin within the upper limit of normal (ULN), and serum transaminase ≤ 2.5 × the ULN). Major exclusion criteria were concurrent cancer, neuropathy, brain, or leptomeningeal involvement, uncontrolled significant comorbid conditions and previous radiotherapy. Participants gave written informed consent before they entered the study, which was approved by the Ethic Committee of Cancer Hospital affiliated with Fudan University.
Patient characteristics
A total of 26 patients (18 males, 8 females, age range, 35–72 years with a median of 53 years) were enrolled in the study between September 2004 and March 2006 (Table 1). The majority (65.4%, n = 17) of patients had low or undifferentiated adenocarcinoma. Eight patients (30.8%) had one organ involved, and 18 (69.2%) had two or more organs involved. The most common metastatic sites were the retroperitoneal and perigastric lymph nodes (69.2%, n = 18). Six patients (23.1%) had undergone previous chemotherapy, thus, EOF5 was administered as first-line therapy in 20 (76.9%) patients (Table 1).
Treatment design
All the patients enrolled in this trial (both the first line and the second line) accepted the treatment of EOF5 regimen. Intravenous epirubicin 50 mg/m2 were given on day 1, combined with a 2-h intravenous infusion of oxaliplatin 130 mg/m2, and followed by 5-FU 375–425 mg/m2 day−1 as a 24-h continuous infusion for 5 days. Such treatment cycle was repeated every 3 weeks (one cycle = 21 days), until disease progression, unacceptable toxicity occurred, treatment withdrawal by the patient or doctor’s decision. Antiemetic prophylaxis was given according to local protocols. Patients were asked to avoid cold exposure during the first week of the treatment.
Evaluation of toxicity and dose adjustments
Toxicity was graded according to National Cancer Institute Common Toxicity Criteria (CTC) Version 2.0 (Trotti et al. 2000) depending on the severity of adverse events observed, the chemotherapy was paused, or the dose was reduced. FU infusion was stopped if diarrhea or mucositis of CTC grade 2 or greater happened during treatment period. FU was reduced by 25% in next cycle if diarrhea, mucositis, or hand and foot syndrome of CTC grade 3 or greater occurred. Oxaliplatin was reduced by 25% in next cycle if neuropathy of CTC grade 3 occurred and was terminated if greater neuropathy occurred. The three drugs had to be reduced by 25% simultaneously in case of CTC grade 4 neutropenia, thrombocytopenia or CTC grade 3 neutropenia accompanied by fever or elevated bilirubin levels. Chemotherapy was terminated when one of the following criteria was met: bilirubin level more than 3.5× the ULN, clearance less than 40 mL/min, cardiac ejection less than 50%, or other severe toxicity. Granulocyte-colony stimulating factor (G-CSF) was not planned as a prophylactic aim and was used if patients developed grade 2 or greater neutropenia.
Monitoring of responses
At the entry of the study, a complete medical history was collected and physical examination, complete blood count (CBC), blood chemical examination and tumor assessment were undertaken, Clinical responses were assessed in every two cycles before the start of next cycle, and graded according to WHO criteria (World Health Organization 1979) as complete response (CR, disappearance of all measurable tumor), partial response (PR, greater or equal to 50% regression of all measurable lesions, with no progression of any lesion), stable disease (SD, less than 50% reduction in tumor volume or the lack of progression), progressive disease (PD, the occurrence of any new lesion during treatment or an increase in size by 25% of one or more lesion), and overall response rate (CR + PR). The best response of a patient was the highest classification that was observed on two consecutive assessments. Patients with PR or CR response had to be confirmed by a second assessment not less than 4 weeks apart. Symptom response was recorded before each cycle of chemotherapy. A symptomatic response was defined as the improvement or resolution of particular symptoms including abdominal pain, distention, anorexia and weight loss for a minimum of 3 weeks. Improvement of weight loss was defined as maintenance or increase in the pretreatment weight. Time to progression (TTP) was measured from the day of assignment to first evidence of progression or death occurring. Overall survival was defined from the date of assignment to death from any cause. Karnofsky performance status (KPS) was assessed at the same intervals as tumor assessments (Rubin et al. 2001).
Statistical methods
Analysis was performed using the SPSS 10.0 program. Time-dependent variables (TTP and OS) were estimated with a Log rank test using the Kaplan–Meier method. Multivariate analysis was performed using Cox’s proportional hazard regression model.
Results
Treatment characteristics and dose intensity
Twenty-six patients received a total of 117 treatment cycles. All patients received study treatment for at least two cycles and the median number of cycles administered per patient was 4.5 (range 2–7). Forty-one cycles were delayed, but only thirteen cycles were delayed by the adverse events caused by chemotherapy, such as hematotoxicity. Twenty-eight cycles were delayed for patients did not present in time or other nonmedical reasons. The median days delayed was 2.12 days (range 0–18). Only in two cycles, the doses were reduced because of the hematological toxicity, and the average relative dose intensity of the regimen was 0.93 (range 0.54–1.0).
Efficacy
All patients were assessable for responses and toxicity. Data from characteristics associated responses are listed in Table 2.
When EOF5 was administered as the first-line chemotherapy, the overall response rate was 40%; eight patients had tumor responses, including two (10%) with CR and six (30%) with PR. Eleven (55%) patients had SD and one (6%) patient had PD. The corresponding numbers in the six patients treated with EOF5 as second-line therapy were 0, 1, 4 and 1, respectively (Table 2).
Symptomatic response of abdominal pain, distention, anorexia and weight loss were 88.2% (15/17), 76.9% (10/13), 89.5% (17/19), 88.9% (16/18), respectively. The mean KPS score of the 26 patients was 72.3 ± 10.7 before treatment and 80 ± 6.9 two cycles later. The KPS score after two cycles was significantly higher than that before treatment (P < 0.001). The KPS score maintained after four cycles and six cycles of treatment, and the average KPS was 82.9 ± 5.6 (after four cycles, n = 21) and 83.3 ± 5.0 (after six cycles, n = 9).
Survival
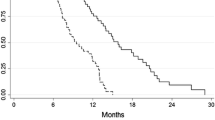
All patients were included in the survival data and analyzed on an intent-to-treat basis. The median follow-up time was 29.1 months (range 19.9–38.8). Follow-up was adequate, as 13 patients of first-line and total 17 (65.4%) patients had died. Overall survival was assessed by Kaplan–Meier analysis reported in Fig. 1. The median time to progress (TTP) and overall survival (OS) of 20 patients in the first-line treated group was 9.7 (95% CI, 5.1–14.3) and 12.5 months (95% CI, 5.1–19.9), respectively, and the 1-year survival rate was 45%. The median survival time of the six patients in the second-line treatment group was 9.5 months (95% CI, 6.1–12.9) (Fig. 2).
It is important to note that seven patients (three with PR, and four with SD), diagnosed in unresectable advanced (n = 2) or metastatic (n = 5) stages, were able to undergo further resectional surgeries after two to four (average 3.6) cycles as first-line treatment. The three PR patients included two patients with metastasis in the rectouterine/rectovesical space, one patient with retroperitoneal metastases. Of the three patients, two patients underwent radical surgery and one received palliative surgery due to multiple abdominal plants. In the two patients who underwent radical surgery, one lived for 17.2 months, and the other was still alive without the disease progression up to 38.8 months at the time of submission of the paper. The survival time of the patient received palliative surgery was 12.5 months (The patient died of cerebral vascular accident). Of the four patients with SD who received surgeries, although none of them had significant reduction (<50%) in primary tumor size as measured on magnetic resonance imaging (MRI), two had complete or remarkable (>50%) metastatic lymph node shrinking in response to EOF5 regimen. At last, three of them underwent palliative surgery for primary tumor. The other one patient, however, underwent exploratory laparotomy only because of the findings of additional multiple abdominal metastases, which were undetectable with the clinical imaging technology.
Toxicity
The major most common adverse events included myelosuppression, nausea/vomiting, constipation, deadlimb, phlebitis and oral mucositis (Table 3). Myelosuppression was usually mild, and the incidence of CTC grade 3 or 4 hematologic toxicities was very low, only included leucopenia (7.7%), neutropenia (15.4%), thrombocytopenia (19.2%), and anemia (3.8%). No patient experienced neutropenic fever. Three patients developed grade I hepatotoxicity after scheduled therapy. None of these patients experienced renal or heart dysfunction. Seven of twelve patients without peripherally inserted central catheter (PICC) experienced grade 2 or higher peripheral phlebitis, while no peripheral phlebitis was observed in the other 14 patients with PICC. Only in one patient, treatment was terminated 24 h prior to the completion of 5-FU infusion at the fourth cycle (the last cycle), owing to numbness in the arms. No patient died of therapy-related complications.
Discussion
Nowadays, chemotherapy has been accepted as a palliative treatment for improvement of survival and of quality of life of metastatic gastric cancer patients. Although the effort to improve the efficacy of combination chemotherapy has never stopped, there is no dramatic progression in this field, and there is still no standard regimen for metastatic gastric cancer.
In the 1990s, a regimen of cisplatin–epirubicin–FU (ECF) produced impressive response rates up to 71% with 12% CR in a phase II setting (Findlay et al.1994; Bamias et al. 1996; Zaniboni et al. 1995), and then it was proposed as a standard practice after the following phase III trial was published (Webb et al. 1997). According to the phase III trial conducted by Webb and his colleagues, ECF regimen showed superior response rates and significantly prolonged survival compared with FAMTX regimen, and the response rates and median survival were 45%, 8.9 months and 21%, 5.7 months, respectively. The response rate and overall survival reached by ECF combination were reproduced in Ross’s phase III trial (Ross et al. 2002) and several phase II trials. Therefore, now ECF is one of the best-investigated regimens for gastric cancer, and is consider as a reference treatment in Europe.
Recently, taxane containing regimens were reported to have good efficacy in metastatic gastric cancer (Roth et al. 2000; Kim et al. 1999). A phase III randomized trial (Moiseyenko et al. 2005) compared DCF regimen (docetaxel, cisplatin and 5-FU) with CF regimen (cisplatin and 5-FU), and the result suggested that DCF was superior to CF. The over all response rate (ORR), time to disease progression (TTP), and over all survival (OS) achieved by DCF and CF were 37%, 5.6 months, 9.2 months versus 25%, 3.7 months, 8.6 months, respectively. However, according to historical comparison the efficacy of DCF was similar to that of ECF. Thus, there is no evidence to conclude that DCF is superior to ECF, and attempts to improve the efficacy and safety profiles of chemotherapy based on ECF regimen are justifiable.
Therefore, after a systemic weighing of the results of some clinical trials using FOLFOX4 to treat patients with MGC (Louvet et al. 2002; De Vita et al. 2005; Lordick et al. 2005), we modified the standard ECF regimen by replacing cisplatin and using 21-day continuous infusion of FU with oxaliplatin and 5-day continuous infusion of FU, based on the hypothesis that the modification may result in less toxicity and better tolerability with equal or even better efficacy.
The shortening of the infusion duration of 5-FU from 21 to 5 days and the replacement of cisplatin with oxaliplatin are likely to contribute to the well tolerability in the present study. The incidence of grade 3/4 toxicity was rather low with neutripenia 15.4%, leucocytopenia 7.7% and thrombocytopenia 19.2%, while in Webb’s trial (Webb et al. 1997) and Ross’s trial (Ross et al. 2002), the incidence were 36, 12, 4 and 32, 13, 4%, respectively. In Webb’s trial treatment delays and dose reductions occurred in 32 and 41% of patients in ECF arm, and one patient died from neutropenic sepsis, while in our trial chemotherapy were delayed only in 27% patinets and dose were reduced in 7.7% patients for chemo-related toxicity. Our data has indicated that the EOF5 regimen has a more desirable hematological toxicity profile than ECF regimen.
Despite mild hematological toxicity, EOF5 regimen showed a very low incidence of severe non-hematological toxicities. Grade 3 or higher of nausea/vomiting and phlebitis only happened in about 2% cycles, respectively. No patient with PICC experienced phlebitis showed that phlebitis can be prevented by PICC. There is no cardiac toxicity, no renal function abnormality or severe hepatic function abnormality recorded in this trial. Although one patient terminated treatment for deadlimb, grade 3 or higher deadlimb only happened in this patient. In addition, no mortality could be attributed to treatment in the current study. So it shows that non-hematological toxicities of our EOF5 regimen were slight and manageable.
The adverse events caused by EOF5 are rather lower than historical control of ECF, but the efficacy does not decreased. As mentioned above, the reported response rates of the original ECF regimen were 42.4–45% by Webb and Ross, respectively. In the present study, equal (45%) response rate was observed. Furthermore, the potential for a curative multimodality approach in responders is noteworthy, because in seven patients six patients had investigator-initiated complementary treatment (radical surgery/palliative surgery), five of them had a over all survival longer than 12 months, and one patient was still alive for 38.8 months at the study cutoff date.
The goal in the treatment of advanced gastric cancer is not only to improve the response rate but also to sustain a longer survival. It is of particular note that 9.7 months median TTP and 12.5 months overall median survival time in the first-line treated group in our study is comparable to those obtained with the original ECF regimen and DCF regimen. Even in second line treatment group, the median overall survival is 9.5 months.
There are also some trials in which FU was administered with a continuous infusion for 5 days, but these trials were reported after we had designed and carried out our trial. We chose the dose of FU 375–425 mg/m2 day−1 for 5 days according to our experience and the results of some phase II trial (Al-Batran et al. 2004; Kim et al. 2003). In 2005, two trials, V325 (Moiseyenko et al. 2005) and FFCD 8801 (Bouché et al. 2005), were reported. In 8801 trial (cisplatin 100 mg/m2 day−1 2 combined with a 5-day continuous infusion of 5-FU 800 mg/m2 day−1), only 48.8% of patients finished more than 80% of the planned dose of FUP regimen. In the trial of V325, FU was given at 750 mg/m2 day−1 for 5 days in DCF arm and 1,000 mg/m2/day for 5 days in CF arm. The grade 3/4 neutropenia, febrile neutropenia (and/or neutropenic infection) and thrombocytopenia in two arms were 82, 29, 8 and 57, 12, 13%, respectively, indicating that the incidence of severe myelosuppression is high in both arms, and the high dose of FU is a important reason. Our EOF5 regimen maintains the efficacy but causes less myelosuppression due to a low dose of FU, which achieves a balance between efficacy and toxicity.
In 2006, Cunningham et al. reported the REAL-2 trial (Cunningham et al. 2006), in which patients were randomized to a 2 × 2 designed trial, in order to prove that capecitabine is better than FU and oxaliplatin is better than cisplatin. About 250 patients were enrolled in EOF arm, and the response rate and PFS were 42.4% and 9.3 months, respectively, which are similar to those obtained with our EOF5 regimen. However, it should be noted that we used a 5-day continuous infusion of FU, and thus our regimen is easier to conduct with a better compliance. In addition, reduction of the time of continuous infusion of FU to 5 days prolongs the intermission period of chemotherapy, which improves patient compliance and reduce the treatment cost.
In conclusion, EOF5 is an innovative and efficient regimen with a favorable toxicity pattern for the treatment of in A/MGC. This regimen may provide an option as palliative chemotherapy, because of its convenience to administer, high activity, and manageable toxicity. Moreover, the ability of this regimen to downstage tumors may enable the resection of some unresectable gastric carcinoma. Based on the results of our phase II trial, we are now proposing a randomized phase III trial to compare the efficacy of EOF5 regimen with ECF regimen in A/MGC patients.
References
Al-Batran SE, Atmaca A, Hegewisch-Becker S, Jaeger D, Hahnfeld S, Rummel MJ, Seipelt G, Rost A, Orth J, Knuth A, Jaeger E (2004) Phase II trial of biweekly infusional fluorouracil, folinic acid, and oxaliplatin in patients with advanced gastric cancer. J Clin Oncol 22:658–663
André T, Bensmaine A, Louvet C, Francois E, Lucas V, Desseigne F, Beerblock K, Bouche O, Carola E, Merrouche Y, Morvan F, Dupont-Andre G, de Gramont A (1999) Multicenter phase II study of bimonthly high-dose leucovorin, 5-fluorouracil infusion and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin regimen (FOLFOX 3/4). J Clin Oncol 17:3560–3568
Bamias A, Hill ME, Cunningham D, Norman AR, Ahmed FY, Webb A, Watson M, Hill AS, Nicolson MC, O’Brien ME, Evans TC, Nicolson V (1996) Epirubicin, cisplatin, and protracted venous infusion of 5-fluorouracil for esophagogastric adenocarcinoma: response, toxicity, quality of life, and survival. Cancer 77:1978–1985
Bouché O, Ychou M, Burtin P, Bedenne L, Ducreux M, Lebreton G, Baulieux J, Nordlinger B, Martin C, Seitz JF, Tigaud JM, Echinard E, Stremsdoerfer N, Milan C, Rougier P (2005) Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the FFCD randomized phase III trial (8801). Ann Oncol 16:1488–1497
Cunningham D, Rao S, Starling N, Iveson T,Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR (2006) Randomised multicentre phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric (OG) cancer: The REAL 2 trial. J Clin Oncol, 2006 ASCO Annual Meeting Proceedings Part I. vol 24, no. 18S (June 20 Supplement), LBA4017
De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, Damiano V, Simeone E, Diadema MR, Lieto E, Castellano P, Pepe S, De Placido S, Galizia G, Di Martino N, Ciardiello F, Catalano G, Bianco AR (2005) A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer 92:1644–1649
Extra JM, Espie M, Calvo F, Ferme C, Mignot L, Marty M (1990) Phase I study of oxaliplatin in patients with advanced cancer. Cancer Chemother Pharmacol 25:299–303
Findlay M, Cunningham D, Norman A, Mansi J, Nicolson M, Hickish T, Nicolson V, Nash A, Sacks N, Ford H (1994) A phase II study in advanced gastro-esophageal cancer using epirubicin and cisplatin in combination with continuous infusion 5-fluorouracil (ECF). Ann Oncol 5:609–616
Kim DY, Kim JH, Lee SH, Kim TY, Heo DS, Bang YJ, Kim NK (2003) Phase II study of oxaliplatin, 5-fluorouracil and leucovorin in previously platinum-treated patients with advanced gastric cancer. Ann Oncol 14:383–387
Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, Kim CS, Rhyu HS, Hyun JH, Kim JS (1999) Paditaxel, 5-fluorouracil and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer 85:295–301
Lordick F, Lorenzen S, Stollfuss J, Vehling-Kaiser U, Kullmann F, Hentrich M, Zumschlinge R, Dietzfelbinger H, Thoedtmann J, Hennig M, Seroneit T, Bredenkamp R, Duyster J, Peschel C (2005) Phase II study of weekly oxaliplatin plus infusional fluorouracil and folinic acid (FUFOX regimen) as first-line treatment in metastatic gastric cancer. Br J Cancer 93:190–194
Louvet C, Andre T, Tigaud JM, Gamelin E, Douillard JY, Brunet R, Francois E, Jacob JH, Levoir D, Taamma A, Rougier P, Cvitkovic E, de Gramont A (2002) Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol 20:4543–4548
MoiseyenkoVM, Ajani JA, Tjulandin SA, Majlis A, Constenla M, BoniC, Anelli A, Yver AJ, Van Cutsem E (2005) Final results of a randomized controlled phase III trial (TAX 325) comparing docetaxel (T) combined with cisplatin (C) and 5-fluorouracil (F) to CF in patients (pts) with metastatic gastric adenocarcinoma (MGC). Proc Am Soc Clin Oncol 24:abstr 4002
Ohtsu A, Shimada Y, Shirao K, Boku N, Hyodo I, Saito H, Yamamichi N, Miyata Y, Ikeda N, Yamamoto S, Fukuda H, Yoshida S (2003) Randomized phase III trial of fluorouracil alone v fluorouracil plus cisplatin v uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol 21:54–59
Parkin DM (1998) Epidemiology of cancer global patterns and trends. Toxicol Lett 102–103:227–234
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Pendyala L, Creaven P (1993) In vitro cytotoxicity, protein binding, red blood cell partitioning, and biotransformation of oxaliplatin. Cancer Res 53:5970–5976
Raymond E, Buquet-Fagot C, Djelloul S, Mester J, Cvitkovic E, Allain P, Louvet C, Gespach C (1997) Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast and ovarian cancers. Anticancer Drugs 8:876–885
Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, Price T, Anderson H, Iveson T, Hickish T, Lofts F, Norman A (2002) Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 20:1996–2004
Roth AD, Maibach R, Martinelli G, Fazio N, Aapro MS, Pagani O, Morant R, Borner MM, Herrmann R, Honegger H, Cavalli F, Alberto P, Castiglione M, Goldhirsch A (2000) Docetaxel (Taxotere)-/cisplatin (TC): an effective drug combination in gastric carcinoma. Swiss Group for Clinical Cancer Research (SAKK), and the European Institute of Oncology (EIO). Ann Oncol 11:301–306
Rubin P, Williams JP, Okunieff P, Rosenblatt JD, Sitzmann JV (2001) Statement of the clinical oncologic problem. In: Rubin P, Williams JP (eds) Clinical oncology A multidisciplinary approach for physicians and students, 8th edn. Elsevier, New York, pp 1–32
Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T, Shipley W, Curran W (2000) Common toxicity criteria:version 2.0. An improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 47:13–47
Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, Planker M, Santos JG, Piedbois P, Paillot B, Bodenstein H, Schmoll HJ, Bleiberg H, Nordlinger B, Couvreur ML, Baron B, Wils JA (2000) Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin v etoposide, leucovorin, and fluorouracil v infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol 18:2648–2657
Waters JS, Norman A, Cunningham D, Scarffe JH, Webb A, Harper P, Joffe JK, Mackean M, Mansi J, Leahy M, Hill A, Oates J, Rao S, Nicolson M, Hickish T (1999) Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: results of a randomized trial. Br J Cancer 80:269–272
Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, Hughes M, Mansi J, Findlay M, Hill A, Oates J, Nicolson M, Hickish T, O’Brien M, Iveson T, Watson M, Underhill C, Wardley A, Meehan M (1997) Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 15:261–267
World Health Organization (1979) WHO handbook for reporting results of cancer treatment. Geneva, Switzerland, World Health Organization, WHO Offset Publication No. 48
Yang L (2006) Incidence and mortality of gastric cancer in China. World J Gastroenterol 12:17–20
Zaniboni A, Barni S, Labianca R, Marini G, Pancera G, Giaccon G, Piazza E, Signaroldi A, Legnani W, Luporini G (1995) Epirubicin, cisplatin, and continuous infusion 5-fluorouracil is an active and safe regimen for patients with advanced gastric cancer. An Italian Group for the Study of Digestive Tract Cancer (GISCAD) report. Cancer 76:1694–1699
Acknowledgments
We thank Professor Jin Li, Dr. Leiping Wang, Dr. Chunlei Zheng for their sincere help in conducting this trial. We also thank Ms. Xiaofeng Gu in the documentation of the study data and the secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, X., Leaw, J., Gu, W. et al. Phase II clinical trial of advanced and metastatic gastric cancer based on continuous infusion of 5-fluorouracil combined with epirubicin and oxaliplatin. J Cancer Res Clin Oncol 134, 929–936 (2008). https://doi.org/10.1007/s00432-008-0376-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-008-0376-4