Abstract
Development and design of agents derived from natural sources with neuroprotective properties have received considerable attention. In the literature, it has been stated that these polyphenolic molecules have low adverse impacts and high efficacy when used in pathological conditions. Dietary flavonoids as a subgroup of polyphenols are bioactive products, extracted from several types of vegetables and fruits. Luteolin (3′,4′,5,7-tetrahydroxyflavone, LUT) is a widespread flavone known to have antioxidant and cytoprotective properties related to nuclear factor erythroid 2-related factor 2-(Nrf2) pathway. Extensive in vitro and in vivo investigations have indicated that LUT exhibits beneficial neuroprotective properties via different mechanisms. However, its psychopharmacological mechanisms are presently investigated in fewer studies. Therefore, we aimed to evaluate the neuroprotective impacts of LUT against central nervous system (CNS) disorders by reviewing available literature. Herein, we also reviewed the studies to understand the underlying mechanisms of LUT for curing CNS disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Consumption of foods of plant origin decreases the risk of developing different pathological conditions, which is attributed to their high content of phytochemicals (Baradaran 2017; Ghorbani et al. 2014; Pandey and Rizvi 2009). Herbal medicine from natural sources has been used to treat a wide range of neurological disorders such as traumatic brain injury (TBI), Alzheimer’s disease (AD), stroke and Parkinson’s disease (PD; Bayat et al. 2012; Feigin 2007; Sarrafchi et al. 2016; Wang et al. 2016b). Herbs contain different types of molecules with antioxidant capacity such as flavonoids (Kr and Christina 2011; Rafieian-Kopaei 2013). The evidence obtained from different levels of studies on animal and human populations has indicated that flavonoids are beneficial for health (Nasri 2016; Rafieian-Kopaei 2013). Due to their abundance in foods, such as fruits, vegetables and medicinal herbs, flavonoids are known as common nutrients which act as antimicrobial agents, antioxidants and regulators of estrogen (Birt et al. 2001; Jafari 2016; Rafieian-Kopaei et al. 2013; Tables 1 and 2).
Luteolin (LUT, 3′,4′,5,7-tetrahydroxyflavone) is a natural flavone compound which can be extracted from different types of plants. Its polyphenolic structures are essential for protecting plant cells against insects, microorganisms and UV irradiation (Harborne and Williams 2000). LUT has a wide range of pharmacological activities and potent anti-oxidant characteristics which scavenge reactive nitrogen and oxygen species. It has been found that the antioxidant properties and neuroprotective effects of LUT are associated with the nuclear factor erythroid 2-related factor 2-(Nrf2) pathway (Wruck et al. 2007). In oxidative stress-induced cell death, a cis-acting element known as the antioxidant responsive element (ARE) regulates the activation of protective transcription factors. Then, Nrf2 binds to ARE and works as the Nrf2-ARE pathway which exhibits resistance to neurotoxicity induced by oxidative stress (Johnson 2008). In the rat model of ischemia, middle cerebral artery occlusion-induced ischemia (MCAo), the anti-oxidative and neuroprotective effects of LUT are associated with its role in the scavenging of free radicals by upregulating Nrf2 protein level, a well-known transcription factor as cells are produced to defend against a variety of detrimental stresses. Subsequently, it can contribute in the inhibition of cell death and decreasing the infarct area (Zhang et al. 2013a, b). In other study, it has been recorded that LUT can protect the glial C6 and dopaminergic neural PC12 cells of a rat against N-methyl-4-phenyl-pyridinium-induced neurotoxicity via the upregulation of Nrf2 protein (Wruck et al. 2007).
Moreover, LUT displays anti-inflammatory and neuroprotective activities in many studies (Ren et al. 2013; Wang et al. 2011). It represents neuroprotection in some animal models such as TBI (Xu et al. 2014a), AD (Paterniti et al. 2014) and anti-inflammation in the model of spinal cord injury (Paterniti et al. 2013). In previous studies, it has been shown that LUT can prevent neuroinflammation by suppressing the microglial inflammation (Andersen and Markham 2005). Moreover, the flavonoid LUT inhibits the lipopolysaccharide (LPS)-stimulated nuclear factor kappa B (NF-κB) pathway (Kim and Jobin 2005; Lee et al. 2009; Weng et al. 2015). In addition, LUT has been shown to attenuate microglial activation and mediate brain-derived neurotrophic factor (BDNF)-like behavior both in vitro and in vivo (Lin and Harnly 2010; Patil and Sathaye 2015; Patil et al. 2014).
Based on these findings, LUT might protect neural cells against impairments included in different conditions. In this study, we reviewed the different sources of literature such as PUBMED to show the mechanisms of LUT neuroprotection against neural damages and subsequent related complications. In this review, we tried to investigate the effects of LUT on central nervous system (CNS) disorders in different types of studies such as animal studies, case reports, open studies and double-blind studies, etc.
Luteolin Source, Structure and Neuropharmacological Features
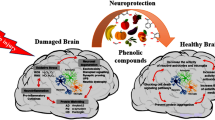
Among the different varieties of flavonoids with pharmacological and biological features, LUT with antioxidant activity (Fig. 1) is one of the most common flavonoids present in vegetables and various fruits such as parsley, celery, perilla leaf, chamomile tea and green pepper (Leung et al. 2006; Paladini et al. 1999; Wang et al. 2005a). Flavonoids (2-phenyl-benzo-ã-pyrones), which are extensively distributed in plant-based foods, are a large group of polyphenolic natural compounds (Andersen and Markham 2005; Shahidi et al. 2008). Flavonoids propose a variety of biological and pharmacological characteristics, including antiallergic, antiulcer, cardiovascular protection, antiviral, anticancer and anti-inflammatory potentials (Dehghan et al. 2007). Moreover, it has been proven that LUT can freely cross the blood–brain barrier (BBB) by modulating the Rho GTPases (Sawmiller et al. 2014; Fig. 2).
Different mechanisms of luteolin action in central nervous system disorders. Inhibition of autophagy by upregulation of beclin-1 and LC3II; Downregulation of proinflammatory factors (IL-1b and TNF-a); Inhibition of the activity of NF-kB; Upregulation of mTOR and p70S6K; Upregulation of Bcl-2 and downregulation of Bax; Inhibition of the activity of NF-κB, TLR4 and TLR5; Decreasing the astrocyte activation by reducing the expression of GFAP; Increasing MMP2; Suppressing the MBP as the main MS antigen; Modulation of factors of cell migration such as MMP-9 and TIMP-1; Increasing the production of IFN- γ; Reducing inducible NO synthase; Suppression of ERK1/2-activation; Increasing the expression of HO-1 levels; Decreasing the transcription of p53 target genes including p21, PUMA and GADD45α; Inhibition of Akt phosphorylation; Anti-oxidative activity (increasing the SOD and GSH-Px levels, and reducing MDA levels); Decreasing the increased activation of caspase-3. MBP: myelin basic protein; TLR: Toll-like receptor; NF-ƘB: nuclear factor kappa B; GFAP: glial fibrillary acidic protein; BDNF: brain-derived neurotrophic factor; PKA: the protein kinase A; NO: nitric oxide; MMP: matrix metalloproteinases; GSH: glutathione levels; SOD: Superoxide dismutase; MDA: malondialdehyde; 6-OHDA: 6-hydroxydopamine; HO-1:heme oxygenase-1
According to the literature, most of the flavonoids have free radical scavenging and metal chelating activities (Dehghan and Khoshkam 2012). Moreover, LUT has been recorded to exhibit anti-inflammatory, antioxidant and anti-cancer properties (Hougee et al. 2005; Romanova et al. 2000). It mediates these actions by different mechanisms such as inhibiting the production of nitric oxide (NO; Kim et al. 1999). LUT is a potent inhibitor of human mast cell activation by suppressing the protein kinase C (PKC) activation and Ca2+ influx (Chowdhury and Rasmusson 2002). LUT has been introduced as an immune reactions modulator, as several studies have compared the anti-inflammatory features of LUT with other flavonoids like quercetin, genistein or hesperetin (Comalada et al. 2006; Xagorari et al. 2001). In addition, LUT has been found to possess anti-inflammatory and neuroprotective activities in microglia (Dirscherl et al. 2010) to reduce the peroxide-induced neurotoxicity (Pavlica and Gebhardt 2010), N-methyl-4-phenyl-pyridinium (MPP+)-induced neurotoxicity (Wruck et al. 2007) and amyloidβ (Aβ) protein (Cheng et al. 2010) in vitro.
LUT inhibits the production of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-8 and tryptase (Kempuraj et al. 2005; Park et al. 2008), and likewise for leukotrienes, histamine and prostaglandin D2 from human cultured mast cells (Kimata et al. 2000). Moreover, LUT suppresses the activation of IL-1-stimulated mast cells (Kandere-Grzybowska et al. 2006) leading to selective release of IL-6. LUT also inhibits release of IL-6 by microglia cells (Jang et al. 2008) and astrocytes (Sharma et al. 2007). In addition, LUT permeates through the BBB, shows anti-amnesic effects against the toxicity of amyloid (β25–35) in mice and attenuates scopolamine-induced amnesia in rats (Liu et al. 2009; Tsai et al. 2007). LUT also activates cyclic AMP (cAMP) response element-binding protein (CREB), which is the mechanism underlying its effects on the facilitation of long-term potentiation (LTP) and memory enhancement (Xu et al. 2010). In addition, LUT protection of neural cells against induced neurotoxicity via the upregulation of Nrf2 protein has received much attention (Wruck et al. 2007). Different pharmacological features have been demonstrated for LUT as follows: (1) the structural component of LUT being similar to other active flavonoids, (2) the activity of some of its glycosylated derivatives (Coleta et al. 2006; Coleta et al. 2008; Fernández et al. 2006) and (3) the similar flavonoid effects in the CNS. The effects of LUT in the CNS are complex and involve different mechanisms such as interaction with the benzodiazepine-binding sites (BDZ-bs) at the gamma-aminobutyric acid (GABA)A receptors (Goutman et al. 2003). Despite the low affinity for the BDZ-R shown in vitro, Coleta et al. (2008) proposed that the CNS activity of LUT is apparently associated with its anxiolytic-like effects via a GABAergic mechanism. Their results suggested that there is a possible interaction between LUT and other neurotransmitter systems (Coleta et al. 2008).
It has been demonstrated that the levels of malondialdehyde (MDA), a marker of lipid peroxidation and the activity of glutathione peroxidase (GSH-Px) were restored after treatment with LUT in heroin-induced oxidative damage in a mice brain (Qiusheng et al. 2005). Inflammatory responses of LUT and LUT-7-O-glucoside by modulatory impacts of NF-κB/AP-1/PI3K-Akt signaling cascades have been confirmed in vitro (Park and Song 2013). In addition, via a reduction in the intracellular Ca2+ levels, LUT also suppresses the expression of TNF-α, IL-6, IL-8 and cyclooxygenase (COX)-2 (Lamy et al. 2015). LUT was reported to inhibit IL-1β function (believed to contribute to glioblastoma cell proliferation) which triggered the expression of the inflammation biomarker of COX-2 in U-87 glioblastoma cells. In a concentration-dependent manner, it also inhibited IL-1β-mediated phosphorylation of inhibitor of NF-κB, inhibitor of κB (IκB), c-Jun amino-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) 1/2 (Lamy et al. 2015).
The Beneficial Effects of Luteolin on Different Nervous System Disorders
Different Mechanisms of Luteolin Against Traumatic Brain Injury
TBI occurs in acute head trauma in events such as falling, motor vehicle accidents or accidental firearm injury. It has been recognized that TBI is a major risk factor for AD (Guo et al. 2000; O'Meara et al. 1997; Plassman et al. 2000). Furthermore, it is attributed to diminished or altered state of consciousness. In addition, it affects the quality of life and expectancy and has been introduced as a common leading cause of morbidity (Brooks et al. 2013). While the outcome of TBI is majorly related to the severity of the primary insult, it is aggravated by secondary events caused by pathological processes, including oxidative stress, excitotoxicity, inflammation and enhanced vascular permeability (Werner and Engelhard 2007). Although different approaches have been tried to cure the secondary insult and improve the outcome of TBI, most of these approaches have failed in clinical trials because of targeting a single injury mechanism of TBI (Sun et al. 2015). It has been proven that LUT, which belongs to the flavonoid family, provides neuroprotection in a variety of neurological diseases, as it is abundant in fruits and vegetables. With no or minimal serious side effects, LUT can treat or prevent the brain against damages following TBI, including neuronal death and subsequent neurological deficits (Dajas et al. 2013). Nrf2–ARE has been proven to be activated in many neurological diseases (Chen et al. 2011; Wang et al. 2007a). This pathway has been introduced as an endogenous and compensatory adaptation against TBI (Yan et al. 2008).
Basic Research Studies (In Vitro & In Vivo)
According to the findings of Xu et al. (2014a, b), LUT with a purity of more than 98% (Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China) cured secondary brain impairments following TBI in a modified Marmarou′s weight-drop model of mice by increasing the neuron survival neuronal apoptosis and improving neurological deficits. They used LUT in three doses of 10, 30 and 50 mg/kg in their in vivo and three doses of 5, 10 and 25 mM in their in vitro studies. Their results showed that administration of LUT reduced oxidative stress by modulating the LDA levels, GSH-Px activity and reactive oxygen species (ROS) production. Moreover, in their study, LUT upregulated the translocation of Nrf2 in both in vitro and in vivo investigations. In addition, administration of LUT failed to provide neuroprotective effects following TBI in transgenic Nrf2(−/−) mice; yet it provided neuroprotective effects possibly via the activation of the Nrf2-ARE pathway (Xu et al. 2014a, b). In another study, Xu et al. (2014a, b) demonstrated that treatment with LUT (30 mg/kg/IP for 15 days, Shanghai Yuan ye Bio-Technology Co., Ltd., Shanghai, China) protected mice from TBI by enhancing the autophagy via expressions of autophagic markers and reducing inflammation by decreasing the nuclear accumulation of P65. Moreover, LUT decreased mRNA and protein expressions of TNF-α and IL-1b, pro-inflammatory factors. Moreover, LUT decreased BBB disruption, neuronal degeneration and alleviated brain edema (Xu et al. 2014b). Cordaro et al. (2016) evaluated the neuroprotective effects of co-ultramicronized compound PEA/LUT (co-ultraPEA/LUT, unknown source, 1 and 10 mg/kg/PI) on secondary events following TBI including inflammatory process and autophagy. Their findings suggest that co-ultraPEA/LUT can counteract the neurodegeneration and neuroinflammation induced by TBI (Cordaro et al. 2016). In a study by Sawmiller et al. (2014), they proved that LUT (unknown source) reduced AD pathologies induced by TBI in Aβ-depositing Tg2576 mice. In this study, they observed that LUT significantly terminated the accelerated pathologies including increased pro-inflammatory cytokines, deposition of Aβ, phospho-tau and also activation of glycogen synthase-3 (GSK-3; Sawmiller et al. 2014).
No clinical trial studies have been investigated to demonstrate the neuroprotective effects of LUT against TBI yet. However, these types of studies are suggested to design and use this agent in a clinical setting to manage the disorders induced by TBI, based on the approved neuroprotective properties of LUT in animal models of TBI.
Different Mechanisms of Luteolin Against Cerebral Ischemic Stroke
Cerebral stroke is one of the common causes of neurological disabilities and the second leading cause of death worldwide (Corbett et al. 2015; Thrift et al. 2014). Cerebral ischemic stroke results from the sudden reduction or obstruction of blood flow to a region of the brain, resulting in a corresponding loss of neurons and subsequent neurological dysfunction including different partial paralysis, difficulties with memory, learning, thinking, language and movement (Mokhtari et al. 2017). Although the restoration of blood circulation to the ischemic region is the current approach for treatment of clinical stroke, this can cause additional impairment and aggravate neurocognitive deficits. Inflammation is introduced as a key characteristic of brain ischemia (Kawabori and Yenari 2015) with major immune system players, namely mast cells (Jin et al. 2009; Silver and Curley 2013) and microglia (Chew et al. 2006; Hanisch and Kettenmann 2007) which act as early responders. These reactions lead to the release of pro-inflammatory mediators and infiltration of other inflammatory cell populations such as T-cell subsets, neutrophils and monocyte/macrophages into the region of the brain with an ischemic condition. In addition, astrocytes activities in the late phase cause the formation of glial scar in the boundary zone of the ischemic core (Sofroniew and Vinters 2010). LUT displays specific anti-inflammatory effects, which are only described by its antioxidant capacities. The anti-inflammatory activity of LUT includes activation of anti-oxidative enzymes, suppression of the NF-κB pathway, and inhibition of pro-inflammatory substances (Middleton et al. 2000; Seelinger et al. 2008). In this study, we reviewed the characteristics of LUT to define the mechanisms of this agent against the pathogenesis of cerebral ischemic stroke based on different studies.
Basic Research Studies (In Vitro & In Vivo)
Different studies have been conducted to determine the mechanism of LUT against pathological changes induced by brain ischemia. Qiao et al. (2012a, b) evaluated the neuroprotective effects of LUT (Rongsheng Biotechnology Co, Xi’an, Shanxi, China, purity of more than 99%) in experimental permanent ischemic stroke 1 or 3 days after surgery and showed that different doses of 10 (middle dose) or 25 mg/kg (high dose) immediately upregulated superoxide dismutase (SOD), catalase (CAT), Bcl-2 and claudin-5 expression, down-regulated Bax and MDA expression and also alleviated the brain water content neurological deficits and volume of infarct 1 day and 3 days after permanent middle cerebral artery occlusion (pMCAO). They concluded that LUT protected the brain from ischemic damage by decreasing oxidative stress and apoptosis (Qiao et al. 2012a, b). In another study, Qiao et al. (2012a, b) confirmed that LUT (10 and 25 mg/kg/IP, Rongsheng Biotechnology Co, Xi’an, Shanxi, China) could protect rat brains against focal ischemia by upregulation of the p-ERK expression and downregulation of NF-κB, Toll-like receptor-4 (TLR-4), TLR5 and p-p38MAPK expression in the pMCAO rat model (Qiao et al. 2012a, b). Zhang et al. (2013a, b) reported that 4 mg/kg of LUT, a flavonoid extracted from Ixeris sonchifolia Hance, provided neuroprotective effects by antioxidant- and Nrf2-inducing activities which resulted in inhibition of neuronal cell death and reducing the infarct area in the rat model of MCAO (Zhang et al. 2013a, b). Additionally, Fang et al. (2009) evaluated the LUT (unknown source) underlying mechanism against ischemia/reperfusion injury and demonstrated that treatment of the cultured neurons of oxygen-glucose deprivation/reperfusion model with LUT increased the cell viability, decreased the apoptotic cells percentage and leakage of lactate dehydrogenase (LDH) rate in a dose-dependent manner (1–100 μmol/L). LUT with a dose of 200 mg/kg/ IP markedly prevented the decrease of ATPase activities in a rat model of global cerebral ischemia/reperfusion (Fang et al. 2009). Moreover, Caltagirone et al. (2016) proved that co-ultraPEA/LUT (Glialia®, 1 mg/kg 1 h after ischemia and 6 h after reperfusion) synergistically improved the neurological index, reduced the infarct area, neuronal cell death, astrocyte activation and mast cell-mediated toxicity, regulated the GDNF- and brain-derived neurotrophic factor (BDNF) expression and elicited neuroprotection in the MCAo model of brain ischemia (Caltagirone et al. 2016).
Clinical Research
Using LUT for curing patients with cerebral ischemia has been evaluated in clinical studies. In a cohort of 250 stroke patients undergoing neurorehabilitation between April 2013 and June 2014, Caltagirone et al. (2016) treated the patients with Glialia® (composed of co-ultramicronized 700 mg of PEA and 70 mg of luteolin, in microgranular form every 12 h) for 60 days. Their results showed improvement in different scores such as Canadian neurological scale (CNS), mini-mental state examination (MMSE), Ashworth scale, numeric rating scale and Barthel index of stroke patients receiving Glialia® for 60 days (Caltagirone et al. 2016).
Different Mechanisms of Luteolin Against Epilepsy
The term epilepsy has been used for a group of disorders that involve hyperexcitable neurons and is described by recurrent spontaneous seizures. It has been suggested that imbalance between excitatory glutamate-mediated neurotransmission and GABA-mediated inhibition leads to epilepsy (Diniz et al. 2015; Grosso et al. 2013). It is usually related to dysfunctions of the brain which lead to numerous behavioral comorbidities (Singh et al. 2012). After the seizure activity during epilepsy, mechanisms of antioxidant defense are reduced in the brain and amount of free radicals is enhanced, which further induces the oxidative stress. During epilepsy, seizure activity diminishes the mechanisms of antioxidant protection in the brain and enhances the amount of free radicals, which further prompts the oxidative stress. Free radicals (FRs) can be defined as molecules or molecular fragments that contain one or more unpaired electrons (Cardenas-Rodriguez et al. 2013). These free radicals are involved in lipid peroxidation, brain edema, and epilepsy, including coma and death (Ramalingam et al. 2013). LPS-mediated activation of Toll-like receptor-4 (TLR-4) receptors can produce epileptiform discharges that can be attenuated by IL-1 receptor antagonists (Rodgers et al. 2009). Inflammation, in the form of microglial activation, generation of the cytokine interleukin 1β (IL-1β) and stimulation of TLR-4 have been reported in epilepsy patients (Maroso et al. 2010). Acute LUT administration has also been shown to attenuate oxidative stress in neuroblastoma cells (Zhou et al. 2011). Anxiolytic-like effects of LUT have been reported following oral and intraperitoneal administration in mice, suggesting that it can cross the BBB (Coleta et al. 2008). In this part, we reviewed the features of LUT to define the mechanisms for this agent against the pathogenesis of epilepsy according to different investigations.
Basic Research Studies (In Vitro & In Vivo)
Birman et al. (2012) demonstrated that pretreatment with LUT (10 mg/kg/IP, Department of Pharmacognosy, Faculty of Pharmacy, Istanbul University) decreased seizure frequency and enhanced reactions of nitric oxide synthases (iNOS) and matrix metalloproteinases (MMP2) in a rat hippocampus after pentylenetetrazole (PTZ) administration (indication of MMP and NOS activities; Birman et al. 2012). In addition, the effects of acute and chronic intraperitoneal LUT injections were evaluated by Shaikh et al. (2013) in four mouse seizure models of 1) maximal electroshock test (MEST), 2) the 6-Hz model, 3) PTZ and 4) second hit PTZ test in the chronic stage of the pilocarpine model. Their study showed that LUT (unknown source) did not exert any significant anti- or pro-convulsant effects after a single dose in the 6-Hz (0.3–10 mg/kg/IP, 3–4 days), PTZ (3 mg/kg/IP) and MEST (0.3–20 mg/kg/Ip) tests and following repeated daily dosing (10 mg/kg/IP) in the 6-Hz model. While TLR4 mRNA levels were enhanced 3 days after pilocarpine-induced status epilepticus, they remained unaltered in the chronic stage of the model. No effect was observed in the second hit PTZ test following repeated LUT injections. These findings suggest that seizure threshold may be independent of TLR4 signaling (Shaikh et al. 2013). Zhen et al. (2016) examined the impacts of LUT (50 or 100 mg/kg/day by oral administration, purity N 98%, CAS: ZL201125, Nanjing Zelang Biotechnology Company, Jiangsu, China) 30 min before PTZ injection on the brain of mice in PTZ-induced seizures. They expressed that LUT reduced the seizure severity and cognitive impairment, decreasing the oxidative stress and subsequent neuronal damages and enhanced phosphoactivation of the protein kinase A (PKA) and CREB and upregulated BDNF expression in the hippocampus region (Zhen et al. 2016). Tambe et al. (2017) assessed the effects of pretreatment with LUT (5, 10 and 20 mg/kg/IP, isolated from Eclipta alba leaves) in PTZ-induced acute and chronic epilepsy models in mice. They found that PTZ-induced kindling was inhibited by LUT (5, 10 and 20 mg/kg/IP) in a dose-dependent manner. LUT decreased the MDA level and restored levels of reduced glutathione (GSH) in these animals (Tambe et al. 2017).
Different Mechanisms of Luteolin Against Multiple Sclerosis
In young adults, multiple sclerosis (MS), as a T cell-mediated demyelinating disease of CNS (Verbeek et al. 2004), is a leading cause of disability and most common inflammatory disease of CNS. Based on strong evidence, it has been suggested that MS pathologically is an autoimmune disease that affects the oligodendrocytes or CNS myelin which is mainly mediated by type 1 T helper (Th1) cell responses to CNS myelin antigens (Alamouti et al. 2015; Milo and Kahana 2010). Interferon-beta (IFN-β), the most effective cure for MS with an unclear mechanism of action, leads to asymptomatic relief in a type of MS in patients called relapsing-remitting MS (RRMS) and administered only parenterally with different undesirable adverse impacts (Bertolotto et al. 2015). LUT as an important member of the flavonoid family has been reported to have immunomodulatory effects that may be beneficial in curing neurodegenerative diseases (Chen et al. 2004) such as MS with autoimmune pathogenesis mediated by T cells (Stadelmann 2007). Based on the findings from different in vitro studies, LUT could suppress T-cell activation (Chen et al. 2004) and decrease the proliferation of autoreactive T cells induced by murine encephalitogen proteolipid protein peptide (PLP) as a candidate autoantigen in MS and an alpha B-crystallin autoantigen in experimental autoimmune encephalomyelitis (EAE; Verbeek et al. 2004). In this section, we reviewed the effects of LUT on pathogenesis of MS to define the mechanisms for it based on different evaluations:
Basic Research Studies (In Vitro & In Vivo)
In an in vitro study, Kempuraj et al. (2008) evaluated the effects of LUT (Sigma) on the human umbilical cord blood-derived cultured mast cells (hCBMCs) and Jurkat T cells activated by myelin basic protein (MBP) and reported that LUT effectively suppressed the main MS antigen, MBP, which induced the activation of human mast cells at 10 and 100 μm. In addition, LUT (1–100 μm) could concentration-dependently inhibit the mast cell-dependent Jurkat T cell activities and stimulate mast cells to produce IL-2, which are associated with the pathogenesis of autoimmune diseases, such as MS, atopic dermatitis and psoriasis (Kempuraj et al. 2008). Moreover, Sternberg et al. (2009) demonstrated that LUT (Sigma Aldrich, St. Louis, MO, USA) also had immunomodulatory impacts on isolated peripheral blood mononuclear cells (PBMC) in MS patients when incubated with this flavonoid. Besides, LUT treatment proposed the additive effects in modulation of pro-inflammatory cytokines secretion such as TNF-α, IL-1β and cell proliferation and as well as effective factors of cell migration, MMP-9 and TIMP-1 (Sternberg et al. 2009). Verbeek et al. (2004) studied the effects of different flavonoids such as LUT on human and murine autoreactive T cells [culturing the spleen cells and lymph node cells 10 days after 40 μg of PLP (139–151) injection] and showed that LUT and apigenin (different concentrations of 3.5, 17.5 and 35 μM, Kaden Biochemicals, Hamburg, Germany) strongly prevented the murine and human T-cell responses in the production of interferon-gamma (IFN-γ) and their in vitro antigen-specific proliferation (Verbeek et al. 2004). In another study, Verbeek et al. (2005) demonstrated that oral flavonoids such as LUT and structurally similar flavonoids (solution of 2.5 mg/ml/daily, Kaden Biochemicals, Hamburg, Germany) delayed recovery from experimental autoimmune encephalomyelitis (EAE) as a model of MS in SJL mice. Both LUT and apigenin enhanced the production of interferon-gamma (IFN-γ) and inhibited the proliferative responses (Verbeek et al. 2005). Furthermore, in a commentary study, Theoharides (2009) recommended that LUT, as a therapeutic option with antioxidant and anti-inflammatory effects, inhibited the mast cells and T cells implicated in the treatment and pathogenesis of MS (Theoharides 2009).
Different Mechanisms of Luteolin Against Alzheimer’s Disease
AD, as the most common leading cause of aging-related dementia, is a progressive neurodegenerative disease that usually starts slowly in the CNS (Zhu et al. 2013). AD is characterized by irreversible loss of neurons. This disorder clinically causes gradual deterioration in intellectual abilities including cognition and memory and leads to neuropsychiatric symptoms (Zhu et al. 2013). Histopathologically, AD is associated with the loss of cortical neurons and synapses and forming the intracellular hyperphosphorylated tau-containing neurofibrillary tangles (NFTs) fragments (Terry et al. 1991). In this neurodegenerative disease, β-amyloid (Aβ) fragments, as major elements of extracellular neurotic plaques, contain between 39 and 43 amino acids which form the core constituent of these plaques (Masters et al. 1985). Aβ fragments are generated from a sequential cleavage of amyloid precursor protein (APP) by γ-secretase and β-site APP cleavage enzyme (Choi et al. 2014). Although the exact mechanism mediating neuronal death induced by Aβ is unknown, oxidative stress, free radical generation and neuroinflammation appear to play prominent roles in the pathogenesis of AD. Aβ can additionally induce cell death through excitotoxicity and neuroinflammation (Subasinghe et al. 2003). Recently, there has been a concerted effort to develop drugs to ameliorate the different defects observed in AD worldwide. Many studies have demonstrated that compounds with free-radical scavenging activities can attenuate Aβ-induced neuronal death (Choi et al. 2014; Di Domenico et al. 2015). In this section, we reviewed the effects of LUT on pathogenesis of PD in different studies.
Basic Research Studies (In Vitro & In Vivo)
In a similar in vitro study, Zhou et al. (2012) showed that LUT attenuated zinc-induced tau phosphorylation at Ser262/356 (an in vitro model of AD) in a dose-dependent manner in SH-SY5Y cells (Zhou et al. 2012). Recently, Choi et al. (2014) showed that LUT (different concentrations of 10, 20, 40 and 80 μM, Sigma-Aldrich, St. Louis, MO, USA) could decrease the Aβ-induced neurotoxicity in murine cortical neurons (isolated from fetal mice) by its potent antioxidant activity (Choi et al. 2014). LUT can alleviate spatial learning and memory defect in AD animal models. It also prevents the thickness reduction in the pyramidal cell layer of hippocampal CA1. According to the findings of Wang et al. (2016a, b), they suggested that LUT (10 and 20 mg/kg) improved memory impairment and prevents the decrease of the CA1 pyramidal cell layer in a streptozotocin (STZ)-induced AD rat model. They declared that these neuroprotective effects of LUT against AD pathogenesis maybe due to the anti-oxidative characteristic of this agent by inhibiting the production of free radicals and dispersing Aβ plaques (Wang et al. 2016a, b). In both in vitro and ex vivo organotypic models of AD, Paterniti et al. (2014) investigated the impacts of co-ultraPEA/LUT on the pathogenesis of AD. They pre-treated neuron-like cells differentiated from SH-SY5Y cells with different doses of co-ultraPEA/LUT (27, 2.7 and 0.27 μM, unknown source) and used Aβ1–42 stimulation (1 μM) for induction of AD (a suitable in vitro model to investigate the pathogenesis of AD). In an ex vivo organotypic model, mice hippocampal slice cultures were prepared and pre-treated with different doses of co-ultraPEA/LUT (27, 2.7 and 0.27 μM, unknown source) and subsequently incubated with Aβ1–42. Treatment with co-ultraPEA/LUT improved cell viability, significantly reduced inducible NO synthase and glial fibrillary acidic protein (GFAP) expression, restored neuronal synthase of NO and BDNF and subsequently reduced apoptosis. In addition, their results proved that the combination therapy with co-ultraPEA/LUT could attenuate neuroinflammation in an experimental AD model (Paterniti et al. 2014).
Different Mechanisms of Luteolin Against Parkinson’s Disease
PD is a common and slowly progressive neurodegenerative disorder. As pathologic events, degeneration of dopaminergic neurons occurs in the striatum in the substantial nigra pars compacta (SNpc), a brain area involved in controlling movements (Meissner et al. 2011). In addition, the presence of abnormal fibrillary aggregations of α-synuclein (α-Syn) protein within neurons, known as Lewy bodies, have been introduced as other pathological characteristics of PD. Based on evidence, oxidative stress and neuroinflammation play pivotal roles in the neurodegeneration associated with PD and the progression of disease (Jenner and Olanow 1996; Taylor et al. 2013; Zhang et al. 1999). It has been reported that oxidative stress impairs lipids, DNA and proteins of neurons along with decreased SOD, CAT and GSH-Px levels (Jenner and Olanow 1996). PD is commonly characterized by a wide range of cardinal features, including tremor, slowness of movement, rigidity and postural instability as a result of selective and progressive degeneration in the dopaminergic neurons of SN (Liu and Hong 2003; Wang et al. 2005b, 2007b). Based on our knowledge, the related data on the pathogenesis of PD have been found from cell culture studies and experimental models and using the neurotoxins (e.g. 1-methyl-4-phenylpyridinium (MPP+), 6-hydroxydopamine (6-OHDA) and popolysaccharide (LPS); Bové et al. 2005). 6-OHDA with structural features analogous to noradrenaline and dopamine, induces neural death via the generation of cytotoxic quinines and free radicals (Saito et al. 2007). As a therapeutic agent, LUT has been used in different studies to reduce the pathogenesis of PD.
Basic Research Studies (In Vitro & In Vivo)
In a recent study, Wruck et al. (2007) demonstrated that LUT (20 μM, Sigma-Aldrich Co., St. Louis, MO, USA) protected rat glial C6 and neural PC12 cells against MPP+-induced cytotoxicity (an in vitro model of PD) through activation of Nrf2 and suppression of ERK1/2 activation known as a Kelch-like ECH-associating protein 1 (Keap1)-Nrf2-ARE pathway dependent factor (Wruck et al. 2007). Additionally, Chen et al. (2008) found that LUT (1, 2.5 and 5 μM, Sigma-Aldrich Co., St. Louis, MO, USA) protected dopaminergic neurons from LPS-induced injury through suppression of rat microglial activation isolated from whole brains of one-day-old Sprague-Dawley rats. In their study, the neuroprotective effects of LUT were investigated evaluating [(3)H]dopamine uptake and counting tyrosine hydroxylase (TH)-immunoreactive cells in primary mesencephalic neuron-glia cultures exposed to LPS treatment. LUT inhibited the production of proinflammatory factors such as NO, superoxide and TNF-α in these cells (Chen et al. 2008). Moreover, Lin et al. (2010) implicated the cytoprotective and neurotrophic actions of LUT (10 and 20 μM, Sigma-Aldrich Co., St. Louis, MO, USA) which dose-dependently enhanced the expression of the growth-associated protein-43 (GAP-43) and differentiation marker in PC12 cell-induced cytotoxicity by serum withdrawal. Furthermore, LUT reduced apoptosis, enhanced the expression of HO-1 levels and increased the binding of Nrf2 to ARE, as a stimulator sequence of HO-1 promoter (Lin et al. 2010). Park and Song (2013) demonstrated that LUT (5, 10, 25 and 50 μM, Sigma-Aldrich Co., St. Louis, MO, USA) and LUT-7-O-glucoside (5, 10, 25 and 50 μM, Sigma-Aldrich Co., St. Louis, MO, USA) inhibit LPS-induced inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells (Park and Song 2013). Hu et al. (2014) revealed that treatment with LUT (20 μM, Sigma-Aldrich Co., St. Louis, MO, USA) attenuates cytotoxicity in 6-OHDA-induced PC12 cells (an in vitro model of PD) by reducing the oxidative stress and caspase-3 activation. Likewise, LUT reduced the transcription of p53 target genes including p21, PUMA and GADD45α. In a similar finding, they demonstrated that this agent modulated the activated Keap1-Nrf2-ARE pathway mediated by 6-OHDA, leading to a reduction in the expression of glutamate cysteine ligase (GCL) consisting of a catalytic subunit (GCLC) and heme oxygenase-1 (HO-1; Hu et al. 2014). Zhu et al. (2014) showed that LUT (20 μM, purity >98%; molecular weight, 286.24; chemical formula C15H10O6) inhibits SH-SY5Y cell apoptosis through the inhibition of TLR-4, NF-κB, mitogen-activated protein kinase (MAPK) and Akt pathways in LPS-stimulated co-cultured murine microglial BV2 cells (Zhu et al. 2014). Moreover, Lin et al. (2015) proved that LUT (10, 25 and 50 μg/ml, Chengdu Must Biotechnology Co., Ltd. Chengdu, China, purity >98.0%) induced protection against the H2O2-induced apoptosis cell death in PC12 neurons by inhibiting the decrease in cell viability, generation of ROS and releasing the LDH. In addition, the SOD and GSH-Px levels were increased after administration of LUT; however, MDA levels were reduced. Furthermore, LUT enhanced the Bcl-2-to-Bax ratio and increased the phosphorylation of Akt (Lin et al. 2015). Wu et al. (2017) demonstrated the ameliorative properties of dietary flavonoids LUT (20 μM, National Institutes for Food and Drug Control, Beijing, China) which attenuated the increased activation of caspase-3, ROS, expression of γ-H2AX and α-Syn in an in vitro model of PD induced by arsenite in neural PC12 cells (Wu et al. 2017).
Recently, Patil et al. (2014) demonstrated that LUT (10 and 20 mg/kg, A. K. Scientific, Inc., Union City, CA, USA) and apigenin (5, 10 and 20 mg/kg, A. K. Scientific, Inc., Union City, CA, USA) improves locomotor and muscular changes in a mice model of PD exposed to neurotoxin 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP; 25 mg/kg) along with probenecid (250 mg/kg). In addition, LUT and apigenin protected the neurons of SN against the neurotoxicity of MPTP by increasing the BDNF and decreasing the GFAP levels. Their finding assumed that LUT with enhancing potential of neurotrophic factors (NFs) could support the dopaminergic neurons of SN by modulating the oxidative stress, neuroinflammation and glial activation (Patil et al. 2014). Siracusa et al. (2015) found that treatment with co-ultraPEA/LUT (1 mg/kg, Sigma-Aldrich, St, Louis, MO, USA) decreased the tyrosine hydroxylase (TH) immunopositive neurons, neuroinflammation and stimulated autophagy process in a mice model of PD induced by MPTP, actions which may underlie its neuroprotective effect. Furthermore, their results were confirmed via an in vitro study (co-ultraPEA/LUT concentration: 0.1–100 μM) on SH-SY5Y neuroblastoma cells. Pretreatment with co-ultraPEA/LUT decreased cell death and maintained high levels of p62 and beclin-1 (improving the autophagy process; Siracusa et al. 2015).
Conclusion
CNS diseases are leading causes of mortality and morbidity worldwide. Literature suggests that LUT has a therapeutic role in the treatment of neurological disorders. Based on the reviewed literature, LUT has antioxidant and neuroprotective properties. It also suppresses different cell-signaling pathways and regulates inflammation, in part, and may be responsible for its beneficial impacts on damaged nerve functions in different neurological disorders. Although further studies including clinical trials should be conducted to confirm this hypothesis, LUT as a neuroprotective agent is a potential suitable therapeutic candidate against different neurological disorders such as AD, PD, TBI, etc.
Abbreviations
- TBI:
-
Traumatic brain injury
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- ARE:
-
Antioxidant responsive element
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- NF-ƘB:
-
Nuclear factor kappa B
- IκB:
-
Inhibitor of κB
- JNK:
-
c-Jun amino-terminal kinase
- CREB:
-
Cyclic AMP response element-binding protein
- ERK:
-
Extracellular signal-regulated kinase
- PKC:
-
Protein kinase C
- TNF-α:
-
Tumor necrosis factor-α
- IL:
-
Interleukin
- MDA:
-
Malondialdehyde
- GSH-Px:
-
Glutathione peroxidase
- COX:
-
Cyclooxygenase
- MBP:
-
Myelin basic protein
- PBMC:
-
Peripheral blood mononuclear cells
- BDNF:
-
Brain-derived neurotrophic factor
- GFAP:
-
Glial fibrillary acidic protein
- NOS:
-
Nitric oxide synthases
- 6-OHDA:
-
6-hydroxydopamine
- GAP-43:
-
Growth-associated protein-43
References
Alamouti MA, Bakhtiyari M, Moradi F et al (2015) Remyelination of the corpus callosum by olfactory ensheathing cell in an experimental model of multiple sclerosis. Acta Med Iran 53(9):533–539
Andersen OM, Markham KR (2005) Flavonoids: chemistry, biochemistry and applications. CRC press
Baradaran A (2017) Administration of herbal drugs in geriatric individuals; trends on its helps and hazards. Geriatr Persia 1(1)
Bayat M, Tameh AA, Ghahremani MH et al (2012) Neuroprotective properties of Melissa officinalis after hypoxic-ischemic injury both in vitro and in vivo. DARU J Pharm Sci 20(1):42
Bertolotto A, Granieri L, Marnetto F et al (2015) Biological monitoring of IFN-β therapy in multiple sclerosis. Cytokine Growth Factor Rev 26(2):241–248
Birman H, Dar KA, Kapucu A, Acar S, Üzüm G (2012) Effects of luteolin on liver, kidney and brain in pentylentetrazol-induced seizures: involvement of metalloproteinases and NOS activities. Balkan Med J 29(2):188
Birt DF, Hendrich S, Wang W (2001) Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther 90(2):157–177
Bové J, Prou D, Perier C, Przedborski S (2005) Toxin-induced models of Parkinson’s disease. NeuroRx 2(3):484–494
Brooks JC, Strauss DJ, Shavelle RM, Paculdo DR, Hammond FM, Harrison-Felix CL (2013) Long-term disability and survival in traumatic brain injury: results from the national institute on disability and rehabilitation research model systems. Arch Phys Med Rehabil 94(11):2203–2209
Caltagirone C, Cisari C, Schievano C et al (2016) Co-ultramicronized palmitoylethanolamide/luteolin in the treatment of cerebral ischemia: from rodent to man. Transl Stroke Res 7(1):54–69
Cardenas-Rodriguez N, Huerta-Gertrudis B, Rivera-Espinosa L et al (2013) Role of oxidative stress in refractory epilepsy: evidence in patients and experimental models. Int J Mol Sci 14(1):1455–1476
Chen G, Fang Q, Zhang J, Zhou D, Wang Z (2011) Role of the Nrf2-ARE pathway in early brain injury after experimental subarachnoid hemorrhage. J Neurosci Res 89(4):515–523
Chen H-Q, Jin Z-Y, Wang X-J, Xu X-M, Deng L, Zhao J-W (2008) Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. Neurosci Lett 448(2):175–179
Chen X, Krakauer T, Oppenheim JJ, Howard OZ (2004) Yin Zi Huang, an injectable multicomponent Chinese herbal medicine, is a potent inhibitor of T-cell activation. J Altern Complement Med 10(3):519–526
Cheng HY, Hsieh MT, Tsai FS et al (2010) Neuroprotective effect of luteolin on amyloid β protein (25–35)-induced toxicity in cultured rat cortical neurons. Phytother Res 24(S1)
Chew LJ, Takanohashi A, Bell M (2006) Microglia and inflammation: impact on developmental brain injuries. Dev Disabil Res Rev 12(2):105–112
Choi S-M, Kim BC, Cho Y-H et al (2014) Effects of flavonoid compounds on β-amyloid-peptide-induced neuronal death in cultured mouse cortical neurons. Chonnam Med J 50(2):45–51
Chowdhury S, Rasmusson D (2002) Effect of GABAB receptor blockade on receptive fields of raccoon somatosensory cortical neurons during reorganization. Exp Brain Res 145(2):150–157
Coleta M, Batista MT, Campos MG et al (2006) Neuropharmacological evaluation of the putative anxiolytic effects of Passiflora edulis Sims, its sub-fractions and flavonoid constituents. Phytother Res 20(12):1067–1073
Coleta M, Campos MG, Cotrim MD, de Lima TCM, da Cunha AP (2008) Assessment of luteolin (3′, 4′, 5, 7-tetrahydroxyflavone) neuropharmacological activity. Behav Brain Res 189(1):75–82
Comalada M, Ballester I, Bailón E et al (2006) Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure–activity relationship. Biochem Pharmacol 72(8):1010–1021
Corbett D, Jeffers M, Nguemeni C, Gomez-Smith M, Livingston-Thomas J (2015) Lost in translation: rethinking approaches to stroke recovery. Prog Brain Res 218:413–434
Cordaro M, Impellizzeri D, Paterniti I et al (2016) Neuroprotective effects of co-ultraPEALut on secondary inflammatory process and autophagy involved in traumatic brain injury. J Neurotrauma 33(1):132–146
Dajas F, Juan Andres A-C, Florencia A, Carolina E, Felicia R-M (2013) Neuroprotective actions of flavones and flavonols: mechanisms and relationship to flavonoid structural features. Cent Nerv Syst Agents Med Chem (Formerly Curr Med Chem Cent Nerv Syst Agents) 13(1):30–35
Dehghan G, Khoshkam Z (2012) Tin (II)–quercetin complex: synthesis, spectral characterisation and antioxidant activity. Food Chem 131(2):422–426
Dehghan G, Shafiee A, Ghahremani MH, Ardestani SK, Abdollahi M (2007) Antioxidant potential of various extracts from Ferula szovitsiana. In relation to their phenolic content. Pharm Biol 45(9):691–699
Di Domenico F, Barone E, Perluigi M, Butterfield DA (2015) Strategy to reduce free radical species in Alzheimer’s disease: an update of selected antioxidants. Expert Rev Neurother 15(1):19–40
Diniz TC, Silva JC, Lima-Saraiva SRGd et al (2015) The role of flavonoids on oxidative stress in epilepsy. Oxidative Med Cell Longev 2015
Dirscherl K, Karlstetter M, Ebert S et al (2010) Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. J Neuroinflammation 7(1):3
Fang L, Xia Q, Zhang X (2009) Protective effect of luteolin on neurons against cerebral ischemia/reperfusion injury via regulating the activity of ATPase. FASEB J 23(1 Supplement):614.19
Feigin VL (2007) Herbal medicine in stroke. Am Heart Assoc
Fernández SP, Wasowski C, Loscalzo LM et al (2006) Central nervous system depressant action of flavonoid glycosides. Eur J Pharmacol 539(3):168–176
Ghorbani R, Mokhtari T, Khazaei M, Salahshoor M, Jalili C, Bakhtiari M (2014) The effect of walnut on the weight, blood glucose and sex hormones of diabetic male rats. Int J Morphol 32(3)
Goutman JD, Waxemberg MD, Doñate-Oliver F, Pomata PE, Calvo DJ (2003) Flavonoid modulation of ionic currents mediated by GABA a and GABA C receptors. Eur J Pharmacol 461(2):79–87
Grosso C, Valentão P, Ferreres F, Andrade PB (2013) The use of flavonoids in central nervous system disorders. Curr Med Chem 20(37):4694–4719
Guo Z, Cupples L, Kurz A et al (2000) Head injury and the risk of AD in the MIRAGE study. Neurology 54(6):1316–1323
Hanisch U-K, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10(11):1387
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55(6):481–504
Hougee S, Sanders A, Faber J et al (2005) Decreased pro-inflammatory cytokine production by LPS-stimulated PBMC upon in vitro incubation with the flavonoids apigenin, luteolin or chrysin, due to selective elimination of monocytes/macrophages. Biochem Pharmacol 69(2):241–248
Hu L-W, Yen J-H, Shen Y-T, Wu K-Y, Wu M-J (2014) Luteolin modulates 6-hydroxydopamine-induced transcriptional changes of stress response pathways in PC12 cells. PLoS One 9(5):e97880
Jafari T (2016) Antioxidants; helpful or harmful? Ann Res Antioxid 1(2)
Jang S, Kelley KW, Johnson RW (2008) Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci 105(21):7534–7539
Jenner P, Olanow CW (1996) Oxidative stress and the pathogenesis of Parkinson's disease. Neurology 47(6 Suppl 3):161S–170S
Jin Y, Silverman AJ, Vannucci SJ (2009) Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke 40(9):3107–3112
Johnson JAJ (2008) The Nrf2 AREpathway: an indicatorandmodulatorofoxidativestressinneurodegeneration. Ann N Y Acad Sci 1147:61
Kandere-Grzybowska K, Kempuraj D, Cao J, Cetrulo CL, Theoharides TC (2006) Regulation of IL-1-induced selective IL-6 release from human mast cells and inhibition by quercetin. Br J Pharmacol 148(2):208–215
Kawabori M, Yenari MA (2015) Inflammatory responses in brain ischemia. Curr Med Chem 22(10):1258–1277
Kempuraj D, Madhappan B, Christodoulou S et al (2005) Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br J Pharmacol 145(7):934–944
Kempuraj D, Tagen M, Iliopoulou B et al (2008) Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell-dependent stimulation of Jurkat T cells. Br J Pharmacol 155(7):1076–1084
Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP (1999) Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure–activity relationships. Biochem Pharmacol 58(5):759–765
Kim JS, Jobin C (2005) The flavonoid luteolin prevents lipopolysaccharide-induced NF-κB signalling and gene expression by blocking IκB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Immunology 115(3):375–387
Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H (2000) Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy 30(4):501–508
Kr GU, Christina A (2011) Effect of Rotula aquatica Lour. on ethylene-glycol induced urolithiasis in rats. Int J Drug Dev Res
Lamy S, Moldovan PL, Saad AB, Annabi B (2015) Biphasic effects of luteolin on interleukin-1β-induced cyclooxygenase-2 expression in glioblastoma cells. Biochim Biophys Acta (BBA)-Mol Cell Res 1853(1):126–135
Lee JK, Kim SY, Kim YS, Lee W-H, Hwang DH, Lee JY (2009) Suppression of the TRIF-dependent signaling pathway of Toll-like receptors by luteolin. Biochem Pharmacol 77(8):1391–1400
Leung HW-C, Kuo C-L, Yang W-H, Lin C-H, Lee H-Z (2006) Antioxidant enzymes activity involvement in luteolin-induced human lung squamous carcinoma CH27 cell apoptosis. Eur J Pharmacol 534(1):12–18
Lin C-W, Wu M-J, Liu IY-C, Su J-D, Yen J-H (2010) Neurotrophic and cytoprotective action of luteolin in PC12 cells through ERK-dependent induction of Nrf2-driven HO-1 expression. J Agric Food Chem 58(7):4477–4486
Lin L-Z, Harnly JM (2010) Identification of the phenolic components of chrysanthemum flower (Chrysanthemum morifolium Ramat). Food Chem 120(1):319–326
Lin P, Tian XH, Yi YS, Jiang WS, Zhou YJ, Cheng WJ (2015) Luteolin-induced protection of H2O2-induced apoptosis in PC12 cells and the associated pathway. Mol Med Rep 12(5):7699–7704
Liu B, Hong J-S (2003) Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther 304(1):1–7
Liu R, Gao M, Qiang G-F et al (2009) The anti-amnesic effects of luteolin against amyloid β 25–35 peptide-induced toxicity in mice involve the protection of neurovascular unit. Neuroscience 162(4):1232–1243
Maroso M, Balosso S, Ravizza T et al (2010) Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 16(4):413–419
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer disease and down syndrome. Proc Natl Acad Sci 82(12):4245–4249
Meissner WG, Frasier M, Gasser T et al (2011) Priorities in Parkinson's disease research. Nat Rev Drug Discov 10(5):377–394
Middleton E, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52(4):673–751
Milo R, Kahana E (2010) Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev 9(5):A387–A394
Mokhtari T, Akbari M, Malek F et al (2017) Improvement of memory and learning by intracerebroventricular microinjection of T3 in rat model of ischemic brain stroke mediated by upregulation of BDNF and GDNF in CA1 hippocampal region. DARU J Pharm Sci 25(1):4
Nasri H (2016) Herbal drugs and new concepts on its use. J Prev Epidemiol 1(1)
O'Meara ES, Kukull WA, Sheppard L et al (1997) Head injury and risk of Alzheimer's disease by apolipoprotein E genotype. Am J Epidemiol 146(5):373–384
Paladini A, Marder M, Viola H, Wolfman C, Wasowski C, Medina J (1999) Flavonoids and the central nervous system: from forgotten factors to potent anxiolytic compounds. J Pharm Pharmacol 51(5):519–526
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longev 2(5):270–278
Park CM, Song Y-S (2013) Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutr Res Pract 7(6):423–429
Park H-H, Lee S, Son H-Y et al (2008) Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch Pharm Res 31(10):1303–1311
Paterniti I, Cordaro M, Campolo M et al (2014) Neuroprotection by association of palmitoylethanolamide with luteolin in experimental Alzheimer’s disease models: the control of neuroinflammation. CNS Neurol Disord Drug Targets (Formerly Curr Drug Targets CNS Neurol Disord) 13(9):1530–1541
Paterniti I, Impellizzeri D, Di Paola R, Navarra M, Cuzzocrea S, Esposito E (2013) A new co-ultramicronized composite including palmitoylethanolamide and luteolin to prevent neuroinflammation in spinal cord injury. J Neuroinflammation 10(1):858
Patil S, Sathaye S (2015) Neuroprotective and neurotrophic effects of apigenin and luteolin in MPTP induced parkinsonism in mice. Mov Disord 30:S301–S302
Patil SP, Jain PD, Sancheti JS, Ghumatkar PJ, Tambe R, Sathaye S (2014) Neuroprotective and neurotrophic effects of Apigenin and Luteolin in MPTP induced parkinsonism in mice. Neuropharmacology 86:192–202
Pavlica S, Gebhardt R (2010) Protective effects of flavonoids and two metabolites against oxidative stress in neuronal PC12 cells. Life Sci 86(3):79–86
Plassman BL, Havlik R, Steffens D et al (2000) Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 55(8):1158–1166
Qiao H, Dong L, Zhang X et al (2012a) Protective effect of luteolin in experimental ischemic stroke: upregulated SOD1, CAT, Bcl-2 and claudin-5, down-regulated MDA and Bax expression. Neurochem Res 37(9):2014–2024
Qiao H, Zhang X, Zhu C et al (2012b) Luteolin downregulates TLR4, TLR5, NF-κB and p-p38MAPK expression, upregulates the p-ERK expression, and protects rat brains against focal ischemia. Brain Res 1448:71–81
Qiusheng Z, Yuntao Z, Rongliang Z, Dean G, Changling L (2005) Effects of verbascoside and luteolin on oxidative damage in brain of heroin treated mice. Pharm Int J Pharm Sci 60(7):539–543
Rafieian-Kopaei M (2013) Medicinal plants for renal injury prevention. J Renal Inj Prev 2(2):63
Rafieian-Kopaei M, Baradaran A, Rafieian M (2013) Plants antioxidants: from laboratory to clinic. J Nephropathol 2(2):152–153
Ramalingam R, Nath AR, Madhavi BB, Nagulu M, Balasubramaniam A (2013) Free radical scavenging and antiepileptic activity of Leucas lanata. J Pharm Res 6(3):368–372
Ren G, Kong J, Jia N, Shang X (2013) Luteolin attenuates neuronal apoptosis in the hippocampi of diabetic encephalopathy rats. Neural Regen Res 8(12):1071
Rodgers KM, Hutchinson MR, Northcutt A, Maier SF, Watkins LR, Barth DS (2009) The cortical innate immune response increases local neuronal excitability leading to seizures. Brain 132(9):2478–2486
Romanova D, Vachalkova A, Cipak L, Ovesna Z, Rauko P (2000) Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma 48(2):104–107
Saito Y, Nishio K, Ogawa Y et al (2007) Molecular mechanisms of 6-hydroxydopamine-induced cytotoxicity in PC12 cells: involvement of hydrogen peroxide-dependent and-independent action. Free Radic Biol Med 42(5):675–685
Sarrafchi A, Bahmani M, Shirzad H, Rafieian-Kopaei M (2016) Oxidative stress and Parkinson’s disease: new hopes in treatment with herbal antioxidants. Curr Pharm Des 22(2):238–246
Sawmiller D, Li S, Shahaduzzaman M et al (2014) Luteolin reduces Alzheimer’s disease pathologies induced by traumatic brain injury. Int J Mol Sci 15(1):895–904
Seelinger G, Merfort I, Schempp CM (2008) Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med 74(14):1667–1677
Shahidi F, McDonald J, Chandrasekara A, Zhong Y (2008) Phytochemicals of foods, beverages and fruit vinegars: chemistry and health effects. Asia Pac J Clin Nutr 17(S1):380–382
Shaikh MF, Tan KN, Borges K (2013) Anticonvulsant screening of luteolin in four mouse seizure models. Neurosci Lett 29(550):195–199.
Sharma V, Mishra M, Ghosh S et al (2007) Modulation of interleukin-1β mediated inflammatory response in human astrocytes by flavonoids: implications in neuroprotection. Brain Res Bull 73(1):55–63
Silver R, Curley JP (2013) Mast cells on the mind: new insights and opportunities. Trends Neurosci 36(9):513–521
Singh B, Singh D, Goel RK (2012) Dual protective effect of Passiflora incarnata in epilepsy and associated post-ictal depression. J Ethnopharmacol 139(1):273–279
Siracusa R, Paterniti I, Impellizzeri D et al (2015) The association of palmitoylethanolamide with luteolin decreases neuroinflammation and stimulates autophagy in Parkinson’s disease model. CNS Neurol Disord Drug Targets (Formerly Curr Drug Targets CNS Neurol Disord) 14(10):1350–1366
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35
Stadelmann C (2007) Recent advances in the neuropathology of multiple sclerosis. Rev Neurol 163(6–7):657–661
Sternberg Z, Chadha K, Lieberman A et al (2009) Immunomodulatory responses of peripheral blood mononuclear cells from multiple sclerosis patients upon in vitro incubation with the flavonoid luteolin: additive effects of IFN-β. J Neuroinflammation 6(1):28
Subasinghe S, Unabia S, Barrow CJ, Mok SS, Aguilar MI, Small DH (2003) Cholesterol is necessary both for the toxic effect of Aβ peptides on vascular smooth muscle cells and for Aβ binding to vascular smooth muscle cell membranes. J Neurochem 84(3):471–479
Sun M, Zhao Y, Gu Y, Zhang Y (2015) Protective effects of taurine against closed head injury in rats. J Neurotrauma 32(1):66–74
Tambe R, Patil A, Jain P, Sancheti J, Somani G, Sathaye S (2017) Assessment of luteolin isolated from Eclipta alba leaves in animal models of epilepsy. Pharm Biol 55(1):264–268
Taylor JM, Main BS, Crack PJ (2013) Neuroinflammation and oxidative stress: co-conspirators in the pathology of Parkinson’s disease. Neurochem Int 62(5):803–819
Terry RD, Masliah E, Salmon DP et al (1991) Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30(4):572–580
Theoharides TC (2009) Luteolin as a therapeutic option for multiple sclerosis. J Neuroinflammation 6(1):29
Thrift AG, Cadilhac DA, Thayabaranathan T et al (2014) Global stroke statistics. Int J Stroke 9(1):6–18
Tsai F-S, Peng W-H, Wang W-H et al (2007) Effects of luteolin on learning acquisition in rats: involvement of the central cholinergic system. Life Sci 80(18):1692–1698
Verbeek R, Plomp AC, van Tol EA, van Noort JM (2004) The flavones luteolin and apigenin inhibit in vitro antigen-specific proliferation and interferon-gamma production by murine and human autoimmune T cells. Biochem Pharmacol 68(4):621–629
Verbeek R, van Tol EA, van Noort JM (2005) Oral flavonoids delay recovery from experimental autoimmune encephalomyelitis in SJL mice. Biochem Pharmacol 70(2):220–228
Wang F, Shing M, Huen Y, Tsang SY, Xue H (2005a) Neuroactive flavonoids interacting with GABAA receptor complex. Curr Drug Targets CNS Neurol Disord 4(5):575–585
Wang X, Chen S, Ma G, Ye M, Lu G (2005b) Involvement of proinflammatory factors, apoptosis, caspase-3 activation and Ca 2+ disturbance in microglia activation-mediated dopaminergic cell degeneration. Mech Ageing Dev 126(12):1241–1254
Wang J, Fields J, Zhao C et al (2007a) Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med 43(3):408–414
Wang X-J, Yan Z-Q, Lu G-Q, Stuart S, Chen S-D (2007b) Parkinson disease IgG and C5a-induced synergistic dopaminergic neurotoxicity: role of microglia. Neurochem Int 50(1):39–50
Wang GG, Lu XH, Li W, Zhao X, Zhang C (2011) Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evid Based Complement Alternat Med 2011
Wang H, Wang H, Cheng H, Che Z (2016a) Ameliorating effect of luteolin on memory impairment in an Alzheimer's disease model. Mol Med Rep 13(5):4215–4220
Wang W, Li H, Yu J et al (2016b) Protective effects of chinese herbal medicine rhizoma drynariae in rats after traumatic brain injury and identification of active compound. Mol Neurobiol 53(7):4809–4820
Weng Z, Patel AB, Panagiotidou S, Theoharides TC (2015) The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J Allergy Clin Immunol 135(4):1044–1052 e5
Werner C, Engelhard K (2007) Pathophysiology of traumatic brain injury. Br J Anaesth 99(1):4–9
Wruck C, Claussen M, Fuhrmann G et al (2007) Luteolin protects rat PC 12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keapl-Nrf2-ARE pathway. Neuropsychiatr Disord Integrative Approach 72:57–67
Wu Y, Jiang X, Yang K et al (2017) Inhibition of α-Synuclein contributes to the ameliorative effects of dietary flavonoids luteolin on arsenite-induced apoptotic cell death in the dopaminergic PC 12 cells. Toxicol Mech Methods 1–33
Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C (2001) Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther 296(1):181–187
Xu B, Li X-X, He G-R et al (2010) Luteolin promotes long-term potentiation and improves cognitive functions in chronic cerebral hypoperfused rats. Eur J Pharmacol 627(1):99–105
Xu J, Wang H, Ding K et al (2014a) Luteolin provides neuroprotection in models of traumatic brain injury via the Nrf2–ARE pathway. Free Radic Biol Med 71:186–195
Xu J, Wang H, Lu X et al (2014b) Posttraumatic administration of luteolin protects mice from traumatic brain injury: implication of autophagy and inflammation. Brain Res 1582:237–246
Yan W, Wang H-D, Hu Z-G, Wang Q-F, Yin H-X (2008) Activation of Nrf2–ARE pathway in brain after traumatic brain injury. Neurosci Lett 431(2):150–154
Zhang J, Perry G, Smith MA et al (1999) Parkinson's disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol 154(5):1423–1429
Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F (2013a) Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol 100:30–47
Zhang Y-C, Gan F-F, Shelar SB, Ng K-Y, Chew E-H (2013b) Antioxidant and Nrf2 inducing activities of luteolin, a flavonoid constituent in Ixeris sonchifolia Hance, provide neuroprotective effects against ischemia-induced cellular injury. Food Chem Toxicol 59:272–280
Zhen J-L, Chang Y-N, Qu Z-Z, Fu T, Liu J-Q, Wang W-P (2016) Luteolin rescues pentylenetetrazole-induced cognitive impairment in epileptic rats by reducing oxidative stress and activating PKA/CREB/BDNF signaling. Epilepsy Behav 57:177–184
Zhou F, Chen S, Xiong J, Li Y, Qu L (2012) Luteolin reduces zinc-induced tau phosphorylation at Ser262/356 in an ROS-dependent manner in SH-SY5Y cells. Biol Trace Elem Res 149(2):273–279
Zhou F, Qu L, Lv K et al (2011) Luteolin protects against reactive oxygen species-mediated cell death induced by zinc toxicity via the PI3K–Akt–NF-κB–ERK-dependent pathway. J Neurosci Res 89(11):1859–1868
Zhu L, Bi W, Lu D, Zhang C, Shu X, Lu D (2014) Luteolin inhibits SH-SY5Y cell apoptosis through suppression of the nuclear transcription factor-κB, mitogen-activated protein kinase and protein kinase B pathways in lipopolysaccharide-stimulated cocultured BV2 cells. Exp Ther Med 7(5):1065–1070
Zhu Z, Yan J, Jiang W et al (2013) Arctigenin effectively ameliorates memory impairment in Alzheimer's disease model mice targeting both β-amyloid production and clearance. J Neurosci 33(32):13138–13149
Author information
Authors and Affiliations
Contributions
TM, AZ and TA conceived the concept, researched and analyzed the literature and wrote the manuscript; MARH, GH, ZK and BY analyzed the literature and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Availability of Data and Material
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Rights and permissions
About this article
Cite this article
Ashaari, Z., Hadjzadeh, MAR., Hassanzadeh, G. et al. The Flavone Luteolin Improves Central Nervous System Disorders by Different Mechanisms: A Review. J Mol Neurosci 65, 491–506 (2018). https://doi.org/10.1007/s12031-018-1094-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1094-2