Abstract
In brain, excess zinc alters the metabolism of amyloid precursor protein, leading to β-amyloid protein deposition, one of the hallmarks of Alzheimer’s disease (AD) pathology. Recently, it has been reported that zinc accelerates in vitro tau fibrillization, another hallmark of AD. In the current study, we examined the effect of high-concentration zinc on tau phosphorylation in human neuroblastoma SH-SY5Y cells. We found that incubation of cells with zinc resulted in abnormal tau phosphorylation at Ser262/356. Moreover, the current study has investigated whether luteolin (Lu), a bioflavonoid, could decrease zinc-induced tau hyperphosphorylation and its underlying mechanisms. Using Western blot and protein phosphatase activity assay, activities of tau kinases and phosphatase were investigated. Our data suggest (1) that zinc induces tau hyperphosphorylation at Ser262/356 epitope and (2) that Lu efficiently attenuates zinc-induced tau hyperphosphorylation through not only its antioxidant action but also its regulation of the phosphorylation/dephosphorylation system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In brain, zinc is released into the synaptic cleft with synaptic activity, reaching routine concentrations of 10–20 μM and a concentration of 300 μM in extreme conditions. It is well established that zinc has a crucial pathogenic role in promoting the deposition of β-amyloid protein [1]. Zinc seems to accumulate in and around neurofibrillary tangles (NFTs), characterized by hyperphosphorylated tau protein, and correlate with the severity of dementia in neurodegenerative diseases [2]. Moreover, enhanced zinc consumption increases brain levels of zinc and causes memory deficits [3]. Recently, it has been shown that zinc can also accelerate the aggregation of a tau peptide, the major protein subunit of NFTs under reducing conditions [4]. These data suggest that zinc may play a key role in quickening the clinical progression of the dementia associated with Alzheimer’s disease (AD). Whether zinc could trigger tau hyperphosphorylation, however, remains elusive.
Targeting zinc ions or controlling zinc-triggering downstream signal transduction cascades are believed to be efficient therapeutic strategies for zinc-induced toxicity-associated diseases. It was reported that treatment with a zinc chelator markedly and rapidly inhibited β-amyloid accumulation in AD-model mice [5, 6], suggesting that targeting zinc might improve senile plaque lesions. Nevertheless, whether targeting zinc could inhibit tau hyperphosphorylation is obscure.
As a bioflavonoid, luteolin (Lu) has been shown to possess antioxidant and anti-inflammatory properties. It was reported that Lu, when applied to the Tg2576 mouse model of AD, could decrease soluble A β levels, reduce GSK3 activity, and disrupt PS1–amyloid precursor protein (APP) association [7], suggesting that Lu may exert a beneficial effect on neurodegenerative diseases. Our previous study has demonstrated that Lu protects SH-SY5Y cells against reactive oxygen species (ROS)-mediated apoptotic cell death induced by zinc by inhibiting the PI3K/Akt/NF–κB/ERKs pathway [8]. Whether Lu is able to interfere with zinc-induced tau phosphorylation, however, is needed for further investigations.
The aims of the current study are to measure the effect of zinc on tau phosphorylation in SH-SY5Y cells, to elucidate the effect of Lu on zinc-induced tau phosphorylation and its underlying mechanisms. Our data demonstrate that high-concentration zinc triggers tau hyperphosphorylation via the overactivation of tau kinases, and Lu downregulates the kinase activities and recovers the suppressed total phosphatase activity. The findings strongly support the notion that Lu might reduce the zinc-induced tau phosphorylation at Ser262/356 epitope in an ROS-dependent manner by regulating the balance between tau kinases and phosphatases.
Materials and Methods
Materials
Anti-pSer262, anti-pSer356 tau antibodies were purchased from Bioworld Technology (Minneapolis, MN, USA). Anti-tau 5 antibody was purchased from Calbiochem (San Diego, CA, USA). Anti-pCaMKII, anti-total CaMKII, anti-pSer21/9 GSK3α/β, anti-total GSK3α/β, anti-pSer389 p70S6K, anti-total p70S6K, and anti-GAPDH antibodies were purchased from Cell Signal Technology (Beverly, MA). U0126, GSH, LY294002, okadaic acid (OA), and rapamycin were purchased from Beyotime Biotechnology (Beyotime, China). Other reagents including zinc sulfate were obtained from Sigma, unless indicated otherwise.
Cell Culture
To assure cell-cycle synchronization, SH-SY5Y cells were cultured for 24 h in Dulbecco’s modified Eagle’s medium (low glucose) supplemented with HEPES, 0.5% (v/v) fetal bovine serum (Hyclone, Inc.), 100 U/mL penicillin, and 100 mg/mL streptomycin, and maintained in a humidified, 5% CO2 incubator at 37°C. Before treating the cells, the cultures were maintained in medium containing 10% FBS for at least 10 h. Unless otherwise stated, experiments were performed with cells at 80% confluency.
Western Blot Assay
To determine the phosphorylation levels of tau protein and the activities of several tau kinases, Western blot analyses were performed as described previously [9]. Briefly, cells were rinsed with ice-cold PBS and then lysed in buffer containing 1% Triton X-100, 1% sodium dodecyl sulfate (SDS) supplemented with protease inhibitor, centrifuged at 8,000 rpm for 5 min at 4°C. Equal amounts of total protein (measured with a BCA Protein Assay Kit, Pierce) were separated by SDS-PAGE on 10% polyacrylamide gels, and then transferred to PVDF membranes. Membranes were blocked with 5% non-fatty milk in Tris buffered saline (TBS)/0.1% (v/v) and Tween-20 (TBST) and then subsequently probed with appropriate primary antibodies diluted in 5% BSA in TBST for 1 h. Membranes were washed with TBST and incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibodies (1:10,000) for 1 h at room temperature; labeled bands were visualized with an ECL detection system. The relative density of immunoreactive bands on Western blots was measured by using the Quantity One 4.3.0 software (Bio-Rad Laboratories, Hercules, CA, USA).
Protein Phosphatase Activity Measurements
Cellular protein phosphatase 2A (PP2A) and total Ser/Thr phosphatase activity were measured using a colorimetric protein phosphatase assay kit purchased from Enzo Life Sciences Inc. The assay was performed according to the manufacturer’s instructions. Briefly, control and treated cells were scraped off the plates with phosphatase extraction buffer. After brief centrifugation, the supernatant was incubated with the phosphate detection solution offered by the kit. Following incubation, the supernatant transferred to a 96-well plate (Bio-Tek Instruments), and free phosphate was quantified by measuring the absorbance of the mixture at 650 nm. Data were normalized to control values.
Statistical Analysis
Quantitative data were statistically analyzed by independent Student’s t test. Data are presented as the mean ± standard deviation and analyzed using SPSS 10.0 statistical software (SPSS Inc, Chicago, IL, USA). p < 0.05 is regarded as the criterion for significant differences among groups.
Results
Zinc induces abnormal tau phosphorylation at Ser262/356 epitope involved in upregulation of several tau kinases, and it appears to be mediated by ROS in SH-SY5Y cells
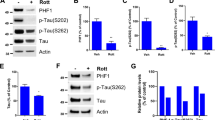
To investigate the effect of zinc on tau phosphorylation in SH-SY5Y cells, the level of tau phosphorylation was examined after cells were treated with different concentrations (0, 100, 200, 300, or 400 μM) of zinc for 1 h. Figure 1a shows that tau phosphorylation at Ser356 is markedly enhanced in the zinc treatment groups compared with the control groups, suggesting that zinc may induce in vitro tau hyperphosphorylation.
Zinc induces abnormal tau phosphorylation at Ser262/356 epitope involved in several tau kinases, and it appears to be mediated by ROS in SH-SY5Y cells. After 0–400 μM zinc treatment for 1 h, the level of tau phosphorylation at Ser356 was examined with Western blot assay (a; n = 3). After 200 μM H2O2 treatment for 0–4 h, the levels of tau phosphorylation at Ser262/356 were also assayed with Western blot (b; n = 3), and the shape of the curve of the changes induced by H2O2 in tau phosphorylation is shown in c. After U0126 (10 μM), GSH (30 mM) and/or Rapa (100 nM) pretreatment for 1 h followed by 300 μM zinc for an additional 1 h, the level of tau phosphorylation was detected (d, n = 3). Cells were pretreated with LY294002 at concentrations of 10, 20, or 40 μM followed by 300 μM zinc for an additional 1 h, and tau phosphorylation at Ser262 was detected (e, n = 3). The graphs in f represent densitometric analysis of the bands from e. Significance indicated by *p < 0.05, **p < 0.01 compared with control groups, #p < 0.05, ##p < 0.01 compared with zinc-treated groups
Exogenous addition of hydrogen peroxide (H2O2) has often been used to mimic the effect of H2O2 production during signal transduction processes [10]. Moreover, our published data reveal that zinc (300 μM, 1 h) significantly induces an increased intracellular ROS level [8]. Thus, in our study, H2O2 treatment was used to mimic the intracellular production of H2O2, which mediated, at least in part, zinc-induced tau phosphorylation. Cells were treated with H2O2 (200 μM) for different lengths of time from 10 min to 4 h, and the levels of phosphorylated tau at Ser262/356 were examined with Western blot assay. As shown in Fig. 1b and c, tau phosphorylation at both sites is elevated at 10 min (relative values, 0.982 ± 0.14 for pSer262, 2.04 ± 0.17 for pSer356, p < 0.05 vs. control groups), peaked at 1 h (relative values, 1.36 ± 0.32 for pSer262 p < 0.05, 2.5 ± 0.16 for pSer356, p < 0.01 vs. control groups), and then returned to control levels within 4 h of exposure to H2O2 (relative values, 1.07 ± 0.18 for pSer262, 0.27 ± 0.37 for pSer356, p > 0.05 vs. control groups), indicating that the changes of phosphorylation levels of tau protein triggered by exogenous H2O2 appear, in case of time course measurement, to be in accordance with that of zinc induction.
To further clarify whether the increased intracellular ROS level mediates zinc-induced tau phosphorylation, a potent free radical scavenger reduced glutathione (GSH) was used to study the effect of ROS on tau phosphorylation. Cells were preincubated with GSH (30 mM, 1 h) followed by 300 μM zinc for an additional 1 h, and the level of pSer356 tau was detected. The result demonstrates that GSH attenuates zinc-induced tau phosphorylation, indicating that the increased intracellular ROS level appears to be tightly correlated with zinc-induced tau phosphorylation. In addition, our published data show that zinc strongly activates the extracellular signal-regulated kinase 1/2 (ERK1/2) [8], also considered as a tau kinase [11, 12]. To determine whether ERK1/2 as well as another tau kinase, the 70 kD S6 protein kinases (p70S6K), mediates zinc-induced tau phosphorylation, cells were pretreated for 1 h with rapamycin (Rapa, 100 nM), an inhibitor of FRAP/mTOR which is the immediate upstream kinase of p70S6K, or with the ERK1/2 inhibitor U0126 (10 μM), followed by 300 μM zinc for an additional 1 h. As shown in Fig. 1c, both Rapa and U0126 significantly decrease tau phosphorylation, suggesting that activations of p70S6K and ERK1/2 are responsible for zinc-induced tau phosphorylation.
We have preciously established that zinc also strongly induced Akt activation, being capable of phosphorylating tau at several sites [13]. To investigate whether Akt mediates zinc-induced tau phosphorylation, cells were pretreatment with LY294002 (0, 10, 20, or 40 μM), an PI3K inhibitor that acts as an upstream inhibitor of Akt activation, followed by 300 μM zinc for an additional 1 h, and tau phosphorylation at Ser262 was detected by Western blot assay. As shown in Fig. 1e and f, ptau is decreased by LY294002 at concentrations of 20 or 40 μM (relative values, 1.32 ± 0.17, 1.08 ± 0.36, p < 0.05 vs. zinc-treated groups), in particular, at a concentration of 40 μM, indicating that Akt is likely to mediate zinc-induced tau phosphorylation.
Luteolin Attenuates Zinc-Induced Abnormal Tau Phosphorylation
To investigate the effect of luteolin on zinc-induced tau phosphorylation, cells were pretreated for 1 h with 5–100 μM luteolin followed by 300 μM zinc for an additional 1 h, and the levels of tau phosphorylation at Ser262/356 were measured with Western blot analysis. Tau phosphorylation was significantly attenuated in the luteolin pretreatment groups compared with the zinc-alone treatment groups (Fig. 2a and b; relative values, 1.746 ± 0.37, 1.52 ± 0.28, 1.42 ± 0.38, 1.39 ± 0.27, 1.0 ± 0.42, and 0.47 ± 0.27, 2.5 ± 0.16 for pSer356; 1.3 ± 0.49, 1.33 ± 0.43, 1.31 ± 0.37, 0.73 ± 0.26, 0.52 ± 0.22, and 0.47 ± 0.27 for pSer262, in the 5, 10, 20, 40, 80, and 100 μM Lu pretreatment groups, respectively, p < 0.05, p < 0.01 vs. zinc-treated groups), in particular, at a concentration of 100 μM.
Luteolin attenuates zinc-induced abnormal tau phosphorylation. Pretreatment with increasing concentrations of luteolin for 1 h followed by zinc (300 μM) for an additional 1 h. The levels of tau phosphorylation at Ser262/356 were examined with Western blot (a). The graphs in b represent densitometric analysis of the bands from three independent experiments of a. Significance indicated by *p < 0.05, **p < 0.01 compared with control groups, #p < 0.05, ##p < 0.01 compared with zinc-treated groups. After cells had been treated with increasing concentrations of luteolin only (0–100 μM) for 1 h, the level of tau phosphorylation at Ser356 was also assayed (c; n = 3)
To test whether luteolin, per se, could affect tau phosphorylation, we treated cells with 5, 20, 80 or 100 μM luteolin alone for 1 h and measured the phosphorylation level of tau at Ser356. We found that luteolin alone did not obviously alter tau phosphorylation, suggesting that luteolin’s effect on tau phosphorylation appears to be dependent upon zinc (Fig. 2c).
Luteolin Regulates the Activities of Tau Protein Kinases and Protein Phosphatases
Since p70S6K and glycogen synthase kinase 3β (GSK3β) are the two major tau kinases, we estimated the effect of luteolin on their activities as assessed by the phosphorylation levels. Cells were pretreated with luteolin (1, 10, or 20 μM) followed by zinc treatment (300 μM) for an additional 1 h. As shown in Fig. 3a, the intensity of the relative immunoreaction of p70S6 kinase is dramatically decreased by luteolin pretreatment. Figure 3b shows GSK3β Ser9 phosphorylation, which inactivates GSK3β, is markedly reduced by luteolin, indicating that luteolin’s inhibitory effect on zinc-induced tau phosphorylation at Ser262/356 appears to be poorly correlated with inhibition of GSK3β.
Regulatory effects of luteolin on the activities of several tau kinases and protein phosphatase. Cells were pretreated for 1 h with 0, 1, 10, or 20 μM Lu for 1 h followed by 300 μM zinc treatment for an additional 1 h, and the phosphorylation levels of p70S6K (a, n = 3) and GSK3β (b, n = 3) were immunoblotted. After luteolin pretreatment with 5–100 μM for 1 h followed by 300 μM zinc treatment for an additional 1 h, the phosphorylation level of CaMKII (c, n = 3) was detected. After luteolin pretreatment with 5–100 μM for 1 h followed by 100 nM OA treatment for an additional 3 h, the phosphorylation level of tau at Ser262/356 (d, n = 3) was detected. After luteolin pretreatment with 20, 40, or 80 μM for 1 h followed by 300 μM zinc treatment for an additional 1 h, the total protein phosphatase activity (e, n = 6) was assayed as described in “Materials and Methods.” Significance indicated by *p < 0.05 compared with control groups, #p < 0.05 compared with zinc-treated groups
It has been reported that calcium/calmodulin-dependent protein kinase II (CaMKII) is an important tau kinase. Thus, in our study, CaMKII activity was also investigated. Cells were pretreated with 5–100 μM luteolin followed by 300 μM zinc for an additional 1 h, and the relative level of pCaMKII was measured with Western blot. As shown in Fig. 3c, the level of pCaMKII is not decreased by luteolin pretreatment, suggesting that CaMKII appears not to be implicated with zinc-induced tau phosphorylation.
A major protein phosphatase implicated in tau dephosphorylation is the PP2A that is reduced in both levels and activity in AD brain. To further define whether luteolin’s inhibition on zinc-induced tau phosphorylation is implicated with the dephosphorylation system, we assayed the role of the protein phosphatase in zinc-induced tau phosphorylation. First, cells were pretreated with 5–100 μM luteolin for 1 h followed by OA (100 nM, 3 h), a specific PP2A inhibitor, and the relative levels of pSer262/356 tau were examined with Western blot. Figure 3d shows that luteolin does not decrease but rather increases OA-induced tau phosphorylation at Ser262/356, indicating that luteolin reduces zinc-induced tau phosphorylation likely independent of recovery of PP2A activity.
Next, to further investigate whether luteolin alters the activities of the PP2A or total protein phosphatase induced by zinc, we pretreated cells with luteolin followed by zinc treatment and examined the activities of the PP2A and total protein phosphatase using a protein phosphatase assay kit. Our results show that neither luteolin nor zinc alters the PP2A activity (data not shown), indicating that PP2A appears not to be involved in zinc-induced tau phosphorylation. Furthermore, the total phosphatase activity decreases in the zinc treatment groups and recovers in the Lu pretreatment groups compared with the zinc treatment groups (Fig. 3e, relative values, 93.1 ± 5.43 for the zinc-treated groups, p < 0.05 vs. control groups; 101.1 ± 5.9 for the 20 μM Lu pretreatment groups, 100 ± 5.8 for the 40 μM Lu pretreatment groups, 98.3 ± 5.7 for the 80 μM Lu pretreatment groups, p < 0.05 vs. zinc-treated groups), suggesting that the recovery total phosphatase activity may be one of mechanisms underlying Lu’s inhibition on zinc-induced tau phosphorylation.
Discussion
In the mammalian brain, excess zinc is a promoter for aggregation of β-amyloid peptide and tau protein or cytolethal. It has been reported that NFTs are apparently required for the clinical expression of AD, and in the absence of amyloid plaques, it leads to dementia in related tauopathies characterized by tau hyperphosphorylation. It is increasingly believed that inhibition of abnormal tau hyperphosphorylation is likely to inhibit neurofibrillary degeneration [14, 15]. Our current study demonstrates that luteolin can inhibit in vitro abnormal tau phosphorylation induced by excess zinc, suggesting that luteolin’s neuroprotection against zinc toxicity is likely implicated with abnormal tau phosphorylation.
Tau phosphorylation at Ser262 in pretangle neurons, as an early event in tauopathology [16], significantly attenuates the ability of tau to bind microtubules approximately by 35% [17]. It has been recently reported that in Drosophila co-expression of human A β42 and mild tau increases tau phosphorylation at Ser262 and enhances tau-induced neurodegeneration; co-expression of A β42 and a Ser262Ala tau mutation, however, do not cause neurodegeneration, suggesting that the Ser262 phosphorylation site is required for the pathogenic interaction between A β42 and tau [18]. Although the precise pathophysiology is not fully known, it is increasingly believed that tau phosphorylation at Ser262 as well as Ser356 may be an important therapeutic target in AD.
Tau hyperphosphorylation is well known to result from an imbalance between tau protein kinases and phosphatases. It has been predicted that selective inhibition of tau kinases and/or activation of tau phosphatases might be the most promising approach to treat tauopathies like AD. Tau is phosphorylated by several protein kinases, and among various kinases, GSK3β is one of the most implicated kinases for tau hyperphosphorylation. Our study reveals that luteolin pretreatment causes an obvious reduction in GSK3β Ser9 phosphorylation, suggesting that luteolin reduces zinc-induced tau phosphorylation independent of GSK3β inactivation according to the study from Meske V [19]. It is well documented that Ser262/356 in tau is a good in vitro substrate for CaMKII. It has been reported that CaMKII accounts for approximately 45% of tau phosphorylation at Ser262 [20], suggesting that tau at Ser262 seems to be primarily phosphorylated by CaMKII [21]. In our study, however, luteolin appears not to inhibit CaMKII activity, and the latter seems not to be implicated with zinc-induced tau phosphorylation at Ser262/356.
The p70S6K whose activity is markedly enhanced in neurons with NFTs mediates tau phosphorylation in AD brain and in vitro [22, 23]. It was reported that in vitro treatment with A β25–35 and in vivo transgenic APP expression could both trigger parallel increases in p70S6K activation and tau phosphorylation at Ser262 [24], suggesting that p70S6K plays an important role in tau phosphorylation at Ser262. Our data reveal that inhibition of zinc-induced tau phosphorylation by luteolin is tightly correlated with p70S6K inactivation. ERK1/2 is also believed to be involved in abnormal tau phosphorylation [25] in A β42-induced tau phosphorylation [26] and in transgenic models co-expressing tau V337M and the familial amyloid precursor protein AD mutation APP V717I [27]. The current study demonstrates that ERK1/2 is likely to mediate zinc-induced tau phosphorylation, whose inhibition may be a mechanism underlying action of luteolin on tau phosphorylation.
Whether oxidative stress contributes to tau hyperphosphorylation is somewhat controversial. It was found that mitochondrial oxidative stress caused tau hyperphosphorylation at PHF-1 (Ser396/Thr404) epitopes in M17 neuroblastoma cells [28] and at Ser-205/396 and Thr404 epitopes in a genetic animal model of oxidative stress [29]. It has been shown that treatment of H2O2 promotes tau dephosphorylation at AT8 (Ser202/Thr205) and PHF1 epitopes accompanied with oxidative stress in neuronal cells [30]. This might be partially determined by cell type as well as phosphorylation sites in tau. In the current study, the concentrations of H2O2 have been referred as a study from Uguz, AC [31]. Our current study supports that ROS may induce tau hyperphosphorylation at Ser262/356. Furthermore, our data demonstrate that luteolin may exert inhibitory effect on zinc-induced tau phosphorylation by antioxidative action; whether, however, ROS exerts an effect through regulation of tau kinases or tau phosphatases needs further investigations.
It is recently reported that selenate treatment can stabilize PP2A-tau complexes, reduce tau hyperphosphorylation, and completely abrogate NFTs formation via targeting PP2A [32, 33], suggesting that a therapy by enhancing phosphatase activity is also promising. Our study reveals that luteolin appears not to affect the PP2A activity but to recover the total phosphatase activity.
Recently, it has been reported that tau phosphorylation may antagonize apoptosis by stabilizing β-catenin via a mechanism involving Alzheimer’s neurodegeneration [34]; by contrast, tau dephosphorylation may promote apoptosis by a mechanism involving a failed dephosphorylation/activation of Bcl-2 [35]. Combined with our previous data showing that zinc triggers cell apoptosis, zinc-induced tau phosphorylation may help to antagonize apoptosis, but, in turn, however, contribute to tau pathology.
More and more evidences have been collected to show a crucial role of tau abnormalities in AD neurodegeneration, and the idea of targeting tau phosphorylation for developing therapeutic disease-modifying drugs of AD for therapeutic measures against zinc toxicity is increasingly becoming a major target for developing neurological disease treatments. Our study supports the notion that luteolin, (1) as a nontoxic and easily bio-available dietary flavonoid and (2) as a potent inhibitor of activated tau kinases should be beneficial for the treatment of chronic neurological diseases associated with zinc toxicity. The findings provide further support for luteolin’s neuroprotective effect on AD-like neurodegenerative diseases.
Abbreviations
- AD:
-
Alzheimer’s disease
- CaMKII:
-
Calcium/calmodulin-dependent protein kinase II
- ERK1/2:
-
Extracellular signal-regulated kinases 1 and 2
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GSH:
-
Reduced glutathione
- GSK3β:
-
Glycogen synthase kinase 3β
- H2O2 :
-
Hydrogen peroxide
- Lu:
-
Luteolin
- mTOR:
-
Mammalian target of rapamycin
- NFTs:
-
Neurofibrillary tangles
- OA:
-
Okadaic acid
- p70S6K:
-
70-kDa Ribosomal protein S6 kinase
- PP2A:
-
Protein phosphatase 2A
- Rapa:
-
Rapamycin
- ROS:
-
Reactive oxygen species
- SH-SY5Y:
-
Human SH-SY5Y neuroblastoma cells
References
Miller Y, Ma B, Nussinov R (2010) Zinc ions promote Alzheimer A beta aggregation via population shift of polymorphic states. Proc Natl Acad Sci U S A 107(21):9490–9495
Religa D, Strozyk D, Cherny RA, Volitakis I, Haroutunian V, Winblad B, Naslund J, Bush AI (2006) Elevated cortical zinc in Alzheimer disease. Neurology 67(1):69–75
Flinn JM, Hunter D, Linkous DH, Lanzirotti A, Smith LN, Brightwell J, Jones BF (2005) Enhanced zinc consumption causes memory deficits and increased brain levels of zinc. Physiol Behav 83(5):793–803
Mo ZY, Zhu YZ, Zhu HL, Fan JB, Chen J, Liang Y (2009) Low micromolar zinc accelerates the fibrillization of human tau via bridging of Cys-291 and Cys-322. J Biol Chem 284(50):34648–34657
Lee JY, Friedman JE, Angel I, Kozak A, Koh JY (2004) The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiol Aging 25(10):1315–1321
Petri S, Calingasan NY, Alsaied OA, Wille E, Kiaei M, Friedman JE, Baranova O, Chavez JC, Beal MF (2007) The lipophilic metal chelators DP-109 and DP-460 are neuroprotective in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem 102(3):991–1000
Rezai-Zadeh K, Douglas SR, Bai Y, Tian J, Hou H, Mori T, Zeng J, Obregon D, Town T, Tan J (2009) Flavonoid-mediated presenilin-1 phosphorylation reduces Alzheimer’s disease beta-amyloid production. J Cell Mol Med 13(3):574–588
Zhou F, Qu L, Lv K, Chen H, Liu J, Liu X, Li Y, Sun X (2011) Luteolin protects against reactive oxygen species-mediated cell death induced by zinc toxicity via the PI3K-Akt-NF-kappaB-ERK-dependent pathway. J Neurosci Res 89(11):1859–1868
Fang J, Zhou Q, Shi XL, Jiang BH (2007) Luteolin inhibits insulin-like growth factor 1 receptor signaling in prostate cancer cells. Carcinogenesis 28(3):713–723
Li Q, Sanlioglu S, Li S, Ritchie T, Oberley L, Engelhardt JF (2001) GPx-1 gene delivery modulates NFkappaB activation following diverse environmental injuries through a specific subunit of the IKK complex. Antioxid Redox Signal 3(3):415–432
Fang H, Zhang LF, Meng FT, Du X, Zhou JN (2010) Acute hypoxia promote the phosphorylation of tau via ERK pathway. Neurosci Lett 474(3):173–177
Harris FM, Brecht WJ, Xu Q, Mahley RW, Huang Y (2004) Increased tau phosphorylation in apolipoprotein E4 transgenic mice is associated with activation of extracellular signal-regulated kinase: modulation by zinc. J Biol Chem 279(43):44795–44801
Kyoung PH, Lovati E, Pasinetti GM, Ksiezak-Reding H (2004) Phosphorylation of tau at THR212 and SER214 in human neuronal and glial cultures: the role of AKT. Neuroscience 127(3):649–658
Gendron TF, Petrucelli L (2009) The role of tau in neurodegeneration. Mol Neurodegener 4:13
Iqbal K, Liu F, Gong CX, Alonso AC, Grundke-Iqbal I (2009) Mechanisms of tau-induced neurodegeneration. Acta Neuropathol 118(1):53–69
Augustinack JC, Schneider A, Mandelkow EM, Hyman BT (2002) Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol 103(1):26–35
Sengupta A, Kabat J, Novak M, Wu Q, Grundke-Iqbal I, Iqbal K (1998) Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch Biochem Biophys 357(2):299–309
Iijima K, Gatt A, Iijima-Ando K (2010) Tau Ser262 phosphorylation is critical for Abeta42-induced tau toxicity in a transgenic Drosophila model of Alzheimer’s disease. Hum Mol Genet 19(15):2947–2957
Meske V, Albert F, Ohm TG (2008) Coupling of mammalian target of rapamycin with phosphoinositide 3-kinase signaling pathway regulates protein phosphatase 2A- and glycogen synthase kinase-3 -dependent phosphorylation of Tau. J Biol Chem 283(1):100–109
Sironi JJ, Yen SH, Gondal JA, Wu Q, Grundke-Iqbal I, Iqbal K (1998) Ser-262 in human recombinant tau protein is a markedly more favorable site for phosphorylation by CaMKII than PKA or PhK. FEBS Lett 436(3):471–475
Bennecib M, Gong CX, Grundke-Iqbal I, Iqbal K (2001) Inhibition of PP-2A upregulates CaMKII in rat forebrain and induces hyperphosphorylation of tau at Ser 262/356. FEBS Lett 490(1–2):15–22
An WL, Cowburn RF, Li L, Braak H, Alafuzoff I, Iqbal K, Iqbal IG, Winblad B, Pei JJ (2003) Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer’s disease. Am J Pathol 163(2):591–607
Ding Q, Markesbery WR, Chen Q, Li F, Keller JN (2005) Ribosome dysfunction is an early event in Alzheimer’s disease. J Neurosci 25(40):9171–9175
Zhou XW, Tanila H, Pei JJ (2008) Parallel increase in p70 kinase activation and tau phosphorylation (S262) with Abeta overproduction. FEBS Lett 582(2):159–164
Guise S, Braguer D, Carles G, Delacourte A, Briand C (2001) Hyperphosphorylation of tau is mediated by ERK activation during anticancer drug-induced apoptosis in neuroblastoma cells. J Neurosci Res 63(3):257–267
Wang HY, Li W, Benedetti NJ, Lee DH (2003) Alpha 7 nicotinic acetylcholine receptors mediate beta-amyloid peptide-induced tau protein phosphorylation. J Biol Chem 278(34):31547–31553
Lambourne SL, Sellers LA, Bush TG, Choudhury SK, Emson PC, Suh YH, Wilkinson LS (2005) Increased tau phosphorylation on mitogen-activated protein kinase consensus sites and cognitive decline in transgenic models for Alzheimer’s disease and FTDP-17: evidence for distinct molecular processes underlying tau abnormalities. Mol Cell Biol 25(1):278–293
Su B, Wang X, Lee HG, Tabaton M, Perry G, Smith MA, Zhu X (2010) Chronic oxidative stress causes increased tau phosphorylation in M17 neuroblastoma cells. Neurosci Lett 468(3):267–271
Melov S, Adlard PA, Morten K, Johnson F, Golden TR, Hinerfeld D, Schilling B, Mavros C, Masters CL, Volitakis I, Li QX, Laughton K, Hubbard A, Cherny RA, Gibson B, Bush AI (2007) Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS One 2(6):e536
Zambrano CA, Egana JT, Nunez MT, Maccioni RB, Gonzalez-Billault C (2004) Oxidative stress promotes tau dephosphorylation in neuronal cells: the roles of cdk5 and PP1. Free Radic Biol Med 36(11):1393–1402
Uguz AC, Naziroglu M, Espino J, Bejarano I, Gonzalez D, Rodriguez AB, Pariente JA (2009) Selenium modulates oxidative stress-induced cell apoptosis in human myeloid HL-60 cells through regulation of calcium release and caspase-3 and -9 activities. J Membr Biol 232(1–3):15–23
van Eersel J, Ke YD, Liu X, Delerue F, Kril JJ, Gotz J, Ittner LM (2010) Sodium selenate mitigates tau pathology, neurodegeneration, and functional deficits in Alzheimer’s disease models. Proc Natl Acad Sci U S A 107(31):13888–13893
Corcoran NM, Martin D, Hutter-Paier B, Windisch M, Nguyen T, Nheu L, Sundstrom LE, Costello AJ, Hovens CM (2010) Sodium selenate specifically activates PP2A phosphatase, dephosphorylates tau and reverses memory deficits in an Alzheimer’s disease model. J Clin Neurosci 17(8):1025–1033
Li HL, Wang HH, Liu SJ, Deng YQ, Zhang YJ, Tian Q, Wang XC, Chen XQ, Yang Y, Zhang JY, Wang Q, Xu H, Liao FF, Wang JZ (2007) Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer’s neurodegeneration. Proc Natl Acad Sci U S A 104(9):3591–3596
Liu XA, Liao K, Liu R, Wang HH, Zhang Y, Zhang Q, Wang Q, Li HL, Tian Q, Wang JZ (2010) Tau dephosphorylation potentiates apoptosis by mechanisms involving a failed dephosphorylation/activation of Bcl-2. J Alzheimers Dis 19(3):953–962
Acknowledgements
This work was supported by Foundation of Doctor Scientific Research of Nanchang Hangkong University, Foundation of State Key Laboratory of Space Medicine Fundamentals and Application, China Astronaut Research and Training Center (no. SMFA10B01), and National Natural Science Foundation of China (no. 30973686).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhou, F., Chen, S., Xiong, J. et al. Luteolin Reduces Zinc-Induced Tau Phosphorylation at Ser262/356 in an ROS-Dependent Manner in SH-SY5Y Cells. Biol Trace Elem Res 149, 273–279 (2012). https://doi.org/10.1007/s12011-012-9411-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9411-z