Abstract
This study examined whether Salvianolic acid B (Sal B), a major active component of Chinese herb Radix Salviae Miltiorrhizae, may exert an anti-inflammatory effect in microglia and may be neuroprotective by regulating microglial activation. Our results showed that Sal B significantly reduced the production of nitric oxide (NO), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and reactive oxygen species (ROS) induced by lipopolysaccharide (LPS) treatment in rat primary microglia in a dose-dependent manner. Sal B had no effects on ATP-dependent IL-1β release and interferon (IFN)-γ-induced NO production. Sal B also suppressed LPS-induced inducible nitric oxide synthase (iNOS), TNF-α, and IL-1β mRNA expression, which was accompanied by inhibiting transcription factor NF-κB activation. Sal B could protect neurons through inhibition of microglial activation in a microglia-neuron coculture system. In conclusion, these data demonstrate that anti-inflammatory activity of Sal B in microglia contributes to its neuroprotective effect and suggest that it may be useful for preventing microglia-mediated neuroinflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microglia, the resident macrophage-like cells of the central nervous system (CNS), are sensitive to even minor disturbances in CNS homeostasis and become readily activated during most neuropathological conditions, including ischemia, AIDS-associated dementia, chronic neurodegenerative diseases. Activated microglia are thought to contribute to neuronal damage either directly through the release of toxic mediators such as cytokines and free radicals or indirectly by attracting activated T cells, monocytes, and neutrophils into the CNS [1–3]. Although the relationship between microglial activation and these brain injuries is not completely understood, many researchers think that inhibition of the microglial over-reaction and the inflammatory processes may represent a therapeutic target to alleviate the progression of neurological diseases, such as stroke [4, 5], and neurodegenerative diseases [6].
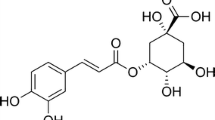
Radix Salviae Miltiorrhizae, known as Danshen, a popular traditional Chinese herb, has been widely used around the world for the treatment of cerebrovascular and cardiovascular disorders [7, 8]. Salvianolic acid B (Sal B; Fig. 1), one of the most abundant and bioactive compounds extracted from Danshen [7], has been shown to exert various neuroprotective and anti-inflammatory activities in vivo as well as in vitro. Previous in vivo studies have demonstrated that Sal B has protective properties against the ischemia–reperfusion induced injury in rat brain [9, 10]. Experimental data have demonstrated that Sal B might inhibit shear and cerebral ischemia induced platelet aggregation [11, 12], ameliorate microcirculatory disturbance and inhibit CD11b/CD18 expression in lipopolysaccharide (LPS)-treated rats [13], attenuates LPS-induced cyclooxygenase-2 (Cox-2), metalloproteinase-2 (MMP-2) and metalloproteinase-9(MMP-9) expression in vitro as well as in vivo [14, 15]. Previous reports also indicated that pretreatment with Sal B can protect tight junction structure and modulate vascular adhesion molecule-1 (VCAM-1) and intercellular cell adhesion molecule-1 (ICAM-1) upregulation in tumor necrosis factor-alpha (TNF-α)-treated endothelial cells [16–18]. Earlier experiments also provided evidence suggesting that inhibition of the NF-κB signaling pathway is a mechanism which mediates the anti-inflammatory effects of Sal B [16, 18, 19].
Based on the known anti-inflammatory activities of Sal B, we hypothesized that Sal B may exert a similar anti-inflammatory effect in microglia and may be neuroprotective via regulating microglial activation. In the present study, the effect of Sal B on microglial activation was first demonstrated. And the neuroprotection of Sal B through its effects on activated microglia was investigated using a coculture system of microglia and neurons.
Experimental Procedure
Chemicals
Sal B was purchased from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), Neurobasal medium, B27 supplement, streptomycin, penicillin and AlamarBlue cell viability reagent were purchased from Invitrogen (Carlsbad, USA). Poly-L-lysine, glutamine, ATP and cytosine arabinoside were got from Sigma (St. Louis, USA). Interferon-γ (IFN-γ) was the product of Peprotech (Rocky Hill, USA). DCFH-DA was obtained from Beyotime (Nanjin, China). Griess Reagent System was the products of Promega (Madison, USA). TNF-α and IL-1β enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, USA). RNAprep pure Cell/Bacteria kit was obtained from Tiangen (Beijing, China). TaqMan Reverse Transcription Reagents and SYBR Green PCR Master Mix reagent kit were obtained from Applied Biosystems (Foster City, USA). Nuclear Extraction kit and NF-κB p65 EZ-TFA transcription factor assay kit were the products of Millipore (Billerica, USA).
Primary Microglial Cell Culture
Use of animals has been approved by the Animal Care and Use Committee at Tianjin University of Traditional Chinese Medicine. Primary microglial cell cultures were prepared from 1-day-old Wistar rats, as described [20]. In brief, whole brains were aseptically removed and the dissected neocortical tissues were incubated with 0.25% trypsin and 0.01% DNase in DMEM for 10 min at 37°C. After addition of FBS, the dissociated cells were passed through a 130-μm mesh, washed twice in DMEM, and plated on poly-L-lysine-coated culture flasks in growth medium (DMEM, 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin). Mixed glia cultures were maintained at 37°C in a 5% carbon dioxide incubator with a renewal of medium twice a week. After 7–10 days of culture, the flasks were shaken at 100 r.p.m. for 10 min. Nonadherent microglia were collected, and seeded on plastic plates and allowed to adhere at 37°C. Unattached cells were removed after 1 h by washing with DMEM. The purity of microglial cultures was >95% as determined by Iba-1 immunocytochemical staining (data not shown).
Primary Cortical Neurons Culture
Primary cultures of cortical neurons were prepared from embryonic (gestational day 17) Wistar rat pups, as described [21]. In brief, dissected cerebral cortices were incubated for 5 min in 0.125% trypsin at 37°C followed by trypsin quenching with FBS. The Cells were dissociated mechanically by repeated gentle pipetting through narrow-bore fire-polished Pasteur pipettes and washed. Then the cells were plated on poly-l-lysine (5 μg/ml)-coated multiwells (24 wells) at a density of 2 × 105 cells per well in culture medium (Neurobasal medium with supplements of 2 mM glutamine, 2% B27, 100 μg/ml streptomycin and 100 U/ml penicillin). On day 3 of culture, cells were treated with cytosine arabinoside (1 μM) for 48 h to reduce the number of proliferating non-neuronal cells. The cultures were maintained for 8 days in vitro (DIV) before experiments.
Measurement of Cytokine and Nitric Oxide (NO) Levels
One day after seeding at a density of 2 × 104 cells per well in 96 well plates, the microglial cells were washed twice with serum-free DMEM and pretreated with Sal B (50, 5, 0.5 μM) for 30 min, and then stimulated with LPS (1 μg/ml) or IFN-γ (10 ng/ml) in serum-free DMEM. The supernatants of the cultured microglia were collected 24 h after LPS or IFN-γ stimulation for measurement of cytokine and nitric oxide (NO) levels. For studying the effect of Sal B on ATP-dependent IL-1β release from LPS-primed microglia, the cells were incubated with LPS (1 μg/ml) for 2 h, washed with PBS, and then changed with 5 mM ATP as previously reported [22]. After 30 min of incubation with ATP, the culture supernatant was collected for measurement of IL-1β. 50 μM Sal B was added 30 min before ATP stimulation in Sal B group. NO production in the medium was determined using the Griess Reagent System. The levels of TNF-α and interleukin-1 beta (IL-1β) were measured using ELISA kits.
Reactive Oxygen Species Assay
Intracellular ROS generation in LPS-stimulated microglia was measured using DCFH-DA assay [23]. The cells (5 × 104 per well in 96 well plate or 2.5 × 105 per wells in 24 well plates containing coverslips) were exposed to HBSS containing the fluorescence probe DCFH-DA (10 μM) for 1 h, followed by pretreated with Sal B for 30 min before LPS stimulation, and ROS measurement was read after 2 h of incubation with LPS at the 488 nm excitation and 535 nm emission on a fluorescence plate reader (Molecular Device, Flaxstation 3; Sunnyvale, USA) or a laser scanning confocal microscopy (Zeiss LSM710; Germany). Cell-free experiments with and without Sal B were conducted to determine that Sal B itself did not alter fluorescence.
Real-time Reverse Transcription Polymerase Chain Reaction
Microglial cells (2.5 × 106 per well in 6 well plate) were treated with LPS in the presence or absence of Sal B (50 μM). Total RNA was isolated from the cells 8 h after LPS stimulation using the RNAprep pure Cell/Bacteria kit, as instructed by the manufacturer (Tiangen; Beijing, China). RNA samples were subsequently reverse-transcribed to cDNA using TaqMan Reverse Transcription Reagents according to the manufacturer’s instructions and the resulting cDNA was used as a template for real-time PCR amplification. Real-time PCR was performed with the ABI PRISM 7300 Sequence Detection System (Applied Biosystems; Foster City, USA), using SYBR Green PCR Master Mix reagent kits and the specific primers (Table 1). Data were analyzed by using the comparative threshold cycle (Ct) method [24].
NF-κB DNA-binding Assay
Microglial cells (2.5 × 106 per 25 cm2 flask) were treated with LPS in the presence or absence of Sal B (50 μM) for 90 min, then nuclear protein extraction was performed using the Nuclear Extraction kit according to the manufacturer’s guidelines. To quantify nuclear NF-κB p65 binding to a consensus NF-κB oligonucleotide, nuclear extracts were analyzed with the NF-κB p65 EZ-TFA transcription factor assay kit. Quantification was performed using the principle of a chemiluminescent ELISA. This assay is specific for NF-kB/p65 activation, and is highly sensitive when compared with the gel-retardation technique.
Neuronal/microglial Cell Coculture System and Cell Viability Assay
Microglia were seeded on the upper inserts of Transwell chambers (4 × 105 per well, pore size of 3 μm; Corning Costar; Cambridge, USA) in 24-well plates and incubated overnight before stimulation. After treatment with LPS and/or Sal B for 6 h, the inserts were thoroughly washed with fresh DMEM. These microglial cells were cocultured for 36 h with neurons by transferring the culture inserts containing microglial cells onto neuronal monolayers. Afterwards, for cytotoxicity assay of neurons, microglial cells culture inserts were removed and AlamarBlue cell viability reagent was added to the remaining neurons at each sampling point at 10 vol% and cultured in a humidified incubator for another 3 h. Thereafter, supernatant samples were transferred into a 96-well plate and read using a fluorescence plate reader (Molecular Device, Flaxstation 3; Sunnyvale, USA). The fluorescence excitation and emission wavelengths were at 545 and 585 nm, respectively.
Data Analysis
Data are expressed as mean ± SD. Statistical comparison between different treatments was done by either Student’s t-test or one-way analysis of variance (ANOVA) with SPSS software (SPSS, Chicago, IL, USA). Differences were considered statistically significant for P < 0.05.
Results
Sal B Inhibits LPS-induced Microglia Activation
First, potential anti-inflammatory activity of Sal B in brain microglia was tested by evaluating the production of inflammatory mediators (NO, TNF-α, and IL-1β) from cultured microglial cells. Rat primary microglial cultures were stimulated with 1 μg/ml LPS in the presence or absence of Sal B for a 24-h incubation period. LPS-stimulated microglial cells showed remarkable increases in the NO, TNF-α, and IL-1β levels in the cell-conditioned media. Pretreatment of microglial cells with Sal B significantly reduced the LPS-induced NO, TNF-α, IL-1β production in a dose-dependent manner (Fig. 2). No cytotoxic effect of Sal B was observed under this experimental condition as measured by AlamarBlue cell viability reagent (data not shown).
Sal B reduces LPS-induced NO, TNF-α and IL-1β production in primary cultured microglia. Primary cultured microglia were pretreated with Sal B (0.5, 5, 50 μM) for 30 min and stimulated with LPS (1 μg/ml) for a 24 h incubation period. Then TNF-α, IL-1β (by ELISA) and NO production (by Griess reagent) in supernatants were measured. The data are expressed as mean ± SD, n = 6 per condition. * P < 0.05, ** P < 0.01, significantly different from the LPS-treated alone
LPS is known to increase the production of ROS, which are the crucial mediators in the inflammation related process [25].To test the effect of Sal B on LPS-induced intracellular ROS increase in microglia, microglial cultures were pretreated with Sal B and exposed to LPS for 2 h. As shown in Fig. 3, the results from both the plate reader and the confocal microscopy showed that LPS induced a significant increase of intracellular ROS. In the presence of Sal B, intracellular ROS was remarkably reduced even at the concentration of 0.5 μM.
Sal B reduces LPS-induced ROS production in primary cultured microglia. The cells were exposed to HBSS containing the fluorescence probe DCFH-DA (10 μM) for 1 h, followed by pretreatment with Sal B for 30 min, and then treated with LPS for 2 h. a Fluorescence intensity was measured on a fluorescence plate reader. The data are expressed as mean ± SD., n = 6 per condition. * p < 0.05, significantly different from control samples. # p < 0.05, ## p < 0.01, significantly different from LPS-treated alone. b Representative images obtained by confocal scanning microscopy of DCF-sensitive intracellular ROS. Bar 100 μm
For LPS is only one form of activating stimuli for microglia, we also investigated the effects of Sal B on microglial cells activated by ATP and IFN-γ. After stimulation with 5 mM ATP, the amount of IL-1β released into the culture supernatant from LPS-primed microglial cells increased markedly compared with control and LPS treatment alone, consistent with previous reports [22, 26]. Sal B (50 μM) had no effect on ATP-dependent IL-1β release (Fig. 4a). Neither could Sal B reduce NO production in IFN-γ-induced microglia (Fig. 4b).
Sal B had no effects on ATP-dependent IL-1β release in LPS-primed microglial cells (a) and NO production in IFN-γ-induced microglia (b). a Primary microglia were incubated with LPS (1 μg/ml) for 2 h, washed with PBS, and then changed with 5 mM ATP. After 30 min of incubation with ATP, the culture supernatant was collected for measurement of IL-1β. 50 μM Sal B was added 30 min before ATP stimulation in Sal B group (“L+A+S”). b Primary microglia were pretreated with Sal B (0.5, 5, 50 μM) for 30 min and stimulated with IFN-γ (10 ng/ml) for a 24 h incubation period. Then NO production in supernatants were measured. The data are expressed as mean ± SD, n = 6 per condition. * p < 0.01, significantly different from control samples. # p < 0.01, significantly different from LPS-treated alone
Sal B Suppresses LPS-induced iNOS, TNF-α and IL-1β mRNA Expression
To determine whether the decreases in LPS-induced NO, TNF-α, and IL-1β measured in supernatants were associated with the decreases in steady-state of iNOS (the enzyme responsible for the production of NO), TNF-α, and IL-1β expression at the transcriptional level, real-time RT–PCR analysis were conducted. Microglial cells were pretreated with Sal B (50 μM) for 30 min and stimulated with LPS (1 μg/ml) for an 8-h incubation period. As anticipated, LPS markedly increased mRNA expressions of iNOS, TNF-α, and IL-1β mRNA in rat primary microglia and pretreatment with Sal B at 50 M significantly downregulated iNOS, TNF-α, and IL-1β mRNA expression (Fig. 5).
Sal B suppresses LPS-induced iNOS, TNF-α and IL-1β mRNA expression in primary cultured microglia. Primary cultured microglia were pretreated with Sal B (50 μM) for 30 min and stimulated with LPS (1 μg/ml) for an 8-h incubation period. iNOS, TNF and IL-1β mRNA levels were measured by real-time RT–PCR. Data are presented as relative-fold change to control samples, n = 6 per condition. * p < 0.05, ** p < 0.01, significantly different from LPS-treated alone
Sal B Inhibits LPS-induced NF-κB Activation in Microglial Cells
NF-κB is an important modulator of cytokines and iNOS expression in microglia. It’s also an important target of several anti-inflammatory drugs [27, 28]. Recent experiment evidence demonstrates that Sal B can suppress NF-κB activation in endothelial cell [16, 18] and mesangial cell [19]. Therefore, we speculated that Sal B may also reduce LPS-induced NF-κB activity in microglial cells. As shown in Fig. 6, LPS (1 μg/ml) treatment significantly increased the NF-κB activity in primary cultured microglia, assessed by the measurement of nuclear p65 DNA binding. And pretreatment with Sal B (50 μM) could significantly inhibit the NF-κB-DNA interactions.
Sal B inhibits LPS-induced NF-κB activation. Microglial cells were pretreated with Sal B (50 μM) for 30 min and stimulated with LPS (1 μg/ml) for a 1 h incubation period. Nuclei were extracted and transcription factor binding activity for NF-κB was determined using the NF-κB p65 EZ-TFA transcription factor chemiluminescent assay. Data are presented as relative-fold activation compared with control samples, n = 3 per condition. * p < 0.01, significantly different from control samples. # p < 0.01, significantly different from LPS-treated alone
Sal B Protects Neurons Through Inhibition of Microglial Activation
Since several lines of evidence indicate that NO and multiple pro-inflammatory cytokines such as TNF-α, and IL-1β releasing from activated microglia, can cause neuronal damage in various neuropathologic processes [1, 3], we next tested whether the inhibition of inflammatory microglia activation by Sal B could protect cocultured neurons against cytotoxic effect of activated microglia in a microglia and neurons coculture system. Microglial cells on the upper inserts of Transwell chambers activated by LPS in the presence or absence of Sal B were washed thoroughly, and then placed above neurons. In this time point, the numbers of living microglia of each group maintained equal (assessed by trypan blue staining and MTT test, data not shown). Microglial cells were then removed after 36 h and the cell viability of neurons was assessed. As shown in Fig. 7, LPS-activated microglial cells significantly decreased neurons viability, consistent with previous reports [29]. Pretreatment with Sal B attenuated the cytotoxicity of activated microglial cells to cocultured neurons, suggesting that Sal B was cytoprotective by inhibiting microglial activation.
Sal B protects neurons through inhibition of microglia activation. Either unstimulated (“Control-MG”) or LPS (1 μg/ml)-stimulated microglial cells in the presence (“Sal B-MG”) or absence (“LPS-MG”) of Sal B (50 μM) were washed thoroughly and cocultured with neurons for 36 h. Then neuronal cell viability was assessed using Alamarblue reagent. Data are presented as percent of control samples, n = 6 per condition.* p < 0.01, significantly different from control samples. # p < 0.05, significantly different from LPS-treated alone
Discussion
Our results showed that Sal B reduced the production of NO, TNF-α, IL-1β and ROS induced by LPS treatment in primary cultured rat microglia in a dose-dependent manner. Cotreatment with Sal B also suppresses LPS-induced iNOS, TNF-α, and IL-1β mRNA expression. Such suppressive effect was accompanied by inhibiting transcription factor NF-κB activation. We also observed that Sal B could afford neuroprotection through inhibition of microglial activation in a coculture system of microglia and neurons. Sal B had no effects on ATP-dependent IL-1β release in LPS-primed microglial cells and NO production in IFN-γ-induced microglia.
Over several decades the question of whether microglia plays harmful or beneficial roles in CNS has been widely debated. The evidence to date suggests that activated microglia function as a “double-edged sword,” with neuroprotective features predominating in the healthy nervous system and neurodestructive properties observed in various disease states [1, 2]. Careful targeting of these cells could have therapeutic benefits for several types of trauma and disease specific to the central nervous system. Previous in vivo or in vitro studies have revealed that drugs such as tetracyclines [30], histone deacetylase inhibitors [31], naloxone [32], dextromethorphan [33], curcumin [28] and several polyphenolic plant-derived flavonoids [27] afford neuroprotection through modulating microglial cells. Of course, we should not seriously hamper their critical role in host defense and their neuroregenerative properties when modulating the deleterious aspects of activated microglia.
Radix Salviae Miltiorrhizae is one of the most widely used traditional Chinese herbs in the treatments of numerous diseases, including cerebrovascular diseases and coronary artery diseases [7]. A lot of studies have been done to elucidate its chemical constituents and pharmacological activities. More than 30 lipophilic diterpene chinone compounds (such as tanshinone I, IIA, IIB; cryptotanshinone), 15 hydrophilic phenolic acids (such as salvianolic acid A, B) and other related compounds (such as danshensu) have been separated and identified from Danshen. Among these compounds, tanshinone IIA and Sal B are the most well studied and bioactive constituents [7]. Previous reports demonstrated that Sal B has anti-inflammatory effects and the NF-κB signal transduction pathway is one of the targets of Sal B in several in vitro inflammatory models [16, 18, 19]. Our results confirmed this pharmacological activity of Sal B in LPS-stimulated microglia. Interestingly, we found that tanshinone IIA, which also exerts anti-inflammatory effects in other cells reported by several investigators [34, 35], including ourselves [36], could not inhibit NO and TNF-α production at concentrations of 0.1, 1 and 10 μM in LPS-induced microglial cells (data not shown). The further mechanisms should be continued to study.
Similar to LPS, stimuli such as Aβ, fibrillar PrP peptides, dsRNA in the form of poly IC, HIV-1 Tat, and MPP+, induce microglial iNOS (the enzyme responsible for the production of NO) mRNA expression through NF-κB pathway [37]. However, IFN-γ is unable to induce the activation of NF-κB in microglia [37]. IFN-γ-induced iNOS expression is primarily through the activation of the transcription factor STAT1α following phosphorylation [38, 39], while independent on NF-κB [37]. Sal B could not suppress IFN-γ-induced NO production (Fig. 4b), suggesting that Sal B was probably unable to interfere with interferon-γ-STAT1α pathway. To our knowledge, no agent except guanosine is capable of inhibiting both NF-κB and STAT1α pathways [39].
Because priming with LPS is required to induce ATP-dependent IL-1β release in microglia [26], primary microglia were primed with LPS to stimulate the release of IL-1β in this study. The P2X7 receptor responds to ATP and facilitates the activation of caspase-1, and this is followed by the release of IL-1β in the LPS-primed microglial cells [26]. Results showed that Sal B had no effect on ATP-dependent IL-1β release (Fig. 4a), suggesting that the ATP-stimulated pathway was insensitive to Sal B.
In the neuronal/microglial cell coculture system of this study, the 3-μm-diameter pores of Transwell chambers allow molecules including cytokines to diffuse between the neurons and microglia without cell contact. It is important to note that, before cocultured with neurons, the LPS-activated microglial cells were washed thoroughly. So the neurons were never exposed to Sal B, suggesting that the cytoprotective effect of Sal B observed in the coculture experiments is not due to its direct protective effect on neurons against toxic inflammatory mediators. Rather, the cytoprotection afforded by Sal B was due to its effect of inhibiting the pro-inflammatory mediators from activated microglia. In fact, as an efficient radical scavenger and antioxidant [40, 41], Sal B exerts direct protective effects against hydrogen peroxide-induced injury in PC12 cell [42]. For the same reason, we proposed that its antioxidant character played a partial role in reducing LPS-induced ROS production even at the concentration of 0.5 μM (Fig. 3). Sal B can also inhibit amyloid β (Aβ) fibril formation, disaggregate preformed fibrils and reduce Aβ mediated cytotoxicity in vitro [43–45]. These multiple neuroprotctive effects of Sal B highlight its potential therapeutic value.
In conclusion, this article reports for the first time the anti-inflammatory activity of Sal B in primary cultured rat microglial cells and its neuroprotective effect through inhibition of microglial activation. Our data provide new mechanism underlying the neuroprotective activity of Sal B and suggest that it may be useful for preventing and treating neuroinflammation mediated by the activated microglia.
References
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394
Rock RB, Gekker G, Hu S et al (2004) Role of microglia in central nervous system infections. Clin Microbiol Rev 17:942–964
Tambuyzer BR, Ponsaerts P, Nouwen EJ (2009) Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol 85:352–370
Kaushal VP, Schlichter LC (2008) Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke. J Neurosci 28:2221–2230
Mabuchi T, Kitagawa K, Ohtsuki T et al (2000) Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke 31:1735–1743
Townsend KP, Pratico D (2005) Novel therapeutic opportunities for Alzheimer’s disease: focus on nonsteroidal anti-inflammatory drugs. FASEB J 19:1592–1601
Zhou L, Zuo Z, Chow MS (2005) Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 45:1345–1359
Imanshahidi M, Hosseinzadeh H (2006) The pharmacological effects of Salvia species on the central nervous system. Phytother Re. 20:427–437
Chen YH, Du GH, Zhang JT (2000) Salvianolic acid B protects brain against injuries caused by ischemia-reperfusion in rats. Acta Pharmacol Sin 21:463–466
Tang M, Feng W, Zhang Y et al (2006) Salvianolic acid B improves motor function after cerebral ischemia in rats. Behav Pharmacol 17:493–498
Li M, Zhao C, Wong RN et al (2004) Inhibition of shear-induced platelet aggregation in rat by tetramethylpyrazine and Salvianolic acid B. Clin Hemorheol Microcirc 31:97–103
Tang MK, Ren DC, Zhang JT et al (2002) Effect of Salvianolic acids from Radix Salviae miltiorrhizae on regional cerebral blood flow and platelet aggregation in rats. Phytomedicine 9:405–409
Guo J, Sun K, Wang CS et al (2008) Protective effects of dihydroxylphenyl lactic acid and Salvianolic acid B on LPS-induced mesenteric microcirculatory disturbance in rats. Shock 29:205–211
Chen YL, Hu CS, Lin FY et al (2006) Salvianolic acid B attenuates cyclooxygenase-2 expression in vitro in LPS-treated human aortic smooth muscle cells and in vivo in the apolipoprotein-E-deficient mouse aorta. J Cell Biochem 98:618–631
Lin SJ, Lee IT, Chen YH et al (2007) Salvianolic acid B attenuates MMP-2 and MMP-9 expression in vivo in apolipoprotein-E-deficient mouse aorta and in vitro in LPS-treated human aortic smooth muscle cells. J Cell Biochem 100:372–384
Zhou Z, Liu Y, Miao AD et al (2005) Salvianolic acid B attenuates plasminogen activator inhibitor type 1 production in TNF-alpha treated human umbilical vein endothelial cells. J Cell Biochem 96:109–116
Ding M, Yuan YJ (2007) Study on the mechanisms of an extract of Salvia miltiorrhiza on the regulation of permeability of endothelial cells exposed to tumour necrosis factor-alpha. J Pharm Pharmacol 59:1027–1033
Chen YH, Lin SJ, Ku HH et al (2001) Salvianolic acid B attenuates VCAM-1 and ICAM-1 expression in TNF-alpha-treated human aortic endothelial cells. J Cell Biochem 82:512–521
Luo P, Tan Z, Zhang Z et al (2008) Inhibitory effects of salvianolic acid B on the high glucose-induced mesangial proliferation via NF-kappaB-dependent pathway. Biol Pharm Bull 31(7):1381–1386
Jang S, Kelley KW, Johnson RW (2008) Luteolin reduces IL-6 production in microglia byinhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci USA 105:7534–7539
Antonelli T, Tomasini M, Fournier J et al (2008) Neurotensin receptor involvement in the rise of extracellular glutamate levels and apoptotic nerve cell death in primary cortical cultures after oxygen and glucose deprivation. Cereb Cortex 18:1748–1757
Sanz JM, Virgilio FD (2000) Kinetics and Mechanism of ATP-Dependent IL-1β Release from Microglial Cells. J Immunol 164:4893–4898
Yang S, Yang J, Yang Z et al (2006) Pituitary adenylate cyclase-activating polypeptide (PACAP) 38 and PACAP4–6 are neuroprotective through inhibition of NADPH oxidase: potent regulators of microglia-mediated oxidative stress. J Pharmacol Exp Ther 319:595–603
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCT method. Methods 25:402–408
Qin L, Liu Y, Wang T et al (2004) NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem 279:1415–1421
Takenouchi T, Iwamaru Y, Sugama S et al (2008) Lysophospholipids and ATP mutually suppress maturation and release of IL-1β in mouse microglial cells using a Rho-dependent pathway. J Immunol 180:7827–7839
Park JS, Woo MS, Kim DH et al (2007) Anti-inflammatory mechanisms of isoflavone metabolites in lipopolysaccharide-stimulated microglial cells. J Pharmacol Exp Ther 320:1237–1245
Yang S, Zhang D, Yang Z et al (2008) Curcumin protects dopaminergic neuron against LPS induced neurotoxicity in primary rat neuron/glia culture. Neurochem Res 33:2044–2053
Fordyce CB, Jagasia R, Zhu X et al (2005) Microglia Kv1.3 channels contribute to their ability to kill neurons. J. Neurosci 25:7139–7149
Yrjänheikki J, Keinänen R, Pellikka M et al (1998) Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA 95:15769–15774
Kim HJ, Rowe M, Ren M et al (2007) Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther 321:892–901
Liu B, Du L, Hong JS (2000) Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther 293:607–617
Liu Y, Qin L, Li G et al (2003) Dextromethorphan protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. J Pharmacol Exp Ther 305:212–218
Xu Y, Feng D, Wang Y et al (2008) Sodium tanshinone IIA sulfonate protects mice from ConA-induced hepatitis via inhibiting NF-kappaB and IFN-gamma/STAT1 pathways. J Clin Immunol 28:512–519
Jeon SJ, Son KH, Kim YS et al (2008) Inhibition of prostaglandin and nitric oxide production in lipopolysaccharide-treated RAW 264.7 cells by tanshinones from the roots of Salvia miltiorrhiza bunge. Arch. Pharm. Res 31:758–763
Fan GW, Gao XM, Wang H et al (2009) The anti-inflammatory activities of Tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J Steroid Biochem Mol Biol 113:275–280
Jana M, Jana A, Liu X et al (2007) Involvement of phosphatidylinositol 3-kinase-mediated up-regulation of IκBα in anti-inflammatory effect of Gemfibrozil in microglia. J Immunol 179:4142–4152
Kleinert H, Wallerath T, Fritz G et al (1998) Cytokine induction of NO synthase II in human DLD-1 cells: roles of the JAK-STAT, AP-1 and NF-kappa B-signaling pathways. Br J Pharmacol 125:193–201
Alimonte ID, Flati V, D’Auro M et al (2007) Guanosine inhibits CD40 receptor expression and function induced by cytokines and β amyloid in mouse microglia cells. J Immunol 178:720–731
Zhao GR, Zhang HM, Ye TX et al (2008) Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem Toxicol 46:73–81
Zhang HS, Wang SQ (2006) Salvianolic acid B from Salvia miltiorrhiza inhibits tumor necrosis factor-α (TNF-α)-induced MMP-2 upregulation in human aortic smooth muscle cells via suppression of NAD(P)H oxidase-derived reactive oxygen species. J Mol Cell Cardiol 41:138–148
Liu CS, Chen NH, Zhang JT (2007) Protection of PC12 cells from hydrogen peroxide-induced cytotoxicity by Salvianolic acid B, a new compound isolated from Radix Salviae miltiorrhizae. Phytomedicine 14:492–497
Lin YH, Liu AH, Wu HL et al (2006) Salvianolic acid B, an antioxidant from Salvia miltiorrhiza, prevents Abeta (25–35)-induced reduction in BPRP in PC12 cells. Biochem Biophys Res Commun 348:593–599
Durairajan SS, Yuan Q, Xie L et al (2008) Salvianolic acid B inhibits Abeta fibril formation and disaggregates preformed fibrils and protects against Abeta-induced cytotoxicty. Neurochem Int 52:741–750
Tang M, Zhang J (2002) Prostate apoptosis response-4 involved in the protective effect of Salvianolic acid B against amyloid β peptide-induced damage in PC12 cells. Jpn J Pharmacol 88:422–427
Acknowledgments
This work is supported by National Natural Science Foundation of China (30572348), the Project of Yunnan Province-Universties Cooperation (2006YX03), National Key Technology R&D Program (2007BAI47B04), University Science & Technology Program of Tianjin (20070314) and National Project 973 (2005CB523400).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, SX., Hu, LM., Gao, XM. et al. Anti-inflammatory Activity of Salvianolic Acid B in Microglia Contributes to its Neuroprotective Effect. Neurochem Res 35, 1029–1037 (2010). https://doi.org/10.1007/s11064-010-0151-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0151-1