Abstract
Purpose
Cytokeratin 19 fragment 21–1 (CYFRA 21–1) and cytokeratin 19 fragment 2G2 (CK 19-2G2) are two soluble fragments of cytokeratin 19 (CK 19) that can be detected in serum. CK 19-positive hepatocellular carcinoma (HCC) is characterized by an aggressive behavior and a poor outcome. This study aimed to assess the prognostic value of serum CYFRA 21–1 and CK 19-2G2 in predicting tumor aggressiveness and overall survival (OS) in patients with hepatic C virus (HCV)-related HCC.
Methods
The current study included 138 patients with HCV-related HCC recruited from the Hepatobiliary and Interventional Radiology Units at Alexandria’s main university hospitals and 40 healthy individuals as controls. Patients were assessed for clinical, radiological tumor characteristics, and aggressiveness index. Baseline serum CYFRA 21–1 and CK 19-2G2 levels were measured by enzyme-linked immunosorbent assay.
Results
Elevated CYFRA 21–1 levels were associated with tumors size ≥ 5 cm (p < 0.001), malignant portal vein thrombosis (mPVT) (p < 0.001), distant metastasis (p = 0.030), ill-defined/infiltrative pattern (p = 0.010), and aggressiveness index > 4 (p = 0.045). Elevated CK19-2G2 levels were not associated with any clinical or radiological characteristics. Either or both elevated serum CYFRA 21–1 and CK 19-2G2 in combination with alpha-feto protein (AFP) ≥ 400 ng/ml have a better predictability for mPVT and ill-defined/infiltrative patterns (sensitivity (10–25%) and specificity (96–100%)). Elevated levels of CYFRA 21–1, CK 19-2G2, or AFP ≥ 400 ng/ml were associated with decreased 1-year OS.
Conclusions
Either or both elevated serum CYFRA 21–1 and CK 19-2G2 levels when added to AFP ≥ 400 ng/ml are specific but less sensitive biomarkers for predicting tumor aggressiveness. These biomarkers can be used independently to predict reduced 1-year OS in Egyptian patients with HCV-related HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a growing major worldwide health problem that represents the sixth and fourth most common malignancies globally and in Egypt, respectively [1, 2], and the fourth most frequent cause of death from cancer globally [3]. Hepatitis C virus (HCV) infection is the most important cause of HCC in Egypt, and the HCV prevalence ranges from 61 to 90.3% in HCC patients [4].
Currently, the treatment modalities for HCC are various, including surgical, locoregional, and systemic therapies (e.g., multikinase inhibitors, molecular target therapy, and immune therapies) [5]. The primary biological characteristic of HCC is heterogeneity, which manifests as a variety of biological behaviors of various molecular phenotypes with hepatocyte and/or hepatic progenitor cell origins [6] and, in turn, is considered one of the factors that influences patient prognosis and treatment effectiveness [7].
Cytokeratins (CKs) are intermediate filament proteins responsible for epithelial cell integrity. Over 20 distinct CKs have been recognized, with CK 19 being a prominent keratin found in simple epithelial cells and various malignant cells [8]. Intact CK polypeptides have low solubility and circulate as a complex in the circulation [9]. Cytokeratin 19 fragment 21–1 (CYFRA 21–1) is a soluble fragment of CK 19, an acid-type CK, with a molecular weight of 40,000 daltons [9].
Caspase-3 cleaves the CK 19 protein during apoptosis, releasing soluble fragments that are detectable in cancer patients. Therefore, the level of CK 19 fragments correlates with the tumor mass or, more accurately, with the necrosis within the tumor [10]. The CYFRA 21–1 assay has broad clinical application in many types of cancers [11, 12]. Cytokeratin 19 fragment 2G2 (CK19-2G2) is a novel CK19 fragment released into circulation. It has been found to be recognized by both CK19 2G2 and CK19 5H2 antibodies. This fragment has been investigated as a surrogate marker of CK 19 in lung and breast cancer [13, 14].
CK 19 is a hepatic progenitor stem cell marker correlated with a poor prognosis in patients with HCC [15]. The presence of CK 19 positivity is observed in approximately 10–30% of HCC patients [16, 17] and is linked to clinical aggressiveness and poor outcomes [18]. Although there is a growing interest in CK 19 as a prognostic biomarker in HCC patients, the need for CK 19 evaluation by immunohistochemistry in liver biopsy is considered one of the challenges to be solved before the routine integration of CK19 assessment into clinical practice [5]. Serum CYFRA 21–1 is the most promising noninvasive predictor of CK 19 expression in HCC [19]. However, data about the value of CK19-2G2 in HCC are scarce.
The number of nodules, maximum tumor size, serum alpha-feto protein (AFP) level, and presence of portal vein thrombosis are four HCC features that commonly influence tumor behavior. Recently, the sum of these variables was reported as the HCC aggressiveness index [20], which is linked to poor overall survival (OS). Therefore, the current study was conducted to explore the value of serum CK 19 fragments (CYFRA 21–1 and CK 19-2G2) in the prediction of tumor aggressiveness in HCV-related HCC and 1-year OS.
Material and Methods
This study included 138 HCV-related HCC patients. Patients were consecutively recruited from the Hepatobiliary Unit, Internal Medicine Department, and Interventional Radiology Unit at Alexandria’s main university hospitals between September 2021 and August 2022. Each patient was followed up for 1 year; the last patient’s final follow-up date was August 30, 2023. Serum CYFRA 21–1 and CK 19-2G2 levels among the group of healthy individuals were measured to set the appropriate cut-off values among the Egyptian population.
Inclusion Criteria
The study included all patients recently diagnosed with HCC according to the European Association for the Study of the Liver (EASL) guidelines with active or previous HCV infection who were able to give informed consent [5].
Exclusion Criteria
Patients were excluded from participation if they had hepatitis B virus (HBV) coinfection (seropositivity to either HBs Ag or HBc ab total) or other causes of chronic liver disease with or without HCV infection, uncontrolled diabetes mellitus, chronic renal or cardiopulmonary diseases, any kind of malignancy other than HCC, or previous therapy for HCC.
Sample Size
The minimum required sample size was determined based on the results of a previous study, which reported that the 1-year OS rates for HCC patients with non-elevated and elevated serum CYFRA 21–1 levels were 66% and 39%, respectively [21]. Additionally, it was noted that elevated serum CYFRA 21–1 levels have been observed in 22–47% (average 34%) of HCCs [22, 23]. Therefore, the expected enrollment ratio was 2:1. A minimum required sample size of 120 patients achieves 80% power for estimating the expected proportion differences with 95% confidence, at a 0.05 significance level using Z-test [24]. We increased the sample size by 18 patients (15% of the estimated sample size) to be 138 patients in total to avoid sampling error and dropout. The sample size was calculated using Power Analysis and Sample Size Software (PASS 2020). We utilized the identical sample size for CK19-2G2, presuming its prognostic value is equivalent to that of serum CYFRA 21–1 due to the absence of data regarding its prognostic significance in patients with HCC.
The Study Protocol
All patients were evaluated at baseline by history taking, complete clinical examination, and laboratory investigations. A complete blood picture was measured automatically by Sysmex Xn 1000, Japan. Renal profile (blood urea and serum creatinine), serum sodium, random blood glucose, and liver profile (total bilirubin, serum albumin, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase (ALP)) were measured automatically by ADVIA 1800 chemistry system, Siemens, Germany. International normalized ratio and prothrombin activity were measured automatically by Sysmex CS-2100i, Japan. Viral markers (HCV ab, HBsAg, and HBcab) were measured using chemiluminescence technique, and the real-time polymerase chain reaction assay was used to detect and measure the level of serum HCV RNA level if it is unavailable. Serum AFP was determined using the chemiluminescence technique and serum CYFRA 21–1 and CK 19-2G2 levels by enzyme-linked immunosorbent assay (ELISA) kits.
All patients underwent abdominal ultrasound, doppler for portal and hepatic circulation, and contrast-enhanced cross-sectional imaging (triphasic computed tomography (CT) or dynamic magnetic resonance imaging (MRI)) to confirm the diagnosis of HCC according to international guidelines and to assess the number, size, pattern, and presence of malignant portal vein thrombosis. Liver biopsy was needed in some cases with atypical radiological features. For all patients, we calculated the Child–Pugh (CP) [25] and Barcelona Clinic Liver Cancer (BCLC) scores [26].
The aggressiveness of the tumor was evaluated using the HCC aggressiveness index described by Carr et al. [20], which is the sum of the following four parameters: AFP, portal vein thrombosis, maximal tumor diameter, and the number of tumor nodules. The aggressive form of HCC was diagnosed if the aggressiveness index was >4 (Supplementary Table 1).
Serum CK 19 fragments (CYFRA 21–1 and CK 19-2G2) were correlated with baseline radiological characteristics, serum AFP, and aggressiveness index score [20].
Serum CYFRA 21–1 and CK19-2G2
To measure serum CYFRA 21–1 and CK19-2G2 levels, venous blood samples were collected at baseline. The serum was centrifuged for 10–20 min at 2000–3000 revolutions per minute after being allowed to clot for 20–30 min. It was then kept at − 20 °C until analysis. The concentrations of serum CYFRA 21–1 and CK19-2G2 were determined using double antibody sandwich ELISA kits supplied by Sino Gene Clon Biotech (Hang Zhou, China) for CK 19 -2G2 and Glory Science (Shanghai, China) for CYFRA 21–1.
CYFRA 21–1 kits have a sensitivity of 0.1 ng/ml and a range of 0.5–20 ng/ml. In contrast, CK 19-2G2 kits have a sensitivity of 7 pg/ml and a range of 28–1800 pg/ml. All protocols followed the manufacturers’ instructions.
In order to address the challenges associated with establishing cut-off values for serum CYFRA 21–1 and CK 19-2G2, as well as variations between studies [19, 22, 23, 27, 28], we measured the serum CYFRA 21–1 and CK 19-2G2 of 40 healthy Egyptian controls to determine cut-off values among Egyptians using the one-sided 95% confidence interval.
Treatment and Follow-up
Patients were classified and managed according to the BCLC staging system, and the HCC board at our institute had to use a multidisciplinary approach in order to select the most effective therapy choice. Patients were followed up for 1 year to estimate the OS. OS was defined as the period of time from the enrollment date to the date of death, regardless of the cause. Patients who were lost to follow-up by the end of the study were censored at their last follow-up date.
Statistical Analysis
Using IBM SPSS version 20.0, the obtained data were coded, processed, and analyzed (IBM Corp, Armonk, NY). The qualitative data was described using percentages and numbers. Quantitative data were presented using the mean and standard deviation (SD) for parametric data and the median and interquartile range for nonparametric data. The chi-square, Fisher’s exact, and Monte Carlo tests were applied to compare the clinical and radiological parameters between patients with elevated and those with normal serum CYFRA 21–1 or CK 19-2G2 levels. An analysis of the degree and direction of a linear association between the AFP and CK 19 fragments was performed using Spearman’s rank-order correlation. Receiver operating characteristic (ROC) curve analysis was used to assess the accuracy of serum AFP, CYFRA 21–1, and CK19-2G2 alone or in combination to predict malignant portal vein thrombosis or ill-defined/infiltrative patterns. The curve was used to measure the sensitivity and specificity, and cross-tabulation was used to measure the accuracy, positive predictive value (PPV), and negative predictive value (NPV). The Kaplan–Meier survival curve with the log-rank test was used to determine the patient’s 1-year OS. The statistical significance of the obtained results was judged at the 0.05 level.
Results
Patient Demographic and Laboratory Data
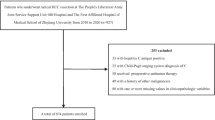
A total of 208 HCV-induced HCC patients were recruited for the study. Seventy patients were excluded initially from the study, including ten patients with hepatitis B-related cirrhosis, 14 patients with NAFLD-related cirrhosis, nine patients with cirrhosis of unknown etiology, 34 with a history of previous therapy for HCC, and three patients with mixed HCC and cholangiocarcinoma diagnosed by biopsy. Finally, the study included 138 HCV-induced HCC patients (Fig. 1).
The median (IQR) age was 63 (58–67) years, with a male predominance of 81.2%. Twenty-five (18%) patients had active HCV infection, 109 (79%) patients received DAA therapy and achieved SVR, and four (2.9%) patients received interferon-based therapy for HCV and SVR. All patients had liver cirrhosis. Laboratory and radiological variables are shown in Table 1.
Serum CYFRA 21–1 and CK 19-2G2 in Normal Controls and HCV-Related HCC Patients
In this study, to determine the normal value of CK 19 serum fragments, serum CYFRA 21–1 and CK 19-2G2 were quantified in a cohort of 40 healthy individuals. The mean value of CYFRA 21–1 was 1.42 ± 0.28 ng/ml, and we used the one-sided 95% confidence interval as the upper limit of the reference range. Therefore, 1.95 ng/ml was set as the cut-off value. Conversely, the mean value of serum CK 19-2G2 was 453.7 ± 59 pg/ml, and the upper limit of the reference range was 573.5 pg/ml.
Based on the previous cut-off values, 43 out of 138 (31%) patients had elevated baseline serum CYFRA 21–1 levels (> 1.95 ng/ml), and 30 out of 138 (22%) patients had elevated baseline serum CK 19-2G2 levels (≥ 573.5 pg/ml).
The percentage of patients with HCC ≥ 5 cm was significantly higher in elevated CYFRA 21–1 cases (83.7%, 36/43) than in normal CYFRA 21–1 cases (52.6%, 50/95) (p < 0.001) (Table 2).
The percentage of patients with ill-defined or infiltrative patterns was significantly higher in elevated CYFRA 21–1 cases (39.5%, 17/43) and (37.2%, 16/43), respectively, than in normal CYFRA 21–1 cases (23.2%, 22/95) and (26.3%, 25/95), respectively (p = 0.010) (Table 2).
The percentage of patients with malignant portal vein thrombosis was significantly higher in elevated CYFRA 21–1 cases (60.5%, 26/43) than in normal CYFRA 21–1 cases (28.4%, 27/95) (p < 0.001) (Table 2).
The presence of distant metastasis was significantly higher in elevated CYFRA 21–1 cases than in normal CYFRA 21–1 cases (p < 0.030) (Table 2).
The percentage of different BCLC stages was significantly variable between patients with elevated serum CYFRA 21–1 levels and patients with normal serum CYFRA 21–1 levels (p < 0.030). However, there were no significant differences between CYFRA 21–1 elevated and normal cases in terms of tumor number, size, and lymph node metastasis.
There were no correlations between elevated CK 19-2G2 levels and tumor number, size, location, BCLC stage, presence of malignant portal vein thrombosis, or lymph node or distant metastasis (Table 2).
The Correlation Between Serum CK-19 fragments and Serum AFP
There was no significant correlation between serum AFP and serum CYFRA 21-1 (rs= 0.095, p = 0.267). Additionally, there was no significant correlation between serum AFP and serum CK 19-2G2 (rs= 0.161, p = 0.059).
The Relation Between the Aggressiveness Index and Serum CK 19 Fragments
The number of patients with aggressive tumors (aggressiveness index > 4) was higher in elevated CYFRA21-1 cases (86%, 37/43) than in normal CYFRA 21–1 cases (71.6%, 68/95) (p = 0.045). However, no correlation existed between elevated serum CK 19-2G2 levels and the number of patients with aggressive tumors (aggressiveness index > 4) (Table 2).
Efficacy of AFP, CK 19-2G2, and CYFRA 21–1 as Tumor Markers for the Detection of Malignant Portal Vein Thrombosis
For the detection of malignant portal vein thrombosis, elevated serum CYFRA 21–1 had a sensitivity, specificity, PPV, and NPV of 49%, 80%, 60%, and 72%, respectively. Elevated serum CK 19-2G2 had a sensitivity, specificity, PPV, and NPV of 30%, 83.5%, 53%, and 66%, respectively. AFP (≥ 400 ng/ml) had a sensitivity, specificity, PPV, and NPV of 57%, 79%, 63%, and 74%, respectively. Serum CK 19-2G2 alone predicted malignant portal vein thrombosis in this study. The combination of AFP with either CYFRA 21–1 or CK19-2G2 increased the specificity from 78.8 to 96.5% and decreased the sensitivity from 56.6 to 25% and 18.9%, respectively. Similarly, when AFP is ≥ 400 ng/ml combined with both serum CYFRA 21–1 and CK19-2G2 levels, the specificity increases to 97.7%, but the sensitivity decreases to 11.32%. The AFP and CYFRA 21–1 combination had the highest ability (AUC = 0.800) for the detection of malignant portal vein thrombosis (Table 3 and Supplementary Fig. 1a).
Efficacy of AFP, CK 19-2G2, and CYFRA 21–1 as Tumor Markers for the Detection of Ill-Defined/Infiltrative Tumor Margins
For the detection of ill-defined/infiltrative tumor margins by dynamic imaging, elevated serum CYFRA 21–1 had a sensitivity, specificity, PPV, and NPV of 41%, 83%, 77%, and 51%, respectively. AFP (≥ 400 ng/ml) had sensitivity, specificity, PPV, and NPV of 48%, 83%, 79%, and 53%, respectively. Serum CK 19-2G2 alone predicted the ill-defined/infiltrative pattern in this study. The combination of AFP ≥ 400 ng/ml with either elevated serum CYFRA 21–1 or CK19-2G2 levels increased the specificity from 83 to 98% and decreased the sensitivity from 47.5 to 18.7% and 15%, respectively. Similarly, when AFP is ≥ 400 ng/ml combined with both serum CYFRA 21–1 and CK19-2G2 levels, the specificity increases to 100%, but the sensitivity decreases to 10%. The AFP ≥ 400 ng/ml and elevated CYFRA 21–1 combination had the highest predictability (AUC = 0.755) of ill-defined/infiltrative patterns (Table 4 and Supplementary Fig. 1b).
Treatment Modalities
As first-line therapy, two (1.4%) patients received radiofrequency ablation (RFA), 64 (46.4%) patients received transarterial chemoembolization (TACE), ten (7.2%) patients received the TACE + RFA combination, 22 (16%) patients received systemic therapy (sorafenib), and 40 (29%) patients received the best supportive care. Unfortunately, none of our patients underwent liver resection or liver transplantation (Fig. 1).
Overall Survival
The median OS for all HCC patients in this study, regardless of the treatment option, was 9.0 months (95% confidence interval 7.6–10.4 months). The overall survival rate was 76.8% at 3 months, 63.8% at 6 months, and 39.1% at 12 months. Using the Kaplan‒Meier method, the median survival for those with elevated CYFRA 21–1 levels (> 1.95 ng/ml) and normal CYFRA 21–1 levels (≤ 1.95 ng/ml) was 6 and 10 months, respectively (p = 0.007) (Fig. 2a and Supplementary Table 2). The median survival for patients with elevated serum CK 19-2G2 levels (> 573.5 pg/ml) and normal serum CK 19-2G2 levels (≤ 573.5 pg/ml) was 4.6 and 10 months, respectively (p = 0.002) (Fig. 2b and Supplementary Table 2). The median survival for patients with serum AFP < 400 ng/ml was not reached, while it was 4 months for patients with serum AFP ≥ 400 ng/ml (p < 0.001) (Fig. 2c and Supplementary Table 2).
Discussion
This study showed a strong statistically significant relationship between CYFRA 21–1 elevated levels and various indicators of tumor aggressiveness such as an aggressiveness index > 4, tumor size ≥ 5 cm, higher BCLC tumor stage, presence of malignant portal vein thrombosis, presence of distant metastasis, and the presence of an ill-defined/infiltrative pattern by dynamic imaging.
This study reported a significant association between elevated serum CYFRA 21–1 levels and an aggressiveness index > 4. This finding was attributed mainly to the reported correlation between elevated serum CYFRA 21–1 levels and larger tumor size or the presence of vascular invasion. There were no significant relationships between elevated serum CYFRA 21–1 levels and serum AFP or the number of tumors. In agreement with these findings, Uenishi et al. [29] showed that serum CYFRA 21–1 levels were significantly higher in patients with malignant vascular invasion than in those without vascular invasion (p = 0.002) and that serum CYFRA 21–1 levels were significantly higher in patients with tumors ≥ 5 cm than in patients with smaller tumors (p = 0.008).
The elevated serum CYFRA 21–1 levels with increased tumor size could be attributed to the release of CK fragments into the bloodstream from the destruction of a substantial percentage of tumor cells [23]. Additionally, CK 19 has been reported by Govaere et al. [17] as a main player in HCC invasion, and CK19-positive HCCs had a higher expression of invasion- or metastasis-related markers. Vascular invasion serves as a marker of aggressiveness and invasive capacity of CK 19-positive HCC [30].
This study showed a strong significant relationship between an ill-defined/infiltrative pattern and higher elevated serum CYFRA 21–1 levels, consistent with previous studies’ findings [30, 31]. These studies demonstrated a strong relationship between irregular tumor margins and CK 19 expression. This might be explained by the fact that HCCs with CK 19 expression have progenitor phenotypes characterized by aggressive growth types and high histological grades, which could lead to the appearance of ill-defined/infiltrative growth patterns. Based on previous studies [31,32,33,34], HCCs express CK 19, exhibiting uneven tumor edges and a lack of tumor capsules, as observed on MRI. This finding is consistent with the infiltrative growth patterns reported on pathology [18, 33].
Serum AFP is routinely measured in patients with HCC and is correlated with aggressive tumor behavior and poor OS [35, 36]. This study showed that the measurement of serum CYFRA 21–1 or CK19-2G2 levels, along with AFP, at the time of diagnosis can help in the prediction of more aggressive tumors (presence of vascular invasion or infiltrative pattern) with high specificity (96–98%) which indicated the clinical usefulness of elevated serum CYFRA 21–1(> 1.95 ng/ml) or CK19-2G2 (> 573.5 pg/ml) as poor prognostic biomarkers.
This study showed that elevated serum CYFRA 21–1 levels, CK19-2G2 levels, and serum AFP ≥ 400 ng/ml at the time of diagnosis allowed the prediction of poor 1-year OS. These results were consistent with a prior meta-analysis that revealed CK-19-positive HCC by immunohistopathology was associated with a reduced 1-year OS rate (odds ratio = 0.32, 95% CI: 0.21–0.50) [37]. Caviglia et al. reported in a retrospective study that elevated serum CYFRA 21–1 and AFP were independent predictors of OS in patients with HCC [21]. In contrast, the current study has the advantage of being a prospective study.
Conclusions
Elevated CYFRA 21–1 levels alone were associated with the presence of malignant portal vein thrombosis, tumor size ≥ 5 cm, ill-defined/infiltrative pattern, and aggressiveness index score > 4. For the prediction of malignant portal vein thrombosis and ill-defined/infiltrative patterns, elevated serum CYFRA 21–1 or CK 19-2G2 levels, either alone or in combination, when combined with AFP at a cut-off value of 400 ng/ml, had higher specificities (96–100%) but lower sensitivities (10–25%) than AFP alone. Elevated levels of CYFRA 21–1, CK 19-2G2, or serum AFP ≥ 400 ng/ml predict a poor 1-year OS in Egyptian patients with HCV-related HCC. Studies with larger sample sizes and multicentric studies are needed to validate the current study results.
Data Availability
The de-identified dataset can be accessed upon a reasonable request from the corresponding author.
References
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14. https://doi.org/10.1016/s0140-6736(18)30010-2.
Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3(12):1683–91. https://doi.org/10.1001/jamaoncol.2017.3055.
Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–62. https://doi.org/10.1056/nejmra1713263.
El-Zayadi AR, Badran HM, Barakat EM, Mel-D A, Shawky S, Mohamed MK, et al. Hepatocellular carcinoma in Egypt: a single center study over a decade. World J Gastroenterol. 2005;11(33):5193–8. https://doi.org/10.3748/wjg.v11.i33.5193.
Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul J-L, et al. European association for the study of the liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019.
Mishra L, Banker T, Murray J, Byers S, Thenappan A, He AR, et al. Liver stem cells and hepatocellular carcinoma. Hepatology. 2009;49:318–29. https://doi.org/10.1002/hep.22704.
Calderaro J, Ziol M, Paradis V, Zucman-Rossi J. Molecular and histological correlations in liver cancer. J Hepatol. 2019;71:616–30. https://doi.org/10.1016/j.jhep.2019.06.001.
Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31(1):11–24. https://doi.org/10.1016/0092-8674(82)90400-7.
Werner S, Keller L, Pantel K. Epithelial keratins: biology and implications as diagnostic markers for liquid biopsies. Mol Aspects Med. 2020;72:100817. https://doi.org/10.1016/j.mam.2019.09.001.
Jose J, Sunil P, Madhavan Nirmal R, Varghese SS. Cyfra 21–1: an Overview. Oral Max Path J. 2013;4(2):368–77.
Nakata B, Ogawa Y, Ishikawa T, Ikeda K, Kato Y, Nishino H, et al. Serum CYFRA 21–1 is one of the most reliable tumor markers for breast carcinoma. Cancer. 2000;89(6):1285–90. https://doi.org/10.1002/1097-0142(20000915)89:6%3C1285::aid-cncr13%3E3.0.co;2-g.
Xu Y, Xu L, Qiu M, Wang J, Zhou Q, Xu L, et al. Prognostic value of serum cytokeratin 19 fragments (Cyfra 21–1) in patients with non-small cell lung cancer. Sci Rep. 2015;5:9444. https://doi.org/10.1038/srep09444.
Kong Y, Wang J, Liu W, Chen Q, Yang J, Wei W, et al. Cytokeratin19-2g2, a novel fragment of cytokeratin19 in serum, indicating a more invasive behavior and worse prognosis in breast cancer patients. PLoS ONE. 2013;8(2):e57092. https://doi.org/10.1371/journal.pone.0057092.
Gao J, Lv F, Li J, Wu Z, Qi J. Serum cytokeratin 19 fragment, CK19-2G2, as a newly identified biomarker for lung cancer. PLoS ONE. 2014;9(7):e101979. https://doi.org/10.1371/journal.pone.0101979.
Zhuo JY, Lu D, Tan WY, Zheng SS, Shen YQ, Xu X. CK19-positive hepatocellular carcinoma is a characteristic subtype. J Cancer. 2020;11(17):5069–77. https://doi.org/10.7150/jca.44697.
Lee JI, Lee JW, Kim JM, Kim JK, Chung HJ, Kim YS. Prognosis of hepatocellular carcinoma expressing cytokeratin 19: comparison with other liver cancers. World J Gastroenterol. 2014;18:4751–7. https://doi.org/10.3748/wjg.v18.i34.4751.
Govaere O, Komuta M, Berkers J, Spee B, Janssen C, de Luca F, et al. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut. 2014;63(4):674–85. https://doi.org/10.1136/gutjnl-2012-304351.
Rhee H, Kim H, Park YN. Clinico-radio-pathological and molecular features of hepatocellular carcinomas with keratin 19 expression. Liver Cancer. 2020;9(6):663–81. https://doi.org/10.1159/000510522.
Kawai T, Yasuchika K, Ishii T, Katayama H, Yoshitoshi EY, Ogiso S, et al. Identification of keratin 19-positive cancer stem cells associating human hepatocellular carcinoma using CYFRA 21–1. Cancer Med. 2017;6(11):2531–40. https://doi.org/10.1002/cam4.1211.
Carr BI, Guerra V, Giannini EG, Farinati F, Ciccarese F, Rapaccini GL, et al. A liver index and its relationship to indices of HCC aggressiveness. J Integr Oncol. 2016;5(4):178. https://doi.org/10.4172/2329-6771.1000178.
Caviglia GP, Ciruolo M, Olivero A, Carucci P, Rolle E, Rosso C, et al. Prognostic role of serum cytokeratin-19 fragment (CYFRA 21–1) in patients with hepatocellular carcinoma. Cancers (Basel). 2020;12(10):2776. https://doi.org/10.3390/cancers12102776.
Nagai T, Murota M, Nishioka M, Fujita J, Ohtsuki Y, Dohmoto K, et al. Elevation of cytokeratin 19 fragment in serum in patients with hepatoma: its clinical significance. Eur J Gastroenterol Hepatol. 2001;13(2):157–61. https://doi.org/10.1097/00042737-200102000-00011.
Li Y, Tang ZY, Tian B, Ye SL, Qin LX, Xue Q, et al. Serum CYFRA 21–1 level reflects hepatocellular carcinoma metastasis: study in nude mice model and clinical patients. J Cancer Res Clin Oncol. 2006;32(8):515–20. https://doi.org/10.1007/s00432-006-0098-4.
Muralidharan K. On sample size determination. Math J Interdiscip Sci. 2014;3(1):55–64. https://doi.org/10.15415/mjis.2014.31005.
Dutta D. Relevance of Child Pugh scoring system as inclusion criteria in hepatocellular carcinoma management. Japanese J Gastro Hepato. 2021;17:1–3.
Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–93. https://doi.org/10.1016/j.jhep.2021.11.018.
Ebied SA, Abdel-Rehim WM, El-Benhawy SA, El-Gawish MA, Hassan MA, El-Settawy II. Serum CYFRA 21–1 in Egyptian women with breast cancer. Alexandria J Med. 2017;53:41–7. https://doi.org/10.1016/j.ajme.2016.02.006.
Yoon S, Lim YK, Kim HR, Lee MK, Kweon OJ. Establishment of reference intervals of cytokeratin 19 fragment antigen 21–1 in Korean adults. Ann Lab Med. 2023;43(1):82–5. https://doi.org/10.3343/alm.2023.43.1.82.
Uenishi T, Yamazaki O, Yamamoto T, Hirohashi K, Tanaka H, Tanaka S, et al. Clinical significance of serum cytokeratin-19 fragment (CYFRA 21–1) in hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2006;13(3):239–44. https://doi.org/10.1007/s00534-005-1069-x.
Qin SD, Zhang J, Qi YP, Zhong JH, Xiang BD. Individual and joint influence of cytokeratin 19 and microvascular invasion on the prognosis of patients with hepatocellular carcinoma after hepatectomy. World J Surg Oncol. 2022;20(1):209. https://doi.org/10.1186/s12957-022-02632-z.
Choi SY, Kim SH, Park CK, Min JH, Lee JE, Choi YH, et al. Imaging features of gadoxetic acid-enhanced and diffusion-weighted MR imaging for identifying cytokeratin 19-positive hepatocellular carcinoma: a retrospective observational study. Radiology. 2018;286(3):897–908. https://doi.org/10.1148/radiol.2017162846.
Hu XX, Wang WT, Yang L, Yang ZX, Liang HY, Ding Y, et al. MR features based on LI-RADS identify cytokeratin 19 status of hepatocellular carcinomas. Eur J Radiol. 2019;113:7–14. https://doi.org/10.1016/j.ejrad.2019.01.036.
Jeong HT, Kim MJ, Kim YE, Park YN, Choi GH, Choi JS. MRI features of hepatocellular carcinoma expressing progenitor cell markers. Liver Int. 2012;32(3):430–40. https://doi.org/10.1111/j.1478-3231.2011.02640.x.
Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee JE, et al. Human hepatocellular carcinomas with “Stemness”-related marker expression: keratin 19 expression and a poor prognosis. Hepatology. 2011;54(5):1707–17. https://doi.org/10.1002/hep.24559.
Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39(12):2214–29. https://doi.org/10.1111/liv.14223.
Bai DS, Zhang C, Chen P, Jin SJ, Jiang GQ. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci Rep. 2017;7(1):12870. https://doi.org/10.1038/s41598-017-12834-1.
Sun DW, Zhang YY, Sun XD, Chen YG, Qiu W, Ji M, et al. Prognostic value of cytokeratin 19 in hepatocellular carcinoma: a meta-analysis. Clin Chim Acta. 2015;448:161–9. https://doi.org/10.1016/j.cca.2015.06.027.
Acknowledgements
The authors would like to express their honest gratitude to the Alexandria HCC Tumour Board team at the Faculty of Medicine, Alexandria University, for their invaluable assistance and unwavering support.
Author information
Authors and Affiliations
Contributions
MYT: concept and design of the study, analysis and interpretation of data for the work. EMH: design of the study, analysis and interpretation of data. ASE-H: performing all laboratory and tumor markers analyses. OSE: acquisition, analysis and categorization of radiological data, main operator for TACE and RFA. MFB: collection of patient data, analysis and interpretation of data for the work. All authors contributed to drafting and revising the manuscript and approved the final submitted version.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
All study participants provided informed consent in compliance with good clinical practice recommendations and the principles of the Declaration of Helsinki. The Institutional Review Board at the Faculty of Medicine, Alexandria University, approved this study (approval number: 201535).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taher, M.Y., Hassouna, E.M., El-Hadidi, A.S. et al. Predictive Value of Serum CYFRA 21-1 and CK19-2G2 for Tumor Aggressiveness and Overall Survival in Hepatitis C-Related Hepatocellular Carcinoma Among Egyptians: A Prospective Study. J Gastrointest Canc 55, 749–758 (2024). https://doi.org/10.1007/s12029-023-01012-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-023-01012-4