Abstract
Non-invasive predictors for the development of cirrhosis-related conditions are needed for patients with primary biliary cholangitis (PBC). We investigated the association between cytokeratin-18 fragments (M30 and M65) and liver histology, treatment response and the development of cirrhosis-related conditions in patients with PBC. We retrospectively reviewed the clinical data of 111 individuals with biopsy-proven PBC. Serum M30 and M65 levels were measured using stored sera. M30 were significantly decreased after treatment, but there was no significant change in the M65 levels. M65 was significantly higher in non-responders according to the Paris-I and Paris-II definitions. In the multivariate analysis, high levels of M65 were significantly associated with advanced Scheuer stage (odds ratio 5.86; 95% confidence interval 0.55–22.2; P = 0.009) and with the development of cirrhosis-related conditions (hazard ratio 3.94; 95% confidence interval: 1.06–14.5, P = 0.039). Among PBC patients without cirrhosis, those with high serum M65 levels at baseline were at higher risk of developing cirrhosis-related conditions (log-rank test; P = 0.001). High levels of serum M65 may be a non-invasive and early predictor of the development of cirrhosis-related conditions in PBC patients. Our findings may help initiate therapies earlier for those at risk for cirrhosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary biliary cholangitis (PBC) is a progressive autoimmune liver disease characterized by portal inflammation, immune-mediated destruction of the intrahepatic bile ducts, and the presence of highly specific anti-mitochondrial antibodies in the serum [1, 2]. Destruction of the intrahepatic bile ducts cause the loss of bile ducts, cirrhosis and liver failure [3]. End-stage liver disease results in liver failure; individuals at this stage either require liver transplantation or die due to liver-related causes [4].
Progress in the clinical examination and treatment for ursodeoxycholic acid (UDCA) has improved the prognosis of PBC. Hard endpoints such as liver transplantation or liver cirrhosis-related death were primary endpoint of treatment [5, 6]. However, most patients with PBC are diagnosed during the early disease stages, and mortality of patients with PBC has improved over the last two decades [7, 8]. Hard endpoints are not thought to be realistic [8]; rather, complications related to cirrhosis and histological evidence of cirrhosis were considered clinically useful endpoints [9]. Liver histology and the UDCA biochemical response are predictors of several poor outcomes in patients with PBC [9, 10]. However, liver biopsy is an invasive procedure, and UDCA response is often determined between 6 months and 2 years after starting treatment for UDCA [11,12,13]. Non-invasive and early predictors for the development of cirrhosis-related conditions are needed.
Cytokeratin (CK)-18 is a major intermediate filament protein in liver cells [14] and is cleaved by caspases during apoptosis [15, 16]. Caspase-cleaved CK-18 fragments are detectable using M30, a monoclonal antibody [17]. In addition, the M65 antibody can detect both caspase-cleaved and uncleaved CK-18 fragments. Serum M30 is used as a marker of apoptosis, whereas serum M65 is used as a marker of overall cell death via apoptosis and necrosis. In patients with PBC, serum M65 is associated with histological fibrosis and hard endpoints [18, 19], but the association between CK-18 fragments and liver histology other than fibrosis, treatment response or the development of cirrhosis-related conditions in patients with PBC is still unknown. The aim of this study was to investigate the association between serum CK-18 fragments (serum M30 and M65) and either liver histology or treatment response and the utility of these fragments as a predictor of the development of cirrhosis-related conditions in patients with PBC.
Materials and methods
Study design and patients
In this retrospective study, we reviewed the clinical records of patients with PBC who were diagnosed at Fukushima Medical University Hospital between January 1988 and December 2014. The inclusion criteria were as follows: (1) diagnosis of PBC confirmed by liver biopsy and (2) available blood sample obtained before biopsy. Patients were diagnosed with PBC features if they met at least two of the following three criteria: (a) chronic elevation of the cholestatic liver enzymes alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGT) for at least 6 months; (b) presence of serum anti-mitochondrial antibody detected via either indirect immunofluorescence or ELISA using commercially available kits; and (c) typical histological findings from biopsied liver specimens [20]. The exclusion criteria comprised evidence of other liver diseases such as chronic hepatitis C (HCV-RNA-positive), chronic hepatitis B (HBV-DNA-positive) and alcoholic liver disease (> 20 g of alcohol/day). Patients with an overlap syndrome or active systemic disorders were also excluded. We initially enrolled 152 patients, but 41 patients were excluded (HCV-RNA-positive, n = 2; no adequate follow-up data, n = 28; no adequate pathological data, n = 5; overlap syndrome, n = 4; active systemic disorder, n = 2 [one was systemic lupus erythematosus and the other was acute myeloid leukemia]). Thus, 111 patients were included in the final analysis, with 15 sex- and age-matched healthy Japanese individuals as controls.
This study was approved for the use of opt-out consent by the ethics committee at Fukushima Medical University School of Medicine and was in accordance with the Declaration of Helsinki. A website with additional information, including an opt-out consent form, was established for this study. The elements of informed consent were presented orally, and all the patients and control subjects agreed to undergo serum testing and have their blood stored for future research. Retrospective testing of blood samples from all the patients and control subjects was performed as part of the approved protocol, and the data were analyzed by individuals blinded to the clinical data according to institutional and national ethics rules.
Liver histology
Liver biopsies were performed using percutaneous ultrasound guidance with an 18-gauge needle. Routine hematoxylin-eosin staining was used to evaluate inflammation and fibrosis. The biopsy specimens were assessed by pathologists without knowledge of prior clinical data. The histological findings were graded according to the Scheuer staging system [21] and the Nakanuma grading and staging system [22]. Three histologic components (fibrosis [grade 0–3], bile duct loss [grade 0–3], and deposition of orcein-positive granules [grade 0–3]) are typically evaluated using the Nakanuma staging system (stage 1–4); however, only 2 components (fibrosis and bile duct loss) were evaluated in the present study. Advanced stage disease was defined as Nakanuma stage 3–4 or Scheuer stage 3–4.
Immunohistochemical M30 staining was performed using the mouse monoclonal antibody (M30 CytoDEATH; Roche Applied Science, Mannheim, Germany) in patients with PBC as reported previously [23]. We used peroxidase-labeled anti-rabbit or anti-mouse antibody (Histofine Simplestain Max PO; Nichirei) as secondary antibody.
Endpoint definitions
The primary endpoint of this study was the development of cirrhosis-related conditions, which are defined by at least one of the following events: histologically proven cirrhosis or cirrhosis-related complications and/or symptoms (i.e., ascites, ruptured and/or endoscopically treated gastroesophageal varices, hepatic encephalopathy, hyperbilirubinemia [≥ 2.0 mg/dL] or hepatocellular carcinoma) [10].
Measurement of CK-18 fragments
Blood samples were obtained from all the patients and control subjects. The blood samples were stored at −20 °C until further testing. Blood samples from patients with PBC were obtained and stored either once (before liver biopsy) or twice (before biopsy and after starting treatment). Serum M30 was measured using an M30-Apoptosense ELISA (CUSABIO, Hubei, China), whereas serum M65 was measured using an M65 ELISA kit (Pevia, Bromma, Sweden).
Definition of biochemical response to treatment
The biochemical response to treatment was evaluated either 6 months or 1 year after treatment initiation according to 5 previously published definitions: Paris-I [24], Paris-II [9], Barcelona [25], Rotterdam [26] and Ehime [11]. Two recently proposed risk scoring systems were also evaluated in patients after 1 year of treatment: the GLOBE score and UK-PBC score [27, 28].
Statistical analysis
The clinical data are expressed as the median and 25th–75th interquartile ranges and were compared between groups with the Mann–Whitney U test or Kruskal–Wallis test. Comparisons of laboratory data before and after treatment were analyzed by the Wilcoxon signed-rank test. Differences in the categorical variables were determined using Fisher’s exact test. Correlations between the data were analyzed with Spearman’s rank correlation test. Univariate and multivariate logistic regression analyses were used to assess predictors of advanced histological stage, whereas univariate and multivariate Cox proportional hazards models were used to assess the predictors of the development of cirrhosis-related conditions. Variables that achieved P < 0.05 in the univariate analysis were included in the multivariate regression analyses. We applied receiver operating characteristic (ROC) curve analysis to determine the ideal cut-off values that could predict the diagnostic performance of advanced histological stage. Kaplan–Meier curves were constructed, and the development of cirrhosis-related conditions of patients was assessed using the log-rank test. Differences with a value of P < 0.05 were considered statistically significant. The data were analyzed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 3.2.1), and a modified version of R commander (version 2.1-7) [29].
Results
Baseline characteristics
The baseline characteristics and histological findings of the 111 patients are shown in Table 1. In patients with PBC, the median age was 55 years, and 89 patients were female (80%). The median follow-up period was 9.1 years. There were 65 patients with Nakanuma stage 1–2 (58%) disease and 81 patients with Scheuer stage 1–2 (72%) disease. The median serum M30 and M65 levels in patients with PBC were 497 and 500 U/L, respectively. Among the 111 patients in the cohort, 13 (11%) had already undergone treatment for UDCA at the time of biopsy. All patients were treated for UDCA after biopsy. Nineteen patients (17%) were diagnosed with liver cirrhosis (n = 16) or recognized cirrhosis-related conditions (gastrointestinal varices: n = 3) at the time of biopsy. Among 92 patients who were not diagnosed with either liver cirrhosis or recognized cirrhosis-related conditions, 15 (16%) developed cirrhosis-related conditions during follow-up period (gastroesophageal varices, n = 9; cirrhosis diagnosis based on biopsy, n = 2; hepatocellular carcinoma, n = 2; hepatic encephalopathy, n = 1; and hyperbilirubinemia, n = 1). The survival rates without adverse outcomes at 5, 10 and 15 years were 95% and 89 and 75%, respectively, among the subgroup of 92 patients.
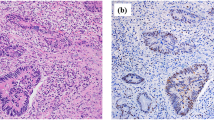
Immunohistological expression of M30 in patients with PBC are shown in Fig. 1. M30 expression in Scheuer stage 1 (Fig. 1a, b), stage 2 (Fig. 1c, d), stage 3 (Fig. 1e, f) and stage 4 (Fig. 1g, h) were shown. M30 was detected in the cytoplasm of biliary epithelial cells and hepatocytes. M30 expression in biliary epithelial cells was detected in patients with PBC stage 1–4. M30 expression in hepatocytes around site of cholangitis was stronger in stage 3–4 than in stage 1–2. Although the nuclei of biliary epithelial cells and hepatocytes looked M30-positive in these patients, nuclear staining was not correlated with apoptosis according to the antibody product information.
Expression of cytokeratin M30 in patients with PBC. a, b Scheuer stage, 1; CA, 0; HA, 0; Nakanuma stage, 1; serum M30, 353 U/L; serum M65. 267 U/L [a: hematoxylin-eosin (HE), ×400. b: M30, ×400]. c, d Scheuer stage, 2; CA, 3; HA, 2; Nakanuma stage, 2; serum M30, 638 U/L; serum M65, 1710 U/L (c HE, ×400. d M30, ×400). e, f Scheuer stage, 3; CA, 2; HA, 2; Nakanuma stage, 3; serum M30, 449 U/L; serum M65, 1630 U/L (e: HE, × 200. f: M30, × 200). g, h Scheuer stage, 4; CA, 3; HA, 3; Nakanuma stage, 3; serum M30, 933 U/L; serum M65 638 U/L (g HE, ×200. h M30, ×200). M30 expression was detected in biliary epithelial cells of PBC patients with Scheuer stage 1–4. M30 expression in hepatocytes around cholangitis was stronger in patients who were stage 3–4 than patients who were stage 1–2
The median age of the control subjects was 55 (52–56) years, and 13 were female (88%). No significant differences in the prevalence of age and sex were observed between the PBC patients and control subjects. The median serum M30 and M65 levels in the healthy subjects were 163 (136–197) and 241 (185–368) U/L, respectively. Additionally, the serum M30 and M65 levels in the PBC patients at baseline were significantly higher than those in the healthy controls (M30: P < 0.001. M65: P = 0.006).
Correlations between cytokeratin-18 fragments and baseline laboratory parameters and liver histology
We assessed the correlations between CK-18 fragments and both laboratory data and liver histology (Table 2). Serum M30 levels were significantly correlated with GGT, TB, CA and Nakanuma stage, whereas serum M65 levels were significantly correlated with AST, ALT, ALP, GGT, TB, Alb, platelet count, fibrosis, bile duct loss, Nakanuma stage and Scheuer stage.
Change in the levels of cytokeratin-18 fragments and laboratory parameters following treatment
Among 111 patients, 55 had blood samples collected and stored before and after treatment. The median period between the first and second blood sampling was 3.9 (1.3–7.2) years. Changes in the levels of CK-18 fragments after treatment are shown in Fig. 2. The median serum M30 values were significantly lower after treatment (497 [414–642] U/L vs 466 [289–587] U/L, P < 0.001), but the serum M65 values did not change significantly after treatment (465 [300–724] U/L vs 420 [320–660] U/L, P = 0.24). The levels of AST (43 [30–79] U/L vs 27 [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37], P < 0.001), ALT (43 [32–87] U/L vs 23 [20,21,22,23,24,25,26,27] U/L, P < 0.001), ALP (440 [358–605] U/L vs 324 [220–453] U/L, P < 0.001) and GGT (160 [86–313] U/L vs 45 [26–74] U/L, P < 0.001) were significantly lower after treatment.
Cytokeratin-18 fragments at baseline and following treatment (N = 55). Serum M30 levels were significantly lower after treatment (497 [414–642] U/L vs 466 [289–587] U/L, P < 0.001); however, serum M65 levels did not change significantly after treatment (465 [300–724] U/L vs 420 [320–660] U/L, P = 0.24)
Association between cytokeratin-18 fragments and response to treatment or risk score
The association between CK-18 fragments and treatment response are shown in Table 3. Serum M65 levels were significantly higher in non-responders than in responders according to the Paris-I (845 vs 485 U/L, P = 0.007) and Paris-II definitions (788 vs 476 U/L, P = 0.005). However, there was no significant difference between responders and non-responders according to the Barcelona, Rotterdam and Ehime definitions. Serum M30 levels were not significantly different between responders and non-responders regardless of the definition used. The UK-PBC risk score was significantly correlated with serum M65 levels at 5, 10 and 15 years (r = 0.36, P = 0.002). Serum M30 levels were significantly correlated with the UK-PBC risk score only at 10 years (r = 0.16, P = 0.019). The GLOBE score did not correlate with any of the CK-18 fragments (Table 4).
Association between cytokeratin-18 fragments and advanced histological stage
We analyzed CK-18 fragments from PBC stages (Fig. 3). Serum M30 levels significantly differed from the Nakanuma stage, but did not differ from the Scheuer stage; in contrast, serum M65 levels significantly differed from both Nakanuma and Scheuer stage, and serum M65 levels were high in advanced stages. We investigated the association between the levels of CK-18 fragments and advanced histological stage according to Nakanuma staging and Scheuer staging (Table 5). We analyzed age, sex, serum M30, serum M65, AST, ALT, ALP, GGT, TB, Alb and PT. The univariate analysis showed that serum M65 ≥ 500 U/L, AST ≥ 50 U/L, ALT ≥ 45 U/L, TB ≥ 0.8 mg/dL and Alb < 4.0 g/dL were significantly associated with advanced Nakanuma stage. However, the multivariate analysis did not identify any independent factors associated with advanced Nakanuma stage. A separate univariate analysis showed that serum M65 ≥ 500 U/L, AST ≥ 50 U/L, ALP ≥ 468 U/L, TB ≥ 0.8 mg/dL and Alb < 4.0 g/dL were significantly associated with advanced Scheuer stage. The subsequent multivariate analysis showed that high serum M65 (odds ratio [OR]: 5.86, 95% confidence interval [CI]: 1.55–22.2, P = 0.009) and low Alb (OR 3.50, 95% CI 1.16–10.6, P = 0.026) were independently associated with advanced Scheuer stage.
ROC curve analysis was performed to determine the optimal cut-off values for serum M65 to distinguish Scheuer stages. The cut-off values of serum M65 for advanced Scheuer stage was 553 U/L. The area under ROC, specificity, and sensitivity for serum M65 are 0.801, 0.828, and 0.671, respectively.
Predictive factors for the development of cirrhosis-related conditions
We analyzed the association between the levels of CK-18 fragments and the development of cirrhosis-related conditions. We analyzed age, sex, serum M30, serum M65, AST, ALT, ALP, GGT, TB, Alb and PT in 92 patients who were not diagnosed with liver cirrhosis at baseline. The univariate analysis identified the following factors as significantly associated with the development of cirrhosis-related conditions: serum M65 ≥ 500 U/L and ALT ≥ 45 U/L (Table 6). The multivariate analysis showed that high levels of serum M65 were independently associated with the development of cirrhosis-related conditions (hazard ratio 3.94, 95% CI 1.06–14.5, P = 0.039).
Kaplan–Meyer analysis of the development of cirrhosis-related conditions was performed using the serum M65 levels that predicted advanced Scheuer stage as the cut-off. Among PBC patients without cirrhosis, those with high serum M65 levels (≥ 500 U/L) at baseline had a higher risk of developing cirrhosis-related conditions (log-rank test; P = 0.001) (Fig. 4). The 10- and 15-year survival rates without the development of cirrhosis-related conditions were 76 and 54% in patients above the serum M65 cut-off (≥ 500 U/L) versus 100 and 92% in patients below the serum M65 cut-off.
Kaplan–Meier plots for predicting the development of cirrhosis-related conditions based on baseline levels of serum M65. The risk of developing cirrhosis-related conditions was significantly higher in patients with high M65 values at baseline (≥ 500 U/L) than those with low M65 values at baseline (P = 0.001)
Discussion
The present study demonstrated that serum M65 levels was associated with the Scheuer staging system and that serum M65 levels were significantly higher in biochemical non-responders than in responders based on the Paris-I and Paris- II criteria and correlated with the UK-PBC risk score. Furthermore, serum M65 levels did not change significantly after treatment and could predict the development of adverse outcomes in PBC patients without cirrhosis. Serum M30 levels were correlated with both histological findings and the UK-PBC score but were not associated with the development of adverse outcomes.
A non-invasive and early predictive factor is desirable to help manage new therapies. In this study, the serum M65 levels at baseline were significantly associated with the development of cirrhosis-related conditions in PBC patients who did not initially have cirrhosis. Serum M65 levels was strongly correlated with AST, ALT, ALP and GGT, but only serum M65 levels did not show a significant decrease after treatment. This result may suggest that serum M65 levels reflect persistent liver injury. Our patients showed good outcomes: the survival rates without adverse outcomes at 5, 10 and 15 years were 95, 89 and 75%, respectively. These outcomes are likely because most patients were in the early histological disease stage, and we only included patients without biopsy-proven cirrhosis. Serum M65 levels could predict the development of outcomes, including patients showing a good prognosis. Serum M65 levels may be a useful, non-invasive and early predictor for the development of cirrhosis-related conditions in non-cirrhotic patients with PBC.
Serum M65 levels was significantly associated with advanced Scheuer stage and strongly correlated with the histological findings of fibrosis, bile duct loss and stage according to the Nakanuma staging system. The use of serum M65 as a predictor of adverse outcomes may be associated with histological findings. Both the Scheuer and Nakanuma staging systems are a good predictor of the development of adverse outcomes [10]. An association between serum M65 and fibrosis was previously reported [19], and we demonstrated that serum M65 was correlated with not only fibrosis but also bile duct loss. Bile duct loss as measured on the baseline biopsy predicted histological progression and the biochemical response to UDCA [12, 30].
In this study, the serum M65 levels at diagnosis were significantly higher in UDCA non-responders than in responders based on the Paris-I and Paris-II definitions and were correlated with the UK-PBC risk score. The biochemical response to UDCA was independent of the histological stage of PBC patients [25]. The UK-PBC risk score has shown good performance for the prediction of death or liver transplantation [28]. Other UDCA response criteria and scores were not associated with serum M65. High serum M65 levels may be associated with persistently elevated transaminase in patients with PBC because the Paris-I and Paris-II definitions and the UK-PBC risk score include an assessment of either AST or ALT during treatment.
Immunohistochemical findings revealed that M30 was expressed in both biliary epithelial cells and hepatocytes in patients with PBC. CK-18 was present in both biliary epithelial cells and hepatocytes. Bcl-2 expression and TUNEL-positive cell were observed in both biliary epithelial cells and hepatocytes [31, 32]. Our results were compatible with these previous reports. M30 expression in hepatocytes around site of cholangitis was stronger in stage 3–4 than in stage 1–2 in this study. Serum M30 was significantly different from the Nakanuma stage, but was not significantly different from the Scheuer stage. These results suggest that M30 expression might be similar in every histological stage of PBC. We noted that M30 expression was strong in advanced histological stages but that differences in M30 expression between stages were not large. Serum M30 levels significantly correlated with CA grading in this study. M30 expression might reflect CA grading than histological stage.
M30 is used as a marker of apoptosis. Although apoptosis is thought to be the primary injury mechanism in patients with PBC [33], serum M30 was not associated with the development of adverse outcomes in this study. The antiapoptotic effects of UDCA have been described in several reports. Decreases in anion exchanger 2 (AE2) activity and HCO3− secretion have been observed, and the former of which leads to apoptosis in patients with PBC by activating adenylyl cyclase [4, 34, 35]. UDCA may protect cholangiocytes by stimulating biliary HCO3− secretion [36] and decrease TGF-β1-induced apoptosis via the glucocorticoid receptor [37]. We demonstrated that serum M30 levels were significantly lower after treatment administration; this finding is consistent with those in previous reports. The association between serum M30 levels and adverse outcomes might decrease after treatment.
There were several limitations in this study. First, this is single-center, retrospective study with a small cohort. Although we only analyzed biopsy-proven PBC patients and the development of cirrhosis-related conditions in patients with no initial signs of cirrhosis; to address this, a large number of subjects and a prospective study are needed. Second, we analyzed the biochemical response to treatment in a subset of patients and demonstrated that non-responders showed significantly higher serum M65 levels than in responders according to the Paris-I and Paris-II definitions. The Paris definitions are recognized as the best validated definitions [8]. The association between serum M65 levels and treatment response were important findings in this study.
In summary, there were associations between serum M65 levels and both liver histology and treatment response. High levels of serum M65 levels at baseline may predict the development of cirrhosis-related conditions in PBC patients without cirrhosis. These findings suggested that serum M65 levels reflected clinicopathological findings such as disease activity, treatment response and prognosis. Our findings may help to guide decisions earlier in the disease progression and implement more optimal therapies.
References
Kaplan MM, Gershwin ME (2005) Primary biliary cirrhosis. N Engl J Med 353:1261–1273
Selmi C, Bowlus CL, Gershwin ME, Coppel RL (2011) Primary biliary cirrhosis. Lancet 377:1600–1609
Poupon R, Chretien Y, Chazouilleres O, Poupon RE (2005) Pregnancy in women with ursodeoxycholic acid-treated primary biliary cirrhosis. J Hepatol 42:418–419
European Association for the Study of the Liver (2017) EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol 67:145–172
Poupon RE, Lindor KD, Cauch-Dudek K, Dickson ER, Poupon R, Heathcote EJ (1997) Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology 113:884–890
Corpechot C, Carrat F, Bonnand AM, Poupon RE, Poupon R (2000) The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis. Hepatology 32:1196–1199
Kuiper EM, Hansen BE, Metselaar HJ, de Man RA, Haagsma EB, van Hoek B, van Buuren HR (2010) Trends in liver transplantation for primary biliary cirrhosis in the Netherlands 1988–2008. BMC Gastroenterol 10:144
Silveira MG, Brunt EM, Heathcote J, Gores GJ, Lindor KD, Mayo MJ (2010) American Association for the Study of Liver Diseases endpoints conference: design and endpoints for clinical trials in primary biliary cirrhosis. Hepatology 52:349–359
Corpechot C, Chazouilleres O, Poupon R (2011) Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J Hepatol 55:1361–1367
Kakuda Y, Harada K, Sawada-Kitamura S, Ikeda H, Sato Y, Sasaki M, Okafuji H, Mizukoshi E, Terasaki S, Ohta H, Kasashima S, Kawashima A, Kaizaki Y, Kaneko S, Nakanuma Y (2013) Evaluation of a new histologic staging and grading system for primary biliary cirrhosis in comparison with classical systems. Hum Pathol 44:1107–1117
Azemoto N, Abe M, Murata Y, Hiasa Y, Hamada M, Matsuura B, Onji M (2009) Early biochemical response to ursodeoxycholic acid predicts symptom development in patients with asymptomatic primary biliary cirrhosis. J Gastroenterol 44:630–634
Kumagi T, Guindi M, Fischer SE, Arenovich T, Abdalian R, Coltescu C, Heathcote EJ, Hirschfield GM (2010) Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol 105:2186–2194
Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD, American Association for the Study of Liver Diseases (2009) Liver biopsy. Hepatology 49:1017–1044
Van Eyken P, Desmet VJ (1993) Cytokeratins and the liver. Liver 13:113–122
Caulin C, Salvesen GS, Oshima RG (1997) Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol 138:1379–1394
Ku NO, Liao J, Omary MB (1997) Apoptosis generates stable fragments of human type I keratins. J Biol Chem 272:33197–33203
Bantel H, Ruck P, Gregor M, Schulze-Osthoff K (2001) Detection of elevated caspase activation and early apoptosis in liver diseases. Eur J Cell Biol 80:230–239
Denk G, Omary AJ, Reiter FP, Hohenester S, Wimmer R, Holdenrieder S, Rust C (2014) Soluble intracellular adhesion molecule, M30 and M65 as serum markers of disease activity and prognosis in cholestatic liver diseases. Hepatol Res 44:1286–1298
Sekiguchi T, Umemura T, Fujimori N, Shibata S, Ichikawa Y, Kimura T, Joshita S, Komatsu M, Matsumoto A, Tanaka E, Ota M (2015) Serum cell death biomarkers for prediction of liver fibrosis and poor prognosis in primary biliary cirrhosis. PLoS One 10:e0131658
Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ, American Association for Study of Liver Diseases (2009) Primary biliary cirrhosis. Hepatology 50:291–308
Scheuer P (1967) Primary biliary cirrhosis. Proc R Soc Med 60:1257–1260
Nakanuma Y, Zen Y, Harada K, Sasaki M, Nonomura A, Uehara T, Sano K, Kondo F, Fukusato T, Tsuneyama K, Ito M, Wakasa K, Nomoto M, Minato H, Haga H, Kage M, Yano H, Haratake J, Aishima S, Masuda T, Aoyama H, Miyakawa-Hayashino A, Matsumoto T, Sanefuji H, Ojima H, Chen TC, Yu E, Kim JH, Park YN, Tsui W (2010) Application of a new histological staging and grading system for primary biliary cirrhosis to liver biopsy specimens: Interobserver agreement. Pathol Int 60:167–174
Bantel H, Lugering A, Heidemann J, Volkmann X, Poremba C, Strassburg CP, Manns MP, Schulze-Osthoff K (2004) Detection of apoptotic caspase activation in sera from patients with chronic HCV infection is associated with fibrotic liver injury. Hepatology 40:1078–1087
Corpechot C, Abenavoli L, Rabahi N, Chretien Y, Andreani T, Johanet C, Chazouilleres O, Poupon R (2008) Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology 48:871–877
Pares A, Caballeria L, Rodes J (2006) Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology 130:715–720
Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, Houben MH, Witteman BJ, van Erpecum KJ, van Buuren HR, Dutch P. B. C. Study Group (2009) Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology 136:1281–1287
Lammers WJ, Hirschfield GM, Corpechot C, Nevens F, Lindor KD, Janssen HL, Floreani A, Ponsioen CY, Mayo MJ, Invernizzi P, Battezzati PM, Pares A, Burroughs AK, Mason AL, Kowdley KV, Kumagi T, Harms MH, Trivedi PJ, Poupon R, Cheung A, Lleo A, Caballeria L, Hansen BE, van Buuren HR, Global PBC Study Group (2015) Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology 149:1804–1812 (e1804)
Carbone M, Sharp SJ, Flack S, Paximadas D, Spiess K, Adgey C, Griffiths L, Lim R, Trembling P, Williamson K, Wareham NJ, Aldersley M, Bathgate A, Burroughs AK, Heneghan MA, Neuberger JM, Thorburn D, Hirschfield GM, Cordell HJ, Alexander GJ, Jones DE, Sandford RN, Mells GF, UK-PBC Consortium (2016) The UK-PBC risk scores: derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology 63:930–950
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Namisaki T, Moriya K, Kitade M, Kawaratani H, Takeda K, Okura Y, Takaya H, Nishimura N, Seki K, Kaji K, Sato S, Sawada Y, Yamao J, Mitoro A, Uejima M, Mashitani T, Shimozato N, Nakanishi K, Furukawa M, Saikawa S, Kubo T, Yoshiji H (2017) Clinical significance of the Scheuer histological staging system for primary biliary cholangitis in Japanese patients. Eur J Gastroenterol Hepatol 29:23–30
Koga H, Sakisaka S, Ohishi M, Sata M, Tanikawa K (1997) Nuclear DNA fragmentation and expression of Bcl-2 in primary biliary cirrhosis. Hepatology 25:1077–1084
Fox CK, Furtwaengler A, Nepomuceno RR, Martinez OM, Krams SM (2001) Apoptotic pathways in primary biliary cirrhosis and autoimmune hepatitis. Liver 21:272–279
Fickert P, Wagner M (2017) Biliary bile acids in hepatobiliary injury—what is the link? J Hepatol 67:619–631
Medina JF, Martinez A, Vazquez JJ, Prieto J (1997) Decreased anion exchanger 2 immunoreactivity in the liver of patients with primary biliary cirrhosis. Hepatology 25:12–17
Prieto J, Garcia N, Marti-Climent JM, Penuelas I, Richter JA, Medina JF (1999) Assessment of biliary bicarbonate secretion in humans by positron emission tomography. Gastroenterology 117:167–172
Beuers U (2006) Drug insight: Mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol 3:318–328
Sola S, Amaral JD, Castro RE, Ramalho RM, Borralho PM, Kren BT, Tanaka H, Steer CJ, Rodrigues CM (2005) Nuclear translocation of UDCA by the glucocorticoid receptor is required to reduce TGF-beta1-induced apoptosis in rat hepatocytes. Hepatology 42:925–934
Acknowledgements
We thank C. Sato and R. Hikichi for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Hayashi, M., Abe, K., Fujita, M. et al. Serum levels of a cell death biomarker predict the development of cirrhosis-related conditions in primary biliary cholangitis. Med Mol Morphol 51, 176–185 (2018). https://doi.org/10.1007/s00795-018-0184-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-018-0184-0