Abstract
Background
Cytokeratin is associated with the recurrence and metastasis of some cancers and tends to increase the malignancy of the disease. It is getting more and more attention in cancer research. Abnormal expression of cytokeratin 19 (CK19) has been reported as an important prognostic factor in cancers. CK19 is a marker of bile duct cells, liver progenitor cells (HPCs), and early hepatoblasts, and its expression is associated with poor prognosis in patients diagnosed with hepatocellular carcinoma (HCC). The purpose of this study was to evaluate the predictive value of CK19 for tumor recurrence after radical resection in patients with hepatitis B virus (HBV) positive HCC.
Methods
This study was a retrospective study conducted in two institutions. A total of 674 patients with HBV positive HCC who underwent radical HCC resection from January 2010 to May 2020 were included in this study. Chi-square test or Fisher’s exact test was used to compare the classification variables and continuous variables were compared by t-test or Wilcoxon rank sum test. Cox regression model was used for univariate and multi-variable survival analyses. Based on the results of the multi-variable analyses of Cox regression, the nomogram of 2-year recurrence-free survival (RFS) was plotted. The model was validated internally in the Hangzhou cohort (training set) and then externally in the Lanzhou cohort (test set) and the effectiveness of the model was tested.
Results
For all 674 patients, 223 cases (33.1%) were positive and 451 cases (66.9%) were negative for CK19. The 2-year RFS rate was higher in patients with CK19 negative than in patients with CK19 positive. In the training set, correlation analysis showed that CK19 expression was correlated with preoperative potassium (P value(P) = 0.030), satellite nodules (P < 0.001) and microvascular invasion (P = 0.020). In the test set, CK19 expression was correlated with postoperative platelet (P = 0.038), satellite nodules (P = 0.003), microvascular invasion (P = 0.011), and maximum tumor size (P = 0.039). Univariate Cox regression correlation analyses showed that CK19 expression was correlated with preoperative potassium (P value(P) = 0.030), satellite nodules (P < 0.001), and microvascular invasion (P = 0.020). Training and test sets showed that postoperative platelet (> 300/L), CK19, satellite nodules in the training set, microvascular invasion, maximum tumor size, and tumor boundary were adverse factors for predicting RFS. Multi-variable analyses showed that in the training set, postoperative platelet > 300/L (hazard ratios (HR) = 2.753, 95% confidence interval (95%CI):1.234–6.142, P = 0.013), CK19 (HR = 1.410, 95%CI:1.006–1.976, P = 0.046), satellite nodule (HR = 1.476, 95%CI:1.026–2.120, P = 0.036), microvascular invasion (HR = 2.927, 95%CI:2.006–4.146, P < 0.001), incomplete tumor capsule (HR = 1.539, 95%CI:1.012–2.341, P = 0.044) were independent prognostic indicator of poor RFS. In the test set, postoperative platelet > 300/L (HR = 2.816, 95%CI:1.043–7.603, P = 0.041), CK19 (HR = 1.586, 95%CI:1.016–2.475, P = 0.042), satellite nodule (HR = 1.706, 95%CI:1.067–2.728, P = 0.026), microvascular invasion (HR = 1.611, 95%CI:1.034–2.510, P = 0.035), and tumor without capsule (HR = 1.870, 95%CI:1.120–3.120, P = 0.017) were independent prognostic indicators of poor RFS. The C-index for the nomogram was 0.698 (95%CI: 0.654–0.742) and the C-index for the test set was 0.670 (95%CI: 0.616–0.724). Both internal and external verification showed good results in identification and calibration.
Conclusion
CK19 plays a key role in tumor malignancy through overexpression and the expression of CK19 is an independent adverse factor affecting recurrence; therefore, CK19 can be used as a potential biomarker to predict adverse prognosis after surgery and adjuvant therapy in HCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver cancer is one of the prevalent types of cancer and is considered a worldwide health problem. Types of primary liver cancer have been defined by the World Health Organization (WHO) as hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and combined hepatocellular and bile duct carcinoma (CHCC-CC).1 HCC is one of the most common malignancies worldwide; it accounts for around 90% 2of liver cancers and has a high mortality rate.3 Hepatitis B virus (HBV)4 infection accounts for approximately 50% of HCC developments, and is the most significant risk factor. At present, early-stage HCC can be treated by surgical resection, local ablation, or transplantation.5,6 However, patients with HCC have a poor postoperative prognosis and a higher frequency of recurrence or metastasis.7 As such, the incidence and mortality rates of HCC continue to increase. It is believed that a number of independent risk factors are closely related to the recurrence and survival of patients with HCC, including tumor size, number of nodules, histological grade, and vascular invasion.8 Therefore, it is important to search for potential biomarkers associated with poor prognosis after surgery and adjuvant therapy.
Cytokeratin (CK) is mainly found in epithelial cells, and its expression patterns in different subtypes help to distinguish between primary and metastatic cancers in different organs.9,10 Studies11–12 have shown that CKs are associated with the recurrence and metastasis of certain types of cancers and tend to increase along the malignancy of the disease.
CK19 is a class of cytokeratin that has a molecular weight of between 40 and 56kD and is usually expressed in ductal epithelium (bile ducts, pancreatic and renal collecting tubules) and gastrointestinal mucosa.13,14 It has been reported that abnormal expressions of CK19 are an important factor when determining the prognosis of a number of cancers, including cutaneous squamous cell carcinoma15, lung cancer16, and oral squamous cell carcinoma [11]. CK19 is a marker for bile duct cells, liver progenitor cells (HPCs), and early hepatoblasts. Additionally, its expression is linked to the poor prognosis of patients diagnosed with HCC.17 CK19 is well correlated with tumor aggressiveness and is an important marker of proliferative subtypes, in which HBV-associated HCC6,18 is usually clustered, suggesting a poor prognosis in patients with HCC.
Studies1920,21 have shown that the overall survival (OS) rate in CK19 negative is greater than CK19 positive patients with HCC. However, so far, no research has been able to show, in a large cohort of patients, that there is an association between CK19 and recurrence-free survival (RFS) in HBV-positive HCC patients. Based on this, we hypothesized that the CK19 expression might be associated with RFS in HCC patients. We have determined to test whether CK19 is a prognostic factor for the recurrence of HCC after hepatectomy in a large sampled cohort and examined the correlation between CK19 expression and prognosis recurrence in patients with HBV-positive HCC. The study explored the prognostic value of CK19 and analyzed the correlation between HCC recurrence and CK19. Our results suggest that CK19 is an important prognostic marker for predicting HCC recurrence and is an independent factor associated with poor prognosis. In fact, CK19 does not only identify malignant characteristics of tumors; it can also be a promising target in the treatment of patients with liver cancer. In addition, we have established a new postoperative nomogram HCC recurrence score to predict 2-year RFS after radical resection of HCC patients.
Materials and Methods
Study Design
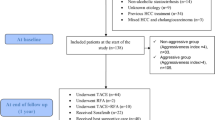
This study was carried out in two institutions. This protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Any personal identity, privacy, and other related information of patients in the study will not be shared with any to commercial interest groups. From January 2010 and May 2020, a total of 674 patients who underwent radical HCC resection and pathologically diagnosed with HCC at The 940th Hospital of Joint Logistics Support force of Chinese People’s Liberation Army and The First Affiliated Hospital of Medical School of Zhejiang University were enrolled in the study. The data from this population of 674 patients were used in this study. HCC is diagnosed using a combination of tumor biopsies, serum tests for tumor markers, and imaging procedures such as ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI). All patients were discharged from hospital after surgery and the last date for follow-up was December 2020. Figure 1 shows the process by which patients were selected for inclusion in the study.
Patients exhibiting the following criteria were included within this study:
(1) hepatitis B antigen positive; (2) diagnosis of A or B, based on the Child–Pugh staging system (score ≤ seven); (3) performance of preoperative abdominal MRI or CT contrast-enhanced scan; (4) confirmation of HCC in postoperative pathology.
The exclusion criteria were as follows:
(1) hepatitis C antigen positive; (2) performance of preoperative antitumor therapy (transcatheter arterial chemoembolization (TACE), radiotherapy, chemotherapy, etc.); (3) history of malignant tumors other than HCC; (4) incomplete clinical data.
Methods
The following methodology was used in this study: the patient’s gender, age, and date of operation were recorded. Potassium levels of patients were recorded 1–3 days before surgery; factors including national nosocomial infections surveillance (NNIS), maximum tumor size, tumor number, tumor boundary, presence of microvascular invasion, and satellite nodules were recorded during surgery. Immunohistochemical tests to detect CK19 expression were performed on the tumors after surgery. Platelet levels were counted 3–5 days after surgery. The positive expression of CK19 is defined as the presence of any membrane, cytoplasm, or membrane-cytoplasm combination in ≥ 10% of tumor cells. A 2-year RFS model for HCC was established in the Hangzhou cohort (training set) and validated internally. Then, the external validation of the model was carried out on the Lanzhou cohort (test set). Early recurrence was defined as any recurrence within 24 months after the initial hepatectomy. Recurrence was diagnosed using imaging techniques including typical CT and/or MRI scans, with or without elevated serum alpha fetoprotein levels. The data on the 2-year RFS covered the time ranges between each patient’s liver resection to their death or recurrence or the last follow-up. The median follow-up time was 45.5 months (range: 1–103 months). The model’s proportional hazards assumption was tested by examining each variable’s plots of scaled Schoenfeld residuals over time.
Statistical Analysis
Statistical analysis was performed with SPSS 25.0 (IBM SPSS). Categorical variables were compared using either the Chi-square test or Fisher’s exact test. To test the differences of non-classified variables between subgroups, the t test for normally distributed continuous variables and the Wilcoxon rank sum test for non-normally distributed variables were used. The RFS period was defined as the period from the date of surgery to the date of recurrence or the last follow-up without recurrence. In terms of survival analysis, univariate and multi-variable survival analyses were performed using Cox regression models to estimate the hazard ratios (HR) and 95% confidence intervals (95%CI), and draw survival curves. Based on the results of multi-variable analyses of Cox regression, the nomogram of 2-year RFS was plotted using the R Studio software. Bilateral tests were used to evaluate the difference with P value (P) < 0.05 considered significant.
Results
Clinical Features
To evaluate the clinical significance of CK19 expression in HCC, patients in the Hangzhou dataset (training set) and Lanzhou dataset (test set) were divided into CK19 positive and CK19 negative groups. The median quartile age (IQR) of all patients was 56 years (range: 48–64). Among 674 HBV-positive HCC patients, 223 patients (33.1%) were CK19 positive and 451 patients (66.9%) were CK19 negative. The follow-up time from surgery to recurrence or study endpoint ranged between 1 and 103 months (median: 45.5 months) and there was recurrence in 221 cases. The relationship between CK19 expression and clinicopathological features is shown in Table 1. In the training set, the 2-year RFS rate was significantly higher in patients with CK19 negative than in patients with CK19 positive (P = 0.001). Correlation analysis showed that there was significant correlation between CK19 expression and preoperative potassium (P = 0.030), satellite nodules (P < 0.001), and microvascular invasion (P = 0.020). However, this analysis also showed that other parameters such as gender, NNIS, postoperative platelet level, maximum tumor size, tumor number, and tumor boundary were not significantly correlated with CK19 expression (P > 0.05). In the test set, the 2-year RFS rate was also significantly higher in patients who had CK19 negative HCC than in patients with CK19 positive HCC (P = 0.001). CK19 expression was also significantly correlated with postoperative platelet (P = 0.038), satellite nodules (P = 0.003), microvascular invasion (P = 0.011), and maximum tumor size (P = 0.039). Other parameters including gender, NNIS, preoperative potassium level, tumor number, and tumor boundary were not significantly associated with CK19 expression (P > 0.05).
Prognostic Significance of CK19
Ten potential prognostic factors (gender, postoperative platelet, preoperative potassium, CK19, NNIS, satellite nodule, microvascular invasion, maximum tumor size, tumor number, tumor boundary) were analyzed for the risk of 2-year RFS (Table 2). In the Cox regression model, the follow-up time was used as a time variable. According to Schoenfeld’s assessment of the proportional residual on the time function, we did not find any significant violation of the proportional hazard assumption.
Using univariate Cox regression analyses of the training set, the following were adverse factors for predicting RFS (Fig. 2): postoperative platelets greater than 300/L (HR = 2.835, 95%CI:1.302–6.173, P = 0.009), CK19 (HR = 1.706, 95%CI:1.229–2.367, P = 0.001); satellite nodules (HR = 1.896, 95%CI:1.331–2.701, P < 0.001); microvascular invasion (HR = 3.145, 95%CI:2.267–4.363, P < 0.001); maximum tumor size (HR = 1.446, 95%CI:1.041–2.008, P = 0.028); and incomplete tumor capsule (HR = 1.786, 95%CI:1.177–2.709, P = 0.006) Table 3 and 4. In addition, in the test set, postoperative platelets greater than 300/L (HR = 3.359, 95%CI:1.272–8.870, P = 0.014), CK19 (HR = 1.892, 95%CI:1.250–2.863, P = 0.003), satellite nodules (HR = 1.998, 95%CI:1.275–3.129, P = 0.003), microvascular infiltration (HR = 2.060, 95%CI:1.352–3.141, P = 0.001), maximum tumor size (HR = 1.211, 95%CI:0.801–1.831, P = 0.028), and tumor without capsule (HR = 1.878, 95%CI:1.141–3.091, P = 0.013) were adverse factors for predicting 2-year RFS.
Multi-variable analyses showed that in the training set, postoperative platelet greater than 300/L (HR = 2.753, 95%CI:1.234–6.142, P = 0.013), CK19 (HR = 1.410, 95%CI:1.006–1.976, P = 0.046), Satellite nodule (HR = 1.476, 95%CI:1.026–2.120, P = 0.036), microvascular invasion (HR = 2.927, 95%CI:2.006–4.146, P < 0.001), incomplete tumor capsule (HR = 1.539, 95%CI:1.012–2.341, P = 0.044) were independent prognostic indicators of poor RFS. In the test set, postoperative platelet greater than 300/L (HR = 2.816, 95%CI:1.043–7.603, P = 0.041), CK19 (HR = 1.586, 95%CI:1.016–2.475, P = 0.042), satellite nodule (HR = 1.706, 95%CI:1.067–2.728, P = 0.026), microvascular invasion (HR = 1.611, 95%CI:1.034–2.510, P = 0.035), and tumor without capsule (HR = 1.870, 95%CI:1.120–3.120, P = 0.017) were independent prognostic indicators of poor RFS.
Prognostic Nomogram
The results of multi-variable analyses of the training set lead to utilization of the following clinical variables in the construction of a prognostic nomogram for 2-year RFS: CK19 expression, postoperative platelets, satellite nodules, microvascular invasion, and tumor boundary (Fig. 3). The C-index for the nomogram in Fig. 3 was 0.698 (95%CI: 0.654–0.742, P < 0.05). By totaling the scores of each nomogram variable, it is possible to gain a more accurate hierarchical prediction of each patient outcome. The higher the total score, the higher the 2-year RFS survival. For a patient with an overall score of 60, the probability of 2-year RFS survival rate was approximately 45%. The calibration curve of the prediction model was plotted to determine the calibration accuracy of the 2-year RFS estimated by the final Cox model (Fig. 4). The test set was also used to externally verify the results. The C-index for the test set was 0.670 (95%CI: 0.616–0.724, P < 0.05) and the calibration curve is shown in Fig. 5. The calibration curves of internal and external validation showed no significant deviation from the perfect fit and there was good correlation between the predicted and the observed results.
Nomogram of 2-year RFS in HCC. Important clinical variables were identified by Cox multi-variable analyses. These include CK19 expression, postoperative platelet, satellite nodule, microvascular invasion, and tumor boundary. To use the nomogram, find the first variable and draw a line along the point axis to determine the score for that variable. Repeat the process for the other four variables and sum up the scores for all the variables. The sum of these numbers is placed on the total point axis and a downward survival axis is plotted to determine the likelihood of 2-year RFS
Internal validation of calibration curves: observation and prediction of 2-year recurrence-free survival plotted, respectively, on the Y-axis and X-axis (per the 2-year RFS Nomogram), with vertical arrows indicating 95% confidence intervals for observed 2-year recurrence-free survival. C-index was 0.698 (95%CI: 0.654–0.742, P < 0.05)
External validation of the calibration curves: observation and prediction of 2-year recurrence-free survival plotted, respectively, on the Y-axis and X-axis (per the 2-year RFS Nomogram), with vertical arrows indicating 95% confidence intervals for observed 2-year RFS. C-index was 0.670 (95%CI: 0.616–0.724, P < 0.05)
Among the 674 patients in this study, 221 patients (32.8%) with HCC had tumor recurrence within 2 years after surgery. Of the 90 patients with recurrence of CK19 positive HCC within 2 years after surgery, 65 patients experienced recurrence only in their liver, 13 patients had metastasis to other liver sites, and 28 patients had extrahepatic recurrence. The recurrence sites of 28 patients with extrahepatic recurrence included the abdomen, lung, brain, lymph, bones, portal vein, adrenal grand, and kidney. Of the 131 patients with recurrence of CK19 negative HCC, 90 had recurrence only in the liver, 17 had metastases elsewhere in the liver, and 44 had extrahepatic recurrence. Therefore, the results of our study show that the expression of CK19 was significantly associated with intrahepatic recurrence (P = 0.005).
Discussion
As diagnostic methods, preoperative and postoperative treatment and surgical techniques develop, more and more patients with HCC choose hepatectomy. However, patients with HCC face a high recurrence rate, even after radical resection.22,23 It is very important to be able to make an accurate prognosis of recurrence or metastasis in patients in order to take timely intervention measures.
CKs compose a polygenic family of keratin proteins that vary in molecular weight and chemical structure; CK1 to CK8 have a high molecular weight and are basic or neutral type II, and CK9 to CK20 have a low molecular weight, and are acidic type I.13 Many CKs are used as biomarkers24 for pathological diagnosis and prediction of prognoses due to their unique expression patterns in tumors. Some CKs have been used as prognostic predictors16,25 for some epithelial tumors. For example, CK17 is a useful marker for differentiating pancreaticobiliary adenocarcinoma from extrabiliary nonmucinous pancreaticobiliary adenocarcinoma.26 Immunohistochemical (IHC) staining of cytokeratin (CK)5/6, CD44, and CK20 is strongly associated with prognosis of urothelial carcinoma of the bladder27, while CK19 is well associated with tumor invasiveness compared with other “stemness” related markers.28,29
Most recent studies have focused on the prognostic or diagnostic significance of CK19 in liver cancer. Such studies have found that CK19 is associated with vascular invasion of HCC, and that the overexpression of CK19 in HCC cells is associated with metastasis.30 The CK19 level in serum can reflect partial pathological progression of HCC, and as such can be used as a useful indicator for predicting tumor metastasis, and act as a therapeutic target for patients with HCC metastasis. Before this study, there has been no research into the specific role of CK19 expression in HBV positive HCC using a large volume of data.
In this study, we found that there was a positive expression of CK19 in instances where there was a recurrence of HCC. This expression was also significantly associated with poor prognosis and malignant characteristics of the tumor. The results of multi-variable analyses show that CK19 is a useful independent prognostic factor for patients with HCC. The risk factors for the recurrence of HCC after surgical operations are related to tumor, host, and operative factors. Our study suggests tumor invasive pathological factors, including postoperative platelets, CK19 expression, satellite nodule, microvascular invasion, and tumor boundary, are risk factors for recurrence. In addition, when analyzing the expression of CK19 and the site of recurrence in HCC patients, we found that the intrahepatic recurrence rate was higher.
To further prove the significance of prognosis, based on multi-variable cox analyses to determine the important related clinical variables (including CK19 expression, postoperative platelets, satellite nodule, microvascular invasion, and tumor boundary), we set up a 2-year RFS nomogram in which the combined score of each variable created an overall score that could accurately predict the patient outcome. The higher the total score meant the greater the likelihood that the patient would have 2-year RFS survival. The performance of the nomogram was assessed using internal verification of the C-index and plotting a calibration curve of the training set, and by external verification of the C-index and plotting a calibration curve of the test set.
In conclusion, CK19 positive HCC patients have a higher incidence of early postoperative recurrence even after radical resection, which may be related to the tumor’s malignant characteristics—progression and spread. Our study clearly demonstrates the prognostic value of CK19 expression in patients with HBV positive HCC, although a larger sample is needed to validate these findings. Our results have provided the evidence that CK19 is an important molecular marker which could be used in aiding the prevention of postoperative recurrence of HCC in patients. Therefore, we recommend the use of CK19 immunohistochemistry test to detect CK19 in patients undergoing surgical resection for HCC. In addition to CK19 expression, our nomogram incorporates multiple clinicopathological factors (including postoperative platelets, satellite nodules, microvascular invasion, and tumor boundary), resulting in a significantly improved accuracy of prognosis. Not only can HCC patients benefit from improved clinical management, but the ability to generate personalized predictions can be used to identify and stratify HCC patients in clinical trials. This in turn can provide clinicians with better prognosis on which they can make improved decisions about the patient’s subsequent treatment. In addition, we acknowledge that the prognosis of any cancer is a dynamic phenomenon as patient outcomes may change based on treatment response, so the nomogram can also be used to improve survival estimates and guide decisions at important turning points in the continuum of care for patients with HCC.
This study has obvious caveats. The main one is that the sample size of HCC metastases is small, which may skew the correlation in our statistical analysis. Second, this is a retrospective study conducted using data collected by only two institutions. We realize that a standardized multicenter collaborative study is needed to highlight the effect of CK19 expression on prognosis and recurrence in HBV-positive HCC patients, which would then generate the strong and reliable medical evidence needed to establish and endorse a uniform set of criteria.
Conclusion
In summary, the expression of CK19 is an independent adverse prognostic factor affecting 2-year RFS in HCC patients and CK19 can be used as a potential biomarker to predict adverse prognosis after surgery and adjuvant therapy. In addition, when analyzing the expression of CK19 and the site of recurrence in liver cancer patients, we found that the intrahepatic recurrence rate was higher.
References
Nagtegaal, I.D., R.D. Odze, D. Klimstra, et al., The 2019 WHO classification of tumours of the digestive system[J]. Histopathology, 2020. 76(2): p. 182-188.
Llovet, J.M., R.K. Kelley, A. Villanueva, et al., Hepatocellular carcinoma[J]. Nat Rev Dis Primers, 2021. 7(1): p. 6.
Yang, J.D., P. Hainaut, G.J. Gores, et al., A global view of hepatocellular carcinoma: trends, risk, prevention and management[J]. Nat Rev Gastroenterol Hepatol, 2019. 16(10): p. 589-604.
T. Akinyemiju, S. Abera, M. Ahmed,et al., The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015[J]. JAMA Oncol, 2017. 3(12): pp. 1683–1691.
Yang, J.D. and J.K. Heimbach, New advances in the diagnosis and management of hepatocellular carcinoma[J]. BMJ, 2020. 371: p. m3544.
Llovet, J.M., A. Villanueva, A. Lachenmayer, et al., Advances in targeted therapies for hepatocellular carcinoma in the genomic era[J]. Nat Rev Clin Oncol, 2015. 12(7): p. 408–24.
Merle, P., The New Immuno-Oncology-Based Therapies and Their Perspectives in Hepatocellular Carcinoma[J]. Cancers, 2021. 13(2): p. 238.
Ang, S.F., E.S. Ng, H. Li, et al., The Singapore Liver Cancer Recurrence (SLICER) Score for relapse prediction in patients with surgically resected hepatocellular carcinoma[J]. PLoS One, 2015. 10(4): p. e0118658.
Kim, M.A., H.S. Lee, H.K. Yang, et al., Cytokeratin expression profile in gastric carcinomas[J]. Hum Pathol, 2004. 35(5): p. 576–81.
Apaydin, R., Y. Gurbuz, D. Bayramgurler, et al., Cytokeratin contents of basal cell carcinoma, epidermis overlying tumour, and associated stromal amyloidosis: an immunohistochemical study[J]. Amyloid, 2005. 12(1): p. 41-7.
R. Shaw., A. Christensen., K. Java., et al., Intraoperative sentinel lymph node evaluation: implications of cytokeratin 19 expression for the adoption of OSNA in oral squamous cell carcinoma[J]. Ann Surg Oncol, 2016. 23(12):p.4043–4048.
Xu, E.S., M.H. Yang, C.Y. Liu, et al., Decreasing cytokeratin 17 expression in head and neck cancer predicts nodal metastasis and poor prognosis: The first evidence[J]. Clin Otolaryngol, 2018. 43(4):p.1010–1018.
Mehrpouya, M., Z. Pourhashem, N. Yardehnavi, et al., Evaluation of cytokeratin 19 as a prognostic tumoral and metastatic marker with focus on improved detection methods[J]. J Cell Physiol, 2019. 234(12): p. 21425–21435.
Jain., R., S. Fische., S. Serra., et al., The Use of Cytokeratin 19 (CK19) Immunohistochemistry in Lesions of the Pancreas, Gastrointestinal Tract, and Liver[J]. Appl Immunohistochem Mol Morphol 2010. 18: p. 9-15.
Kato, J., T. Hida, S. Sugita, et al., Cytokeratin 19 expression is a risk factor for metastasis in cutaneous squamous cell carcinoma[J]. J Eur Acad Dermatol Venereol, 2018. 32(7): p. e299-e301.
Oezkan, F., A.M. Khan, T. Hager, et al., OSNA: A Fast Molecular Test Based on CK19 mRNA Concentration for Assessment of EBUS-TBNA Samples in Lung Cancer Patients[J]. Clin Lung Cancer, 2016. 17(3): p. 198-204.
Govaere, O., M. Komuta, J. Berkers, et al., Keratin 19: a key role player in the invasion of human hepatocellular carcinomas[J]. Gut, 2014. 63(4): p. 674-85.
Zhuo, J.Y., D. Lu, W.Y. Tan, et al., CK19-positive Hepatocellular Carcinoma is a Characteristic Subtype[J]. J Cancer, 2020. 11(17): p. 5069-5077.
Lee, J.I., J.W. Lee, J.M. Kim, et al., Prognosis of hepatocellular carcinoma expressing cytokeratin 19: comparison with other liver cancers[J]. World J Gastroenterol, 2012. 18(34): p. 4751-7.
Yang, X.R., Y. Xu, G.M. Shi, et al., Cytokeratin 10 and cytokeratin 19: predictive markers for poor prognosis in hepatocellular carcinoma patients after curative resection[J]. Clin Cancer Res, 2008. 14(12): p. 3850-9.
Uenishi., T., S. Kubo., Tanaka., et al., Cytokeratin 19 expression in hepatocellular carcinoma predicts early postoperative recurrence[J]. Cancer Sci 2003. 94(10): p. 851-857.
Yang, Y., H. Nagano, H. Ota, et al., Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection[J]. Surgery, 2007. 141(2): p. 196-202.
Ochiai, T., H. Ikoma, K. Okamoto, et al., Clinicopathologic features and risk factors for extrahepatic recurrences of hepatocellular carcinoma after curative resection[J]. World J Surg, 2012. 36(1): p. 136-43.
Schweizer, J., P.E. Bowden, P.A. Coulombe, et al., New consensus nomenclature for mammalian keratins[J]. J Cell Biol, 2006. 174(2): p. 169-74.
Kaliszewski., K., M. Rzeszutko., B. Wojtczak., et al., Expression of cytokeratin-19 (CK19) in the classical subtype of papillary thyroid carcinoma: the experience of one center in the Silesian region[J]. Folia Histochem Cytobiol, 2016. 54(4): p. 193-201.
Chu., P.G., R.E. Schwarz., S.K. Lau., et al., Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of vater adenocarcinoma application of CDX2, CK17, MUC1, and MUC2[J]. Am J Surg Pathol, 2005. 29(3): p. 359–367.
Jung, M., B. Kim, and K.C. Moon, Immunohistochemistry of cytokeratin (CK) 5/6, CD44 and CK20 as prognostic biomarkers of non-muscle-invasive papillary upper tract urothelial carcinoma[J]. Histopathology, 2019. 74(3): p. 483-493.
MJ., H., S. EA., C. YW., et al., Role of intermediate filaments in migration, invasion and metastasis[J]. Cancer and Metastasis Reviews, 1996. 15: p. 507-525.
Kim, H., G.H. Choi, D.C. Na, et al., Human hepatocellular carcinomas with "Stemness"-related marker expression: keratin 19 expression and a poor prognosis[J]. Hepatology, 2011. 54(5): p. 1707-17.
Ding, S.J., Y. Li, Y.X. Tan, et al., From proteomic analysis to clinical significance: overexpression of cytokeratin 19 correlates with hepatocellular carcinoma metastasis[J]. Mol Cell Proteomics, 2004. 3(1): p. 73-81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, with the 1964 Helsinki Declaration and its later amendments, or comparable ethical standards. This article does not include any animal research conducted by the authors.
Informed Consent
Informed consent was obtained from all the individual participants that were included in the study.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei Shuyao and Bao Mingyang contributed to the work equally and should be regarded as co-first authors.
Rights and permissions
About this article
Cite this article
Shuyao, W., Mingyang, B., Feifei, M. et al. CK19 Predicts Recurrence and Prognosis of HBV Positive HCC. J Gastrointest Surg 26, 341–351 (2022). https://doi.org/10.1007/s11605-021-05107-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-021-05107-w