Abstract
Background
Head elevation is recommended as a tier zero measure to decrease high intracranial pressure (ICP) in neurocritical patients. However, its quantitative effects on cerebral perfusion pressure (CPP), jugular bulb oxygen saturation (SjvO2), brain tissue partial pressure of oxygen (PbtO2), and arteriovenous difference of oxygen (AVDO2) are uncertain. Our objective was to evaluate the effects of head elevation on ICP, CPP, SjvO2, PbtO2, and AVDO2 among patients with acute brain injury.
Methods
We conducted a systematic review and meta-analysis on PubMed, Scopus, and Cochrane Library of studies comparing the effects of different degrees of head elevation on ICP, CPP, SjvO2, PbtO2, and AVDO2.
Results
A total of 25 articles were included in the systematic review. Of these, 16 provided quantitative data regarding outcomes of interest and underwent meta-analyses. The mean ICP of patients with acute brain injury was lower in group with 30° of head elevation than in the supine position group (mean difference [MD] − 5.58 mm Hg; 95% confidence interval [CI] − 6.74 to − 4.41 mm Hg; p < 0.00001). The only comparison in which a greater degree of head elevation did not significantly reduce the ICP was 45° vs. 30°. The mean CPP remained similar between 30° of head elevation and supine position (MD − 2.48 mm Hg; 95% CI − 5.69 to 0.73 mm Hg; p = 0.13). Similar findings were observed in all other comparisons. The mean SjvO2 was similar between the 30° of head elevation and supine position groups (MD 0.32%; 95% CI − 1.67% to 2.32%; p = 0.75), as was the mean PbtO2 (MD − 1.50 mm Hg; 95% CI − 4.62 to 1.62 mm Hg; p = 0.36), and the mean AVDO2 (MD 0.06 µmol/L; 95% CI − 0.20 to 0.32 µmol/L; p = 0.65).The mean ICP of patients with traumatic brain injury was also lower with 30° of head elevation when compared to the supine position. There was no difference in the mean values of mean arterial pressure, CPP, SjvO2, and PbtO2 between these groups.
Conclusions
Increasing degrees of head elevation were associated, in general, with a lower ICP, whereas CPP and brain oxygenation parameters remained unchanged. The severe traumatic brain injury subanalysis found similar results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Historically, studies have focused on intracranial pressure (ICP) and cerebral perfusion pressure (CPP) as targets in the management of patients with acute brain injury. In general, the treatment thresholds in the setting of intracranial hypertension are mainly derived from traumatic brain injury (TBI) guidelines because targets for nontraumatic etiologies were not adequately studied [1,2,3]. The fourth edition of Guidelines for the Management of Severe TBI [1], published by the Brain Trauma Foundation, recommends treating an ICP > 22 mm Hg and targeting a CPP between 60 and 70 mm Hg, values that are associated with favorable outcomes [4].
By considering only ICP and CPP, important data regarding the physiologic and metabolic state of the brain are overlooked, and significant parenchymal hypoxia may occur even when ICP and CPP are normal [5, 6]. Data regarding cerebral oxygenation can be mainly assessed by jugular bulb oxygen saturation (SjvO2) or by brain tissue partial pressure of oxygen (PbtO2). Moreover, the arteriovenous difference of oxygen (AVDO2) can also be determined by calculating the difference between the arterial oxygen saturation and SjvO2 [7]. The last severe TBI guidelines [1] recommend that the use of SjvO2 or AVDO2 as a source of information for management decisions may be considered to reduce mortality and improve outcomes at 3 and 6 months post injury [1, 8,9,10]. This guideline provides no recommendations regarding the PbtO2 for such purposes, although there is increasing interest in this parameter and ongoing phase III clinical trials evaluating whether its use is associated with better functional outcomes [11,12,13].
A variety of measures may be adopted to reduce ICP of patients with acute brain injury, including pharmacological and nonpharmacological interventions as well as emergent surgery [3]. Head elevation is generally recommended as a tier zero measure [3, 14, 15] in this setting and was demonstrated as an effective measure to reduce ICP in a previous meta-analysis [16]. However, by simultaneously decreasing mean arterial pressure (MAP), head elevation may theoretically reduce CPP and/or cerebral oxygenation [17]. The repercussions of head elevation on these parameters on CPP, as well as on cerebral oxygenation, are uncertain. In fact, we are unaware of meta-analyses addressing such parameters. Therefore, we aim to analyze the effects of different degrees of head elevation on ICP, CPP, SjvO2, PbtO2, and AVDO2 among patients with acute brain injury through a systematic review and meta-analysis.
Methods
This systematic review and meta-analysis was performed in line with recommendations from the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guidelines. The protocol was registered and made publicly available on the PROSPERO database (CRD42023391072) on January 22, 2023. This article complies with ethical standards, and institutional review board approval was not required.
Search Strategy and Selection Process
We systematically searched for studies on PubMed, Scopus, and Cochrane Library from inception to January 17, 2023. The exact search string is presented in Supplementary Table 1. Two independent reviewers analyzed all titles and abstracts for eligibility criteria. Articles were included if they assessed the effect of head elevation on any of the main outcomes in the setting of acute brain injury, defined as the life threatening acute neurological condition requiring the use of an invasive ICP measurement device. The main outcomes were ICP (direct measurements), CPP, SjvO2, PbtO2, and AVDO2. Articles were excluded (1) if they were editorials, letters, book chapters, brief reports, or protocols and (2) if they were not available in the English language. When necessary, the full articles were also analyzed. Discrepancies were resolved by consensus between the reviewers.
Risk of Bias and Publication Bias Assessment
We used the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool for risk of bias assessment. The risk of bias was evaluated by two independent reviewers. Discrepancies were resolved by consensus between the reviewers. Publication bias was assessed through funnel plots.
Data Retrieval
The following main outcomes were collected and analyzed from each report: (1) ICP, (2) CPP, (3) SjvO2, (4) PbtO2, and (5) AVDO2. Other data were also retrieved: (1) number of patients, (2) invasive ICP monitoring type, (3) age distribution, (4) degree of head elevation, (5) type of brain injury, (6) mean invasive MAP value before and after intervention, (7) site of insertion of MAP catheter (e.g., radial artery or femoral artery), (8) level of MAP transducer (e.g., foramen of Monro or right atrium), (9) timing of intervention, and (10) timing of measurement of main outcomes after head positioning. Patients who underwent the intervention served as their own controls, with different degrees of elevation. When studies reported multiple timings of outcome measurements, we considered the first measurement. When studies reported more than one MAP transducer level, we considered the one measured at the level of Monro foramen.
Statistical Analysis
We used Cochrane’s Review Manager version 5.4 (Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) for statistical analysis. Weighted mean differences (MDs) were used to pool continuous outcomes that appeared in two or more studies. Heterogeneity was evaluated with the Cochran Q test and the I2 statistic. A p < 0.10 and an I2 statistic > 25% were considered as heterogeneous. Overall estimates of effect and 95% confidence intervals (CIs) were calculated using a random-effects model and inverse variance weighting. When outcomes were present only on charts and did not show the exact values, we used an online resource to predict the values (https://apps.automeris.io/wpd/). When articles reported median and interquartile range, we estimated means and standard deviations (SDs) according to the methodology described by Luo et al. [18] and Wan et al. [19].
Subgroup and Sensitivity Analyses
We performed a subanalysis of studies that included only patients with TBI. When both the Cochran Q test p value and the I2 statistic indicated heterogeneity, we performed sensitivity analyses. This consisted of (1) leaving individual studies out of the analysis (leave-one-out analysis) and (2) performing a meta-analysis of studies in which the baseline mean ICP (i.e., the ICP in the supine position) plus 1 SD reached the value of at least 22 mm Hg (higher ICP analysis).
Results
Study Selection, Baseline Characteristics, and Qualitative Analysis
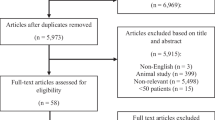
The initial search yielded 1,610 results (Fig. 1). After the removal of duplicates and applications of eligibility criteria, 25 articles were included in the systematic review. Of these, 16 provided quantitative data regarding outcomes of interest, allowing for meta-analysis (quantitative analysis) [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. Each outcome was analyzed in each comparison of 15° increments of head elevation when there were two or more included studies (Fig. 1). Baseline characteristics of the nine studies [36,37,38,39,40,41,42,43,44] included in the qualitative analysis are shown in Supplementary Table 2, and their main findings are presented in Supplementary Table 3. These studies lacked sufficient information to undergo a meta-analysis, such as those that underwent the quantitative analysis. The baseline characteristics of studies included in the quantitative analysis are shown in Table 1. All included studies were prospective cohort studies.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for the identification of studies evaluating the effects of head elevation on intracranial pressure (ICP), cerebral perfusion pressure (CPP), jugular bulb oxygen saturation (SjvO2), brain tissue partial pressure of oxygen (PbtO2), and arteriovenous difference of oxygen (AVDO2)
ICP, MAP, and CPP
The mean ICP of patients with acute brain injury was lower at 30° of head elevation than in the supine position (MD − 5.58 mm Hg; 95% CI − 6.74 to − 4.41 mm Hg; p < 0.00001; Fig. 2a). The only comparison in which a greater degree of head elevation did not significantly reduce the ICP was 45° vs. 30°. In all other comparisons, increments of ≥ 15° resulted in significantly lower ICP values (Supplementary Figs. 1–5). Increments of ≥ 15° also resulted in lower MAP values, except for the 45° vs. 30° comparison (Fig. 2b and Supplementary Figs. 1–5). The mean CPP remained similar between 30° of head elevation and the supine position (MD − 2.48 mm Hg; 95% CI − 5.69 to 0.73 mm Hg; p = 0.13; Fig. 2c). Similar findings were observed in all other comparisons (Supplementary Figs. 1–5).
Forest plots showing the mean differences between 30° of head elevation and supine positions in the setting of acute brain injury on intracranial pressure (ICP) (a), mean arterial pressure (MAP) (b), and cerebral perfusion pressure (CPP) (c). CI confidence interval, IV inverse variance, SD standard deviation
Brain Oxygenation
The mean SjvO2 was similar between the 30° of head elevation and supine position groups. There was no statistically significant difference between groups (MD 0.32%; 95% CI − 1.67% to 2.32%; p = 0.75; Fig. 3a). The mean PbtO2 was similar between the 30° of head elevation and supine position groups (MD − 1.50 mm Hg; 95% CI − 4.62 to 1.62 mm Hg; p = 0.36; Fig. 3b), as well as between the 30° and 15° of head elevation groups (MD − 0.99 mm Hg; 95% CI − 5.02 to 3.05 mm Hg; p = 0.63; Supplementary Fig. 3). The mean AVDO2 was also similar between the 30° of head elevation and supine position groups (MD 0.06 µmol/L; 95% CI − 0.20 to 0.32 µmol/L; p = 0.65; Fig. 3c).
Forest plots showing the mean differences between 30° of head elevation and supine positions in the setting of acute brain injury on jugular bulb oxygen saturation (SjvO2) (a), brain tissue partial pressure of oxygen (PbtO2) (b), and arteriovenous difference of oxygen (AVDO2) (c). CI confidence interval, IV inverse variance, SD standard deviation
Severe TBI Subanalysis
A total of five articles provided quantitative data regarding outcomes of interest among patients with severe TBI, allowing for meta-analysis. This subanalysis was only possible in the 30° of head elevation group because outcomes were not present in ≥ 2 studies for other comparisons. The mean ICP of patients with TBI was lower with 30° of head elevation when compared with the supine position (MD − 4.78 mm Hg; 95% CI − 6.21 to − 3.36 mm Hg; p < 0.00001; Fig. 4a). There was no difference in the mean values of MAP, CPP, SjvO2, and PbtO2 between these groups (Fig. 4b–e). Other studies that included patients with TBI as part of their sample did not provide data particularly for this condition.
Forest plots showing the mean differences between 30° of head elevation and supine positions in the subanalysis of severe traumatic brain injury (TBI) on intracranial pressure (ICP) (a), mean arterial pressure (MAP) (b), cerebral perfusion pressure (CPP) (c), jugular bulb oxygen saturation (SjvO2) (d), brain tissue partial pressure of oxygen (PbtO2) (e), and arteriovenous difference of oxygen (AVDO2) (f). CI confidence interval, IV inverse variance, SD standard deviation
Risk of Bias and Publication Bias
The overall risk of bias was low in 24% (n = 6 of 25), moderate in 48% (n = 12 of 25), serious in 28% (n = 7 of 25), and critical in zero studies. The analysis of each study is presented in Supplementary Table 4. Funnel plots for each publication bias analysis are shown in Supplementary Figs. 6–11.
Heterogeneity
For the main outcomes, there was high heterogeneity (demonstrated by both the Cochran Q test p value and the I2 statistic) in the analysis of ICP and CPP between 30° of head elevation and the supine position (Fig. 2a, c, respectively). In the TBI subanalysis, there was also high heterogeneity in the analysis of CPP between 30° of head elevation and the supine position (Fig. 4c).
Sensitivity Analysis
Leave-one-out Analysis
When removing the study by Schwarz et al. [34] from the ICP analysis between 30° and the supine position, the I2 statistic dropped to 0% and the Cochran Q test p value increased to 0.56, meaning low heterogeneity. The removal of the study by Moraine et al. [29] also reduced in a lesser degree the heterogeneity, with an I2 statistic of 17% and a Cochran Q test p value of 0.27. In the CPP analysis between 30° and the supine position, the study by Schwarz et al. [34] was the only study that, when removed, reduced the heterogeneity significantly, with an I2 statistic of 27% and a Cochran Q test p value of 0.18. In the CPP analysis of the TBI subanalysis between 30° and the supine position, the removal of the study by Dagod et al. [23] significantly reduced the heterogeneity, with an I2 statistic of 0% and a p value of 0.87.
Higher ICP Analysis
For this approach, we removed studies with a lower mean ICP from analyses with a high heterogeneity (the studies by Brimioulle et al. [22], Dagod et al. [23], and Schwarz et al. [34]). The heterogeneity of the ICP analysis between 30° and the supine position reduced substantially (the I2 statistic dropped to 0%, and the Cochran Q test p value increased to 0.49). The analysis of CPP between 30° and the supine position found similar results (the I2 statistic dropped to 0%, and the Cochran Q test p value increased to 0.71). In the severe TBI subanalysis, we removed the study by Dagod et al. [23], and the heterogeneity of the CPP analysis between 30° and the supine position decreased significantly (the I2 statistic dropped to 0%, and the Cochran Q test p value increased to 0.87).
Discussion
Main Findings
We conducted a systematic review and meta-analysis regarding the effect of head elevation on ICP, CPP, and brain oxygenation in the acute brain injury setting. Increasing degrees of head elevation was associated, in general, with a lower ICP, whereas CPP and brain oxygenation parameters remained unchanged. The severe TBI subanalysis found similar results.
ICP and CPP
Our results demonstrated that increasing degrees of head elevation decreases ICP in patients with acute brain injury (Fig. 2a and Supplementary Figs. 1–5). This fact was also demonstrated by the severe TBI subanalysis (Fig. 4a). The exception was the comparison between 45° and 30° of head elevation, in which no statistical difference was found in the MD between groups. The CPP remained unchanged in all analyses (Figs. 2c and 4c and Supplementary Figs. 1–5). The MAP values decreased or tended to decrease with head elevation.
Of note, absolute CPP measurements may be affected by some MAP monitoring details, such as site of catheter insertion and level of measurements, which are not consistent across studies and sometimes are not even reported (Supplementary Table 2 and Table 1). In fact, measurements through the radial artery may underestimate MAP when compared to measurements through the femoral artery [45]. However, the differences in CPP measurements according to different degrees of head elevation should not be affected, regardless of the site of insertion. In addition, an MAP transducer at the level of the Monro foramen (approximately at the level of the tragus) tends to generate lower values than an MAP transducer placed at the level of right atrium when the head is elevated. Therefore, when an MAP transducer is placed at the level of right atrium, CPP values may be overestimated during head elevation. For purposes of accurate CPP calculations, councils by the Neuroanaesthesia and Critical Care Society of Great Britain and Ireland and the Society of British Neurological Surgeons endorse positioning (leveling) the arterial transducer at the level of the middle cranial fossa, which can be approximated to the tragus of the ear [46]. Moreover, we included studies of patients with different conditions and, hence, with different pathophysiology. For instance, the study by Schwarz et al. [34] notably increased heterogeneity in the analysis of ICP and CPP between 30° of head elevation and the supine position by showing no effect on ICP and impairment on CPP (Fig. 2a, c). Interestingly, this was the only study that included exclusively patients with hemispheric ischemic stroke. In other articles, patients with ischemic stroke represented a small portion of the sample. In addition, the study by Schwarz et al. [34] was the one in which patients presented the lowest mean ICP in the supine position. Possibly, these factors were the most responsible for these discrepancies, and additional caution should be taken when extrapolating our results to the ischemic stroke population. Indeed, a prior meta-analysis [17] demonstrated that the middle cerebral artery mean flow velocity among patients with acute ischemic stroke increased significantly in the side affected but not in the unaffected side when they were positioned in a lying-flat head position at the supine position or at 15° of head elevation in comparison with 30° of head elevation.
In the severe TBI analysis between 30° of head elevation and the supine position, the study by Dagod et al. [23] increased the heterogeneity of the CPP results by showing a deleterious effect. Conversely, other severe TBI studies showed no significant effect of head elevation on CPP (Fig. 4c). We did not find a specific reason for these discrepancies because we did not detect patient characteristics, measurement methods, or interventional approaches that were exclusive to this specific study.
Brain Oxygenation
There are various types of brain oxygenation monitoring. The most used are the SjvO2 and the PbtO2. The SjvO2 can be used for the indirect measurement of oxygen supply to the brain as a whole and its consumption. It also allows for the calculation of the AVDO2, whose alterations may reflect changes in cerebral blood flow. SjvO2 and the AVDO2 monitoring can be considered to reduce mortality and improve outcomes at 3 and 6 months after severe TBI [1, 8,9,10].
The PbtO2 values reflect a regional oxygenation of the brain tissue, and there is increasing research interest in such a parameter. In fact, three phase III clinical trials are underway to study the benefits of PbtO2 monitoring in the setting of severe TBI: the BOOST-3 trial [12] (NCT03754114), the OXY-TC trial [11] (NCT02754063), and the BONANZA trial [13] (ACTRN12619001328167). In our study, we did not find a statistically significant difference of brain oxygenation parameters (SjvO2, PbtO2, and AVDO2) in all comparisons that we made across different degrees of head elevation (Fig. 3 and Supplementary Fig. 3d). The severe TBI analysis also showed no difference in SjvO2 and PbtO2 parameters (Fig. 4d, e) between 30° of head elevation and the supine position.
The Timing Factor
Although the timing of head elevation since acute brain injury or since patient admission may play an important role in the findings, many studies did not mention it or did not detail it adequately. Among studies that mentioned it, this timing varied substantially (Supplementary Table 2 and Table 1). It is not clear whether the outcomes of interest remain steady during the first days after injury [21, 23]. Also, the timing of parameter measurement after intervention varied widely across studies (Supplementary Table 2 and Table 1), which may also influence the results.
ICP Measurement Methods
The most common methods of ICP monitoring were intraparenchymal and intraventricular probes (Supplementary Table 2 and Table 1). The intraventricular measurement is considered the gold standard because of its accuracy [47, 48]. In addition, it also allows the simultaneous drainage of cerebrospinal fluid. Intraparenchymal probes tend to reflect a local cerebral pressure rather than the ventricular pressure. However, its placement is generally easier and faster, especially in patients with small ventricles or severe brain edema [47, 48]. The included studies did not provide comparisons of outcomes according to different types of ICP monitoring.
Strengths and Limitations
This study presents limitations. First, we analyzed patients with acute brain injuries due to pathologies with different pathophysiology altogether, although many included studies also used this approach. We performed a subanalysis of patients with severe TBI to minimize heterogeneity. Subanalyses of other conditions were not possible because of the low or inexistent number of articles analyzing only patients with specific pathologies. Second, we only assessed invasive methods of neuromonitoring and did not perform comparisons among them. Methods such as transcranial Doppler, optic nerve sheath diameter, near-infrared spectroscopy, pupillometry, and skull elasticity-based measurements were beyond the scope of this article. Third, we did not assess clinical outcomes, such as mortality or disability. However, measuring the effect of head elevation on values of brain monitoring is clinically relevant because it allows us to avoid values associated with increased mortality and/or disability, for instance. To the authors’ best knowledge, only one randomized trial (HeadPoST trial [49]) assessed the clinical effects of head elevation among neurocritically ill patients. This study found no difference on disability outcomes between patients with acute ischemic stroke assigned to a lying-flat position for 24 h and patients assigned to a sitting-up position with the head elevated to at least 30° for 24 h. Fourth, we included only English-language studies. This was probably the only exclusion criterion for some articles. Fifth, several aspects may influence our findings and were not quantitatively assessed, such as additional therapies (e.g., hyperosmolar therapy, temperature management, vasoactive drugs, ventilatory parameters, PaCO2, PaO2, sedation, decompressive craniectomy) as well as the timing of measurements and interventions. Decompressive craniectomy may heavily affect brain hemodynamics [50] and was only assessed by Burnol et al. [21] and Schwarz et al. [34], whose findings demonstrated no effect of this therapy on postural induced ICP changes. Other studies that included patients who underwent decompressive craniectomy did not perform analysis in this subgroup [24, 32, 34, 36, 38]. Sixth, only 7 of the 25 included studies described how the degrees of head elevation was obtained (by using a goniometer or a protractor). Other studies did not mention the method.
Recommendations for Future Studies
Future studies on head elevation in the setting of acute brain injury should include a more homogeneous sample. For instance, articles should include only patients with a specific condition (e.g., subarachnoid hemorrhage, TBI, or intracerebral hemorrhage) instead of analyzing them together. When more than one pathology is included, subanalyses of each condition or individual patient data reporting would be reasonable approaches. Even within a same pathology, however, important characteristics should be clearly described (e.g., isolated TBI and TBI with concomitant polytrauma) because they may potentially affect the analysis of outcomes. A clear and detailed methodology is essential. Information such as the site of MAP insertion, the level where the MAP transducer was placed, the type of ICP monitoring, the timing of parameter measurement since patient admission, and the timing of parameter measurement after head positioning is imperative.
Conclusions
Our results suggest that head elevation is an effective measure to reduce ICP, without significant effect on CPP and brain oxygenation parameters. We are unaware of previous meta-analyses addressing all these parameters. In the severe TBI subanalysis, we also found similar results. Regarding general clinical practice, head elevation also decreases the rates of ventilator-associated pneumonia [51]. However, studies analyzing the effects of head elevation on brain hemodynamics and oxygenation with other specific conditions (e.g., subarachnoid hemorrhage, intracerebral hemorrhage, and stroke) are scarce. Therefore, additional caution is important when performing head elevation in these scenarios, with the purpose of improving brain hemodynamics and oxygenation.
References
Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury. Fourth Edition Neurosurg. 2017;80(1):6–15. https://doi.org/10.1227/neu.0000000000001432.
Greenberg SM, Ziai WC, Cordonnier C, et al. Guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2022;53(7):e282–361.
Cadena R, Shoykhet M, Ratcliff JJ. Emergency neurological life support: intracranial hypertension and herniation. Neurocrit Care. 2017;27(Suppl 1):82–8. https://doi.org/10.1007/s12028-017-0454-z.
Sorrentino E, Diedler J, Kasprowicz M, et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care. 2012;16(2):258–66. https://doi.org/10.1007/s12028-011-9630-8.
Chang JJ, Youn TS, Benson D, et al. Physiologic and functional outcome correlates of brain tissue hypoxia in traumatic brain injury. Crit Care Med. 2009;37(1):283–90. https://doi.org/10.1097/CCM.0b013e318192fbd7.
Longhi L, Pagan F, Valeriani V, et al. Monitoring brain tissue oxygen tension in brain-injured patients reveals hypoxic episodes in normal-appearing and in peri-focal tissue. Intensive Care Med. 2007;33(12):2136–42. https://doi.org/10.1007/s00134-007-0845-2.
Zhong W, Ji Z, Sun C. A review of monitoring methods for cerebral blood oxygen saturation. Healthcare (Basel, Switzerland). 2021;9(9):52. https://doi.org/10.3390/healthcare9091104.
Robertson CS, Gopinath SP, Goodman JC, Contant CF, Valadka AB, Narayan RK. SjvO2 monitoring in head-injured patients. J Neurotrauma. 1995;12(5):891–6. https://doi.org/10.1089/neu.1995.12.891.
Stocchetti N, Canavesi K, Magnoni S, et al. Arterio-jugular difference of oxygen content and outcome after head injury. Anesth Analg. 2004;99(1):230–4. https://doi.org/10.1213/01.ane.0000130393.08355.d4.
Robertson C. Desaturation episodes after severe head injury: influence on outcome. Acta Neurochir Suppl (Wien). 1993;59:98–101. https://doi.org/10.1007/978-3-7091-9302-0_17.
Payen JF, Richard M, Francony G, et al. Comparison of strategies for monitoring and treating patients at the early phase of severe traumatic brain injury: the multicentre randomised controlled OXY-TC trial study protocol. BMJ Open. 2020;10(8):e040550. https://doi.org/10.1136/bmjopen-2020-040550.
Bernard F, Barsan W, Diaz-Arrastia R, Merck LH, Yeatts S, Shutter LA. Brain oxygen optimization in severe traumatic brain injury (BOOST-3): a multicentre, randomised, blinded-endpoint, comparative effectiveness study of brain tissue oxygen and intracranial pressure monitoring versus intracranial pressure alone. BMJ Open. 2022;12(3):e060188. https://doi.org/10.1136/bmjopen-2021-060188.
Actrn. The BONANZA trial- a randomised controlled trial that is testing whether a management strategy guided by early brain tissue oxygen monitoring in patients in with severe traumatic brain injury improves long term neurological and functional outcomes. Trial registry record; Clinical trial protocol. 2019
Hawryluk GWJ, Aguilera S, Buki A, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). 2019;45(12):1783–1794. https://doi.org/10.1007/s00134-019-05805-9
Chesnut R, Aguilera S, Buki A, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). 2020;46(5):919–929. https://doi.org/10.1007/s00134-019-05900-x
Jiang Y, Ye Z, You C, et al. Systematic review of decreased intracranial pressure with optimal head elevation in postcraniotomy patients: a meta-analysis. J Adv Nurs. 2015;71(10):2237–46. https://doi.org/10.1111/jan.12679.
Olavarría VV, Arima H, Anderson CS, et al. Head position and cerebral blood flow velocity in acute ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis. 2014;37(6):401–8. https://doi.org/10.1159/000362533.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805. https://doi.org/10.1177/0962280216669183.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. https://doi.org/10.1186/1471-2288-14-135.
Feldman Z, Kanter MJ, Robertson CS, et al. Effect of head elevation on intracranial pressure, cerebral perfusion pressure, and cerebral blood flow in head-injured patients. J Neurosurg. 1992;76(2):207–11. https://doi.org/10.3171/jns.1992.76.2.0207.
Burnol L, Payen JF, Francony G, et al. Impact of head-of-bed posture on brain oxygenation in patients with acute brain injury: a prospective cohort study. Neurocrit Care. 2021;35(3):662–8. https://doi.org/10.1007/s12028-021-01240-1.
Brimioulle S, Moraine JJ, Norrenberg D, Kahn RJ. Effects of positioning and exercise on intracranial pressure in a neurosurgical intensive care unit. Phys Ther. 1997;77(12):1682–9. https://doi.org/10.1093/ptj/77.12.1682.
Dagod G, Roustan JP, Bringuier-Branchereau S, et al. Effect of a temporary lying position on cerebral hemodynamic and cerebral oxygenation parameters in patients with severe brain trauma. Acta Neurochir (Wien). 2021;163(9):2595–602. https://doi.org/10.1007/s00701-021-04851-x.
Kiening KL, Härtl R, Unterberg AW, Schneider GH, Bardt T, Lanksch WR. Brain tissue pO2-monitoring in comatose patients: implications for therapy. Article Neurol Res. 1997;19(3):233–40. https://doi.org/10.1080/01616412.1997.11740805.
Kim MN, Edlow BL, Durduran T, et al. Continuous optical monitoring of cerebral hemodynamics during head-of-bed manipulation in brain-injured adults. Neurocrit Care. 2014;20(3):443–53. https://doi.org/10.1007/s12028-013-9849-7.
Ledwith MB, Bloom S, Maloney-Wilensky E, Coyle B, Polomano RC, Le Roux PD. Effect of body position on cerebral oxygenation and physiologic parameters in patients with acute neurological conditions. J Neurosci Nurs. 2010;42(5):280–7. https://doi.org/10.1097/jnn.0b013e3181ecafd4.
Mahfoud F, Beck J, Raabe A. Intracranial pressure pulse amplitude during changes in head elevation: a new parameter for determining optimum cerebral perfusion pressure? Acta Neurochir (Wien). 2010;152(3):443–50. https://doi.org/10.1007/s00701-009-0520-1.
Meixensberger J, Baunach S, Amschler J, Dings J, Roosen K. Influence of body position on tissue-pO2, cerebral perfusion pressure and intracranial pressure in patients with acute brain injury. Neurol Res. 1997;19(3):249–53. https://doi.org/10.1080/01616412.1997.11740808.
Moraine JJ, Berré J, Mélot C. Is cerebral perfusion pressure a major determinant of cerebral blood flow during head elevation in comatose patients with severe intracranial lesions? J Neurosurg. 2000;92(4):606–14. https://doi.org/10.3171/jns.2000.92.4.0606.
Ng I, Lim J, Wong HB. Effects of head posture on cerebral hemodynamics: its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery. 2004;54(3):593–7. https://doi.org/10.1227/01.neu.0000108639.16783.39.
Park CO, Ha YS. Clinical analysis of 34 diffuse axonal injured (DAI) patients below GCS 8. Yonsei Med J. 1992;33(4):326–36. https://doi.org/10.3349/ymj.1992.33.4.326.
Rosner MJ, Coley IB. Cerebral perfusion pressure, intracranial pressure, and head elevation. J Neurosurg. 1986;65(5):636–41. https://doi.org/10.3171/jns.1986.65.5.0636.
Schneider GH, von Helden GH, Franke R, Lanksch WR, Unterberg A. Influence of body position on jugular venous oxygen saturation, intracranial pressure and cerebral perfusion pressure. Acta Neurochir Suppl (Wien). 1993;59:107–12. https://doi.org/10.1007/978-3-7091-9302-0_19.
Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of body position on intracranial pressure and cerebral perfusion in patients with large hemispheric stroke. Stroke. 2002;33(2):497–501. https://doi.org/10.1161/hs0202.102376.
Altun Uğraş G, Yüksel S, Temiz Z, Eroğlu S, Şirin K, Turan Y. Effects of different head-of-bed elevations and body positions on intracranial pressure and cerebral perfusion pressure in neurosurgical patients. J Neurosci Nurs. 2018;50(4):247–51. https://doi.org/10.1097/jnn.0000000000000386.
Agbeko RS, Pearson S, Peters MJ, McNames J, Goldstein B. Intracranial pressure and cerebral perfusion pressure responses to head elevation changes in pediatric traumatic brain injury. J Pediatr Crit Care Med. 2012. https://doi.org/10.1097/PCC.0b013e31820ac2ad.
Durward QJ, Amacher AL, Del Maestro RF, Sibbald WJ. Cerebral and cardiovascular responses to changes in head elevation in patients with intracranial hypertension. J Neurosurg. 1983;59(6):938–44. https://doi.org/10.3171/jns.1983.59.6.0938.
Kenning JA, Toutant SM, Saunders RL. Upright patient positioning in the management of intracranial hypertension. Article Surg Neurol. 1981;15(2):148–52. https://doi.org/10.1016/0090-3019(81)90037-9.
Lang SS, Valeri A, Zhang B, et al. Head of bed elevation in pediatric patients with severe traumatic brain injury. J Neurosurg Pediatr. 2020;26(5):465–75. https://doi.org/10.3171/2020.4.peds20102.
March K, Mitchell P, Grady S, Winn R. Effect of backrest position on intracranial and cerebral perfusion pressures. J Neurosci Nurs. 1990;22(6):375–81. https://doi.org/10.1097/01376517-199012000-00008.
McNett M, Livesay S, Yeager S, et al. The impact of head-of-bed positioning and transducer location on cerebral perfusion pressure measurement. J Neurosci Nurs. 2018;50(6):322–6. https://doi.org/10.1097/jnn.0000000000000398.
Parsons LC, Wilson MM. Cerebrovascular status of severe closed head injured patients following passive position changes. Nurs Res. 1984;33(2):68–75.
Raabe A, Czosnyka M, Piper I, Seifert V. Monitoring of intracranial compliance: correction for a change in body position. Acta Neurochir (Wien). 1999;141(1):31–6. https://doi.org/10.1007/s007010050263.
Zhang Y, Rabinstein AA. Lower head of the bed position does not change blood flow velocity in subarachnoid hemorrhage. Neurocrit Care. 2011;14(1):73–6. https://doi.org/10.1007/s12028-010-9444-0.
Wisanusattra H, Khwannimit B. Agreements between mean arterial pressure from radial and femoral artery measurements in refractory shock patients. Sci Rep. 2022;12(1):8825. https://doi.org/10.1038/s41598-022-12975-y.
Thomas E, Czosnyka M, Hutchinson P. Calculation of cerebral perfusion pressure in the management of traumatic brain injury: joint position statement by the councils of the Neuroanaesthesia and Critical Care Society of Great Britain and Ireland (NACCS) and the Society of British Neurological Surgeons (SBNS). Br J Anaesth. 2015;115(4):487–8. https://doi.org/10.1093/bja/aev233.
Harary M, Dolmans RGF, Gormley WB. Intracranial pressure monitoring-review and avenues for development. 2018;18(2): 52. https://doi.org/10.3390/s18020465
Padayachy LC, Figaji AA, Bullock MR. Intracranial pressure monitoring for traumatic brain injury in the modern era. Childs Nerv Syst. 2010;26(4):441–52. https://doi.org/10.1007/s00381-009-1034-0.
Anderson CS, Arima H, Lavados P, et al. Cluster-randomized, crossover trial of head positioning in acute stroke. N Engl J Med. 2017;376(25):2437–47. https://doi.org/10.1056/NEJMoa1615715.
Parichay PJ, Khanapure K, Joshi KC, Aniruddha TJ, Sandhya M, Hegde AS. Clinical and radiological assessment of cerebral hemodynamics after cranioplasty for decompressive craniectomy-a clinical study. J Clin Neurosci. 2017;42:97–101. https://doi.org/10.1016/j.jocn.2017.04.005.
Wang L, Li X, Yang Z, et al. Semi-recumbent position versus supine position for the prevention of ventilator-associated pneumonia in adults requiring mechanical ventilation. The Cochrane Database Syst Rev. 2016;2016(1):5009946. https://doi.org/10.1002/14651858.CD009946.pub2.
Funding
There are no funding sources for this study.
Author information
Authors and Affiliations
Contributions
MBR: conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, supervision, validation, visualization, writing the original draft, review, editing; JPEB: data curation, formal analysis, investigation, methodology, project administration, validation, visualization, writing the original draft, editing; JPMT: conceptualization, data curation, formal analysis, investigation, methodology, software, supervision, validation, visualization, writing the original draft, review, editing; GBN: data curation, formal analysis, investigation, validation, visualization, writing the original draft, review, editing; GIC: data curation, formal analysis, investigation, validation, visualization, writing the original draft, review, editing; CBR: conceptualization, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing the original draft, review, editing; MJT: conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing the original draft, review, editing; EGF: conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing the original draft, review, editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval/informed consent
This article complies with ethical standards, and institutional review board approval was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramos, M.B., Britz, J.P.E., Telles, J.P.M. et al. The Effects of Head Elevation on Intracranial Pressure, Cerebral Perfusion Pressure, and Cerebral Oxygenation Among Patients with Acute Brain Injury: A Systematic Review and Meta-Analysis. Neurocrit Care (2024). https://doi.org/10.1007/s12028-024-02020-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12028-024-02020-3