Summary
The pressure reactivity index (PRx) and the pulse amplitude index (PAx) are invasively determined parameters that are commonly used to describe autoregulation following traumatic brain injury (TBI). Using a transcranial Doppler ultrasound (TCD) technique, it is possible to approximate cerebral arterial blood volume (CaBV) solely from cerebral blood flow velocities, and further, to calculate non-invasive markers of autoregulation. In this brief study, we aimed to investigate whether the estimation of relative CaBV with different models could describe the cerebrovascular reactivity of TBI patients. PRx, PAx and their non-invasive counterparts (nPRx and nPAx) were calculated retrospectively from data collected during the monitoring of TBI patients. CaBV, an essential parameter for the calculation of nPRx and nPAx, was determined with both a continuous flow forward (CFF) model—considering a non-pulsatile blood outflow from the brain—and a pulsatile flow forward (PFF) model, presuming a pulsatile outflow. We found that the estimated CaBV demonstrates good coherence with ICP and that nPRx and nPAx can describe cerebrovascular reactivity similarly to PRx and PAx. Continuous monitoring with TCD is difficult, so the usability of PRx and PAx is limited. However, they might become useful for clinicians in the near future owing to rapid advances in these technologies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cerebral autoregulation
- Pressure reactivity index
- Pulse amplitude index
- Transcranial Doppler
- Cerebral blood volume
Introduction
The pressure reactivity index (PRx) is one of the commonly used parameters to describe autoregulation in traumatic brain injury (TBI). It quantifies the changes in vascular smooth muscle tone that occur as a result of variations in transmural pressure. It is calculated as the moving linear correlation coefficient between mean arterial blood pressure (ABP) and intracranial pressure (ICP) [1].
In certain cases (i.e. after craniectomy), PRx might falsely indicate good autoregulation due to the increased compliance of the intracranial space and the altered status of ICP. In these situations, the correlation of ABP and the pulse amplitude of ICP (AMP) could be a better descriptor of cerebrovascular reactivity. This index is called the pressure-amplitude index (PAx) [2].
Since ICP is needed to calculate both PRx and PAx, both indices are considered to be invasively quantified markers of cerebral autoregulation. PRx and PAx are applicable for this purpose because a change in cerebral arterial blood volume (CaBV) results in a corresponding change in ICP. Therefore, PRx and PAx are indirect descriptors of the relationship between the mean arterial blood pressure (ABP) and the instantaneous blood volume inside the cranial space. However, with the help of the transcranial Doppler ultrasound (TCD) technique, it is possible to approximate CaBV noninvasively solely from cerebral blood flow velocities. The disadvantage of this method is that because of the unknown cross-sectional area of the insonated blood vessels, the direct calculation of blood volume is not possible. In this brief study, we aimed to investigate whether noninvasive estimation of relative CaBV with different models could be used to describe the cerebrovascular reactivity of TBI patients.
Materials and Methods
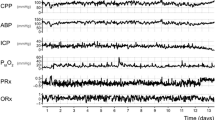
TBI patients received both continuous invasive (ABP and ICP) monitoring and daily noninvasive monitoring with TCD over the duration of admission to the Neurosciences Critical Care Unit (NCCU) at Addenbrooke’s Hospital, Cambridge, United Kingdom. Data registered prospectively as a part of standard care were retrospectively reviewed with ICM+ software (Cambridge Enterprise, Cambridge, United Kingdom; http://www.neurosurg.cam.ac.uk/icmplus). The database was fully anonymized, no data on patient identifiers were available, and therefore no additional ethical approval or formal patient or proxy consent was needed. PRx and PAx were calculated as the correlation coefficients between 30 samples of 10-s averages of ABP and ICP (or the amplitude of ICP in the case of PAx).
The change in CaBV at any given time is determined by the volume of inflow and the volume of outflow from the cranial space. With TCD, only the velocity of the blood inflow is monitored. Based on the assumption made about the nature of outflow, two different methods can be used to model changes in CaBV [3]:
-
1.
\( \Delta {C}_{\mathrm{a}}{BV}_{CFF}(t)={\int}_{t_0}^t\left( CB{\mathrm{F}}_{\mathrm{a}}(s)-\mathrm{meanCBFa}\right)\mathrm{d}s \)
-
2.
\( \Delta {C}_{\mathrm{a}}{BV}_{PFF}(t)={\int}_{{\mathrm{t}}_0}^t\left( CB{\mathrm{F}}_{\mathrm{a}}(s)-\frac{ABP(s)}{CVR}\right)\mathrm{d}s \)
where: s—the arbitrary time variable of integration, CBFa—cerebral blood flow, ABP—arterial blood pressure, and CVR—cerebrovascular resistance (CVR = meanABP/meanCBFa).
In a continuous flow forward (CFF) model, a non-pulsatile blood outflow is considered. The pulsatile inflow is equilibrated by a continuous outflow through the dural sinuses. Over a longer period, the outflow is considered to be equal to the inflow, so it can be calculated by averaging the inflow over several cardiac cycles (in this study, we used 5-min intervals).
The second equation presumes that the outflow—similarly to the inflow—is also pulsatile, becoming the pulsatile flow forward (PFF) model. The idea behind this theory is that the outflow is affected by the vasomotor tone of the regulating arterioles and the pulsatile ABP and can be determined by the ratio between ABP and cerebrovascular resistance.
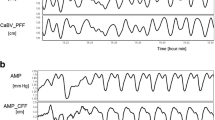
With TCD monitoring, the cross-sectional area of the middle cerebral artery is unknown, and the CBF cannot be precisely calculated. In these equations, CBF can be replaced with CBFV, so the relative changes in CaBV can be estimated (Fig. 1).
The noninvasive counterparts of PRx (nPRx) and PAx (nPAx) were derived similarly, but with help of the estimated cerebral volumes. nPRx is calculated with CaBV instead of ICP, and nPAx with the pulse amplitude of CaBV instead of AMP. Both nPRx and nPAx were calculated using both the CFF and PFF models.
Results
Discussion
With TCD it is possible to derive noninvasive indices – nPRx and nPAx – of cerebrovascular reactivity by estimating the relative changes in CaBV. These indices can be calculated similarly to PRx and PAx if ICP is changed to CaBV. Figure 2 demonstrates that a change in CaBV is reflected in a corresponding change in ICP – which is the rationale of the usability of PRx [1]—but this could also explain the similarities between the invasive and noninvasive indices shown in Fig. 3. This analogous behavior opens up possibilities for the use of these noninvasive cerebrovascular reactivity indices: they may become clinically useful in the subacute phase of neuro-intensive care because they can provide further information about autoregulation even after the removal of invasive ICP monitors. With other noninvasive techniques (continuous ABP monitoring via finger-cuff), cerebrovascular reactivity can be described without the necessity for invasive measurements, a PRx-like index can be quantified on a long-term follow-up and can be compared to PRx derived from early clinical care. In less severe cases of TBI, if invasive parameters are not available, noninvasive optimal cerebral perfusion pressure (nCPPopt) instead of traditionally invasive optimal cerebral perfusion pressure (CPPopt) could be determined and used to guide treatment.
The usability of either nPRx or nPAx is limited because these indices depend on continuous TCD monitoring technology. However, these techniques develop quickly, so further studies aimed at the investigation of nPRx and nPAx would be useful, which would enable clinicians to utilize the previously mentioned advantages immediately after the necessary improvements are made.
References
Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD (1997) Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 41(1):11–19
Aries MJH, Czosnyka M, Budohoski KP, Kolias AG, Radolovich DK, Lavinio A, Pickard JD, Smielewski P (2012) Continuous monitoring of cerebrovascular reactivity using pulse waveform of intracranial pressure. Neurocrit Care 17(1):67–76
Uryga A, Kasprowicz M, Calviello L, Diehl RR, Kaczmarska K, Czosnyka M (2019) Assessment of cerebral hemodynamic parameters using pulsatile versus non-pulsatile cerebral blood outflow models. J Clin Monit Comput 33(1):85–94
Acknowledgement
Marek Czosnyka is supported by National Institute of Health Research, Cambridge Biomedical Research Centre.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Ethics declarations
ICM+ is a software licensed by Cambridge Enterprise Ltd. (https://icmplus.neurosurg.cam.ac.uk). PS and MC have a financial interest in a fraction of licensing fees.
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Czigler, A., Calviello, L.A., Zeiler, F.A., Toth, P., Smielewski, P., Czosnyka, M. (2021). Usability of Noninvasive Counterparts of Traditional Autoregulation Indices in Traumatic Brain Injury. In: Depreitere, B., Meyfroidt, G., Güiza, F. (eds) Intracranial Pressure and Neuromonitoring XVII. Acta Neurochirurgica Supplement, vol 131. Springer, Cham. https://doi.org/10.1007/978-3-030-59436-7_33
Download citation
DOI: https://doi.org/10.1007/978-3-030-59436-7_33
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-59435-0
Online ISBN: 978-3-030-59436-7
eBook Packages: MedicineMedicine (R0)