Abstract
Background/Objectives
Epileptiform abnormalities (EA) on continuous electroencephalography (cEEG) are associated with increased risk of acute seizures; however, data on their association with development of long-term epilepsy are limited. We aimed to investigate the association of EA in patients with acute brain injury (ABI): ischemic or hemorrhagic stroke, traumatic brain injury, encephalitis, or posterior reversible encephalopathy syndrome, and subsequent development of epilepsy.
Methods
This was a retrospective, single-center study of patients with ABI who had at least 6 hours of cEEG during the index admission between 1/1/2017 and 12/31/2018 and at least 12 months of follow-up. We compared patients with EAs; defined as lateralized periodic discharges (LPDs), lateralized rhythmic delta activity (LRDA), generalized periodic discharges (GPDs), and sporadic interictal epileptiform discharges (sIEDs) to patients without EAs on cEEG. The primary outcome was the new development of epilepsy, defined as the occurrence of spontaneous clinical seizures following hospital discharge. Secondary outcomes included time to development of epilepsy and use of anti-seizure medications (ASMs) at the time of last follow-up visit.
Results
One hundred and one patients with ABI met study inclusion criteria. Thirty-one patients (30.7%) had EAs on cEEG. The median (IQR) time to cEEG was 2 (1–5) days. During a median (IQR) follow-up period of 19.1 (16.2–24.3) months, 25.7% of patients developed epilepsy; the percentage of patients who developed epilepsy was higher in those with EAs compared to those without EAs (41.9% vs. 18.6%, p = 0.025). Patients with EAs were more likely to be continued on ASMs during follow-up compared to patients without EAs (67.7% vs. 38.6%, p = 0.009). Using multivariable Cox regression analysis, after adjusting for age, mental status, electrographic seizures on cEEG, sex, ABI etiology, and ASM treatment on discharge, patients with EAs had a significantly increased risk of developing epilepsy compared to patients without EA (hazard ratio 3.39; 95% CI 1.39–8.26; p = 0.007).
Conclusions
EAs on cEEG in patients with ABI are associated with a greater than three-fold increased risk of new-onset epilepsy. cEEG findings in ABI may therefore be a useful risk stratification tool for assessing long-term risk of seizures and serve as a biomarker for new-onset epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Continuous EEG (cEEG) monitoring has increased exponentially in the last decade and has led to increased detection of seizures in critically ill patients [1]. In addition to electrographic seizures, approximately 30% of cEEG recordings identify other epileptiform abnormalities (EAs) such as lateralized periodic discharges (LPDs), lateralized rhythmic delta activity (LRDA), generalized periodic discharges (GPDs), brief potentially ictal rhythmic discharges (BIRDs), bilateral independent periodic discharges (BIPDs), or sporadic epileptiform discharges (sEDs) [2]. A large multicenter study of > 4500 patients demonstrated a strong association of EAs with increased risk of acute seizures [2]. Further, some recent studies have found that higher burden of EAs is associated with worse functional outcomes in patients with subarachnoid hemorrhage and acute ischemic stroke [3, 4]. While these EAs are conclusively associated with an increased risk of acute seizures, the investigation of their long-term significance is only beginning [5].
Recent studies in patients with a range of underlying etiologies have demonstrated an association between the presence of LPDs on cEEG with later development of epilepsy [6, 7]. Moreover, the presence of early seizures is associated with long-term development of post-traumatic epilepsy in patients with moderate-to-severe traumatic brain injury (TBI) [8]. In fact, acquired epilepsy is a frequent and potentially debilitating complication of acute brain injury (ABI) with 20–60% of epilepsies caused by an identified brain insult [9]. Further, since it is unclear how long the risk of seizure remains high, a large proportion of patients who undergo cEEG are treated with anti-seizure medications (ASMs), and 50–90% of them stay on ASMs for 12 months or longer [6, 10,11,12]. Due to the cognitive adverse effects of ASMs, the potentially harmful effects of polypharmacy in the elderly, and added healthcare costs, it is critical to understand long-term post-hospitalization outcomes of these patients. Therefore, we aimed to explore the association between EAs on cEEG and subsequent development of long-term epilepsy in patients with ABI who underwent cEEG monitoring during index hospitalization.
Methods
Study Population
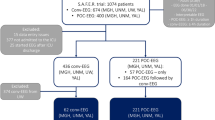
This was a retrospective study of patients admitted to Grady Memorial Hospital from January 1, 2017 to December 31, 2018. We included patients who had: (1) ABI on presentation defined as acute ischemic stroke (AIS), intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), TBI, or “other,” with “other” including posterior reversible encephalopathy syndrome or encephalitis, (2) cEEG for 6 or more hours, and (3) at least 12 months of follow-up as determined by review of all electronic medical records (EMR) at our institution, which integrates with several local hospital EMRs. We excluded patients with (1) history of epilepsy, (2) prior remote brain injury or other central nervous system lesion with new-onset clinical or electrographic seizures at the time of presentation meeting criteria for epilepsy, (3) anoxic brain injury during index admission, and (4) less than 12 months of follow-up. This study was approved by the Emory University Institutional Review Board and Grady Memorial Hospital research oversight committee.
Clinical and EEG Variables
We collected clinical variables including age, sex, ABI etiology, Glasgow Coma Scale (GCS) at the time of EEG, clinical seizure prior to cEEG, time-to-cEEG, hospital length of stay, ASM use on discharge, and time to last follow-up. Clinical seizures were identified as events witnessed by treating teams that were documented in the EMR and defined as generalized tonic–clonic activity or focal events (jerking of the arms, legs, or facial twitching), with/without gaze deviation consistent with focal seizure. Time-to-cEEG was calculated from the day of arrival to emergency department. All patients underwent cEEG using the conventional international 10–20 system of electrode placement with 21 disk scalp electrodes. The interictal findings were interpreted in accordance with the 2012 American Clinical Neurophysiology Society standardized critical care EEG terminology [13]. EEG variables included the presence of electrographic or electroclinical seizures, the type of EA (LPDs, LRDA, GPDs, and sEDs). Generalized rhythmic delta activity (GRDA) was not included due to its non-epileptiform nature as shown in the recent studies [2]. We considered EA to be a binary variable—present or absent. Hence, patients with multiple EA such as having both LRDA and LPDs were counted only once (EA present).
Outcomes
The primary outcome was development of epilepsy, defined as the occurrence of spontaneous clinical seizures following hospital discharge. Occurrence of seizure was determined by electronic medical record notes documenting witnessed clinical events by clinicians and other staff in the hospital or an unequivocal description by the patient/family/bystander deemed by the treating clinician to be consistent with a generalized or focal seizure. Secondary outcomes included, time to development of epilepsy and the use of ASMs at the time of last follow-up.
Statistical Analysis
We compared baseline characteristics between patients with and without EA using Pearson Chi-square test or Fisher’s exact test for categorical variables and Mann–Whitney U test for continuous variables. Kaplan–Meier curve and log-rank test were used to compare time-to-epilepsy development in patients with and without EA. Patients who did not develop epilepsy were censored at their last follow-up. Multivariable Cox proportional hazards regression models were used to determine the association of EAs with new-onset epilepsy, adjusting for age, sex, mental status at the time of EEG (stratified by GCS), ABI etiology, discharge ASMs, clinical seizure prior to cEEG, and electrographic seizure on cEEG. Results are reported as hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). All statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
Results
The study included 101 patients with ABI with median [IQR] age of 56 [45–65] years of which 37.6% were women (Table 1). We included patients with a varying degree of severity; AIS patients had National Institute of Health Stroke Scale (NIHSS) scores of 4–25, ICH patients had ICH scores of 0–4, subarachnoid hemorrhage (SAH) patients had Hunt and Hess scores of 3–4, and TBI patients had intracranial injuries of varying severity and GCS scores of 3–15. The median (IQR) time to cEEG was two (1–5) days. Thirty-one (30.7%) patients had EAs on cEEG: 15 (14.9%) had LPDs, 12 (11.9%) had LRDA, 7 (6.9%) had GPDs, and 2 (2%) had sEDs. Nine (8.9%) patients had acute electrographic seizures and 40 (39.6%) demonstrated focal slowing on cEEG. Patients with EAs were more likely to develop acute seizures (19.4 vs. 4.3%, p = 0.023) and were more likely to be discharged on ASMs (74.2 vs. 32.9% p = 0.001). The median duration of monitoring in the EA group was significantly longer compared to the duration of monitoring in the non-EA group (48.0 IQR 2.8–84.0 vs. 22.8, IQR 17.4–42.9, p = 0.01). There were no other differences in the baseline characteristics of patients with EA and without EA.
Overall, 26 (25.7%) patients with ABI developed new-onset epilepsy during a median (IQR) follow-up duration of 19.1 (16.2–24.3) months. Patients without EAs had a longer duration of follow-up (19.3 [16.67–24.44] months) compared to those with EAs (15.3 [13.0–20.9] months), p = 0.02. The proportion of patients who developed epilepsy was significantly higher in those with vs. without EAs (41.9 vs. 18.6%, p = 0.025). Patients with EAs were more likely to be continued on ASMs during follow-up, as compared with patients without EAs. On multivariable Cox regression analysis, after adjusting for covariates, the risk of new-onset epilepsy following ABI was significantly higher in patients with vs. without EAs (adjusted HR 3.39; 95% CI 1.39–8.26; p = 0.007) (Table 2 and Fig. 1). Clinical seizure prior to cEEG was also associated with an increased risk of development of epilepsy (adjusted HR 2.70; 95% CI 1.07–6.79; p = 0.035).
Discussion
Our study demonstrates that in patients with ABI, the presence of EAs on cEEG is associated with a greater than three-fold increased risk of new-onset epilepsy, independent of clinical factors. This elevated risk was seen despite most patients being discharged on ASMs. Of note, the median time from admission to cEEG in our study was two days, suggesting a role for early cEEG within the first week of injury to stratify epilepsy risk in patients with ABI.
Our findings are consistent with prior studies in patients with TBI and acute ischemic stroke showing EAs on early EEG independently predicted post-stroke and post-traumatic epilepsy [14, 15]. However, a recent study found no difference in risk of new-onset epilepsy between patients with LPDs and/or LRDA versus patients without LPDs or LRDA [5]. There are several possible reasons for the discrepancies in the findings across these studies. Firstly, the study population in each study was variable and ranging from a specific population which may be higher risk such as acute brain injury to all critically ill patients including those without ABI in whom the risk of epilepsy might be lower. Secondly, we included all types of EAs in our study, whereas other studies examined only LPDs and LRDA, which could have influenced the results. Lastly, the duration of follow-up was also different among the studies and hence, the results are not comparable. These findings indicate the need for a larger prospective study which can overcome these limitations and also allow for subgroup analyses for definitive conclusions.
While EAs are associated with an increased risk of acute seizure [2, 16], there is a dearth of information about their long-term significance. Consequently, there are no guideline recommendations for long-term ASM management and post-hospitalization care of ABI patients with EAs. Our finding of EAs as a prognostic marker for epilepsy development may be used to counsel patients and families about the elevated risk of seizures in those with EAs and potentially guide ASM management as well. Interestingly, most of the patients in our study who developed epilepsy were already on ASMs at the time of discharge. Since the ASMs are often associated with cognitive and other adverse effects [17], further studies should investigate how ASM treatment affects long-term risk of epilepsy development and outcomes in ABI patients with EAs.
Recently, there have been increased efforts to discover biomarkers for epileptogenesis which are common across patients with ABI such as stroke and TBI [18]. While some clinical risk factors for the development of epilepsy after ABI, such as injury severity, cortical location, and presence of hemorrhage, have been identified, it remains challenging to predict which ABI patients will develop epilepsy, hindering the development of clinical trials and interventions to prevent this adverse outcome. Continuous EEG is a candidate tool due to its widespread use and similar EEG findings across the spectrum of ABI. In experimental rat models of TBI, high frequency oscillations (HFOs) and repetitive spikes recorded via depth electrodes were seen early after fluid percussion injury in the most severe cases and resulted in a high incidence of late seizures [19]. These HFOs can also be detected on scalp EEG [20], and their role in epileptogenesis in TBI is being explored [21]. Although invasive EEG is not commonly employed for management of conditions beyond severe TBI and SAH, it is plausible that a subset of EAs seen on scalp EEG, such as periodic discharges with overriding fast activity, represent the correlate of HFOs and may be a marker of future development of epilepsy following ABI. Moreover, it is now apparent that periodic discharges (PDs) produce metabolic changes and hypoxia similar to that seen in electrographic seizures [22]. Therefore, it is possible that PDs insinuate cellular and metabolic changes similar to the early seizures after ABI and increase the long-term risk of developing epilepsy.
Early clinical seizures after TBI and ICH have been well known to be associated with development of epilepsy [8, 23]. In a cohort of 46 TBI patients, 24 had early seizures and 41.7% of those patients developed epilepsy; early seizure was a strong predictor of post-traumatic epilepsy in a multivariate model as well [8]. Similarly, early seizures are part of the CAVE score for predicting risk of epilepsy after ICH [23]. Our study confirms this association between early clinical seizures and development of epilepsy across the broad spectrum of ABI. We hypothesize that recruiting patients with early seizures after ABI may offer an avenue to maximize the yield of anti-epileptogenesis trials.
Our study has several limitations. Firstly, due to the single-center retrospective design, our findings may not be generalizable to all centers and patients. Secondly, we excluded patients with < 1-year follow-up, which may have affected our results. Similarly, the rate of detection of EA may have been affected by the duration of monitoring, which was shorter in patients without EA. However, recent studies have shown that the probability of detecting EA and seizures decays with time and 24 h of screening is recommended even for high-risk patients, as the likelihood of seizure beyond this time frame is less than 5% if no EA or seizures have been detected within 24 h [24, 25]. Since the patients without EA also had a median duration of cEEG monitoring of 22.75 h, we believe that most were adequately screened. It is possible that the longer duration of monitoring in the EA group is due to the tendency to monitor patients with EA longer to detect and manage seizures. Thirdly, due to the heterogenous etiology, we were unable to compare other variables such as severity of injury and location of injury which are known risk factors for development of epilepsy. Lastly, our small sample size precluded further subgroup analysis by type of EA or their plus modifiers. Despite these limitations, our study is one of the few which contributes to the scant literature on the long-term outcomes and lays the groundwork for a larger prospective study.
Conclusion
In this retrospective cohort study of patients with ABI, EA on cEEG was associated with > 3-fold increased risk of developing new onset epilepsy. Early cEEG in the ABI population may therefore be a useful tool for assessing risk of future development of epilepsy.
References
Hill CE, Blank LJ, Thibault D, et al. Continuous EEG is associated with favorable hospitalization outcomes for critically ill patients. Neurology. 2019;92:e9–18.
Rodriguez Ruiz A, Vlachy J, Lee JW, et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol. 2017;74:181–8.
Tabaeizadeh M, Aboul Nour H, Shoukat M, et al. Burden of epileptiform activity predicts discharge neurologic outcomes in severe acute ischemic stroke. Neurocrit Care. 2020;32:697–706.
Zafar SF, Postma EN, Biswal S, et al. Effect of epileptiform abnormality burden on neurologic outcome and antiepileptic drug management after subarachnoid hemorrhage. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2018;129:2219–27.
Husari KS, Johnson EL, Ritzl EK. Acute and long-term outcomes of lateralized rhythmic delta activity (LRDA) versus lateralized periodic discharges (LPDs) in critically ill patients. Neurocrit Care. 2020. https://doi.org/10.1007/s12028-020-01017-y.
Punia V, Bena J, Krishnan B, Newey C, Hantus S. New onset epilepsy among patients with periodic discharges on continuous electroencephalographic monitoring. Epilepsia. 2018;59:1612–20.
Punia V, Fitzgerald Z, Zhang X, et al. Electroencephalographic biomarkers of epilepsy development in patients with acute brain injury: a matched, parallel cohort study. Ann Clin Transl Neurol. 2019;6:2230–9.
Tubi MA, Lutkenhoff E, Blanco MB, et al. Early seizures and temporal lobe trauma predict post-traumatic epilepsy: a longitudinal study. Neurobiol Dis. 2019;123:115–21.
Banerjee PN, Filippi D, Allen Hauser W. The descriptive epidemiology of epilepsy: a review. Epilepsy Res. 2009;85:31–45.
Punia V, Garcia CG, Hantus S. Incidence of recurrent seizures following hospital discharge in patients with LPDs (PLEDs) and nonconvulsive seizures recorded on continuous EEG in the critical care setting. Epilepsy Behav. 2015;49:250–4.
Punia V, Vakani R, Burgess R, Hantus S. Electrographic and clinical natural history of lateralized periodic discharges. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2018;35:71–6.
Alvarez V, Rodriguez Ruiz AA, LaRoche S, et al. The use and yield of continuous EEG in critically ill patients: a comparative study of three centers. Clin Neurophysiol Off J Int Feder Clin Neurophysiol. 2017;128:570–8.
Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27.
Bentes C, Martins H, Peralta AR, et al. Early EEG predicts poststroke epilepsy. Epilepsia Open. 2018;3:203–12.
Kim JA, Boyle EJ, Wu AC, et al. Epileptiform activity in traumatic brain injury predicts post-traumatic epilepsy. Ann Neurol. 2018;83:858–62.
Struck AF, Ustun B, Ruiz AR, et al. Association of an electroencephalography-based risk score with seizure probability in hospitalized patients. JAMA Neurol. 2017;74:1419–24.
Naidech AM, Beaumont J, Muldoon K, et al. Prophylactic seizure medication and health-related quality of life after intracerebral hemorrhage. Crit Care Med. 2018;46:1480–5.
Klein P, Dingledine R, Aronica E, et al. Commonalities in epileptogenic processes from different acute brain insults: Do they translate? Epilepsia. 2018;59:37–66.
Reid AY, Bragin A, Giza CC, Staba RJ, Engel J Jr. The progression of electrophysiologic abnormalities during epileptogenesis after experimental traumatic brain injury. Epilepsia. 2016;57:1558–67.
Andrade-Valenca LP, Dubeau F, Mari F, Zelmann R, Gotman J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology. 2011;77:524–31.
Vespa PM, Shrestha V, Abend N, et al. The epilepsy bioinformatics study for anti-epileptogenic therapy (EpiBioS4Rx) clinical biomarker: study design and protocol. Neurobiol Dis. 2019;123:110–4.
Vespa P, Tubi M, Claassen J, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016;79:579–90.
Haapaniemi E, Strbian D, Rossi C, et al. The CAVE score for predicting late seizures after intracerebral hemorrhage. Stroke. 2014;45:1971–6.
Struck AF, Osman G, Rampal N, et al. Time-dependent risk of seizures in critically ill patients on continuous electroencephalogram. Ann Neurol. 2017;82:177–85.
Struck AF, Tabaeizadeh M, Schmitt SE, et al. Assessment of the validity of the 2HELPS2B score for inpatient seizure risk prediction. JAMA Neurol. 2020;77:500–7.
Funding
No grant support was received from any funding agency for this work in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Dr. Monica B. Dhakar has full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the concept and design. Polly Kumari, Julia Lega, Denise F. Chen and Monica B. Dhakar contributed to the acquisition, analysis, or interpretation of data. Denise F. Chen and Monica B. Dhakar contributed to the drafting of the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content. Monica B. Dhakar contributed to the statistical analysis.
Corresponding author
Ethics declarations
Ethical Approval
Emory University Institutional Review Board and Grady Memorial Hospital Research Oversight Committee approved the study and granted waiver of consent.
Conflict of interest
Denise F. Chen reports no disclosures. Polly Kumari reports no disclosures. Hiba A. Haider receives consultant support from Ceribell, Inc., author royalties from UpToDate, Inc. and Springer Publishing. Andres Rodriguez has participated in an education symposium sponsored by Neuropace Inc and has financial stake at Rodzi LLC. Julia Lega reports no disclosures. Monica B. Dhakar has received honoraria for consultancy from Adamas Pharmaceuticals and research support from Marinus Pharmaceuticals, UCB Biopharma for clinical trials. She also receives funding from NIH for work unrelated to this project.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, D.F., Kumari, P., Haider, H.A. et al. Association of Epileptiform Abnormality on Electroencephalography with Development of Epilepsy After Acute Brain Injury. Neurocrit Care 35, 428–433 (2021). https://doi.org/10.1007/s12028-020-01182-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-020-01182-0