Abstract
Importance
The pathophysiological mechanisms of Posterior Reversible Encephalopathy Syndrome (PRES) and related seizures remain poorly understood. The prevalence and clinical significance of nonconvulsive seizures (NCSz) and related epileptiform patterns during continuous electroencephalography monitoring (CEEG) in PRES have not been well described.
Objective
To report the prevalence, characteristics and risk factors for NCSz and related highly epileptiform patterns in patients with PRES, and to determine their relation to imaging abnormalities and outcome.
Design, Setting and Participants
From a prospective CEEG database, we retrospectively identified patients with PRES and reviewed their medical charts. Based on CEEG findings, we designed a retrospective cohort study comparing two groups defined based on the presence or the absence of NCSz and/or periodic discharges (PDs).
Main outcomes and Measures
The prevalence and risk factors for PDs and NCSz, description of EEG and magnetic resonance imaging (MRI) abnormalities and functional outcome as measured by the Glasgow Outcome Scale (GOS) at hospital discharge.
Results
Among 37 eligible patients, 23 (62%) had PDs or NCSz. The presence of NCSz was associated with the presence of PDs (15/22 vs. 1/15; p = 0.0002). NCSz and PDs were usually either lateralized or bilateral independent and predominated in the posterior regions. No clinical features were associated with the occurrence of PDs or NCSz. Cortical restricted diffusion on MRI was more frequent in the PDs/NCSz group (17/23 vs. 1/14; p < 0.001). PDs/NCSz were associated with worse outcome, with 3 deaths vs. 0 in the no PDs/NCSz group and fewer cases with low disability (4 vs. 9 cases with GOS = 5, p < 0.04).
Conclusions and Relevance
Our results reveal a high prevalence of NCSz and PDs in critically ill patients with PRES and an association with restricted diffusion and worse outcome, whether treating or preventing these EEG findings can improve outcome requires further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posterior Reversible Encephalopathy Syndrome (PRES) is a clinical-radiographic diagnosis often presenting as acute neurological symptoms with evidence of vasogenic subcortical edema caused by an endothelial dysfunction in the setting of blood pressure fluctuations, acute renal failure, drug toxicity, autoimmune disorders, or eclampsia [1]. It is generally reversible, both radiographically and clinically, and carries a favorable prognosis [2]. A minority of patients exhibit cytotoxic edema on imaging, whose nature and significance are unclear [3,4,5,6]. Clinical seizures are a common presentation of PRES and occur in approximately 70% of cases [7]. Status epilepticus (SE), including nonconvulsive SE (NCSE) is less common, occurring in 5–15% of cases [1, 8]. Most studies have shown that clinical seizures are usually easily controlled, do not affect outcome, and do not recur after PRES is resolved. Risk factors for seizures in PRES are unknown. In particular, there is no known association between the risk of convulsive or nonconvulsive seizures and clinical or imaging features in PRES. A shortcoming in most studies is that only clinical seizures were investigated. In contrast to imaging, studies on electroencephalographic (EEG) findings are scarce and mostly limited to short-term EEG recordings [9, 10, 12]. The majority of seizures in patients in the intensive care unit (ICU) are nonconvulsive and are reliably detected only with continuous EEG (CEEG) [13]. The prevalence and clinical significance of nonconvulsive seizures (NCSz) and NCSE during CEEG in PRES have not been investigated beyond case reports and small case series [8, 14].
The aims of this study were to report the prevalence, characteristics and risk factors for NCSz and related highly epileptiform findings in patients with PRES, and to describe to the associated imaging abnormalities and their impact on outcome.
Methods
Study Design and Population
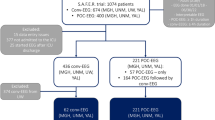
The study was approved by the institutional review boards of Hôpital Erasme and of Yale University. From our prospective CEEG databases, we retrospectively identified a cohort of patients with a clinical diagnosis of PRES during the period between 10/01/2011 and 05/31/2014 at Yale University, and between 01/01/2008 and 08/01/2017 at Hôpital Erasme. We reviewed their medical charts to confirm that they met the following criteria: typical neurological symptoms (encephalopathy, defined as altered consciousness or confusion, either with seizures, headache, or visual disturbances) in a compatible etiological setting (blood pressure fluctuations, acute renal failure, drug toxicity, autoimmune disorders, or eclampsia) [1].
We excluded patients who did not have brain magnetic resonance imaging (MRI) during the acute phase. We divided the cohort into two groups based on EEG findings of interest: a group of patients with NCSz or periodic discharges (PDs) and another one without NCSz or PDs (see EEG definitions below).
Clinical Variables
We reviewed medical charts to collect the following variables: age, sex, etiology of PRES (categorized as hypertensive emergency, acute renal failure, eclampsia, drug toxicity, autoimmune disorder, other), clinical manifestations at onset (encephalopathy, presence and type of clinical seizures [including SE], headache, visual disturbances), mental status (categorized as awake and following command [Glasgow Coma Scale—Motor Subscale (GCS-M) = 6], not following command but with purposeful response to stimulation (GCS-M = 4 or 5), or lack of any purposeful response (GCS-M < 4) on admission and on the day of CEEG, Sequential Organ Failure Assessment score [15] on the day of CEEG, the presence of a focal neurological deficit, mean arterial pressure (MAP) on admission and at CEEG onset, time from first symptoms to CEEG (in days), therapeutic strategies (blood pressure management, drug discontinuation, number of anti-seizure medications, use of continuous infusion of anesthetics for seizure control), discharge disposition (home, rehabilitation facility, skilled nursing facility, nursing home) and functional outcome using the Glasgow Outcome Scale (GOS) at discharge and at 6 months after discharge.
Electroencephalography
CEEG studies were performed as requested by the clinical team, using 21 electrodes placed according to the International 10–20 System. Two authors (L.B. and N.G.) reviewed the entire CEEG recording for the occurrence and location of NCSz and related highly epileptiform patterns, including PDs and lateralized rhythmic delta activity (LRDA) [16]. Both NCSz onsets and PDs were defined as lateralized, bilateral independent, multifocal or generalized [16]. In addition, we localized all patterns to 1 of 4 pre-defined regions (left anterior, left posterior, right anterior or right posterior). We considered the Fp1/2, F3/4/7/8/z, C3/4/z and T3/4 (also known as T7/T8) as anterior, and T5/6 (also known as P7/P8), P3/4/z, O1/2 as posterior, respectively. NCSE was defined according to published criteria [17].
We also recorded background EEG features, including state changes, posterior dominant rhythm, and reactivity [16]. Severity of encephalopathy was graded as mild (the presence of a posterior dominant rhythm), moderate (absence of posterior dominant rhythm but the presence of reactivity or spontaneous state changes), or severe (absence of posterior dominant rhythm, reactivity, and spontaneous state changes).
Magnetic Resonance Imaging
All included patients underwent an initial MRI requested by the clinical team on a 1.5-T or 3.0-T scanner. In all cases, imaging sequences included spin-echo T1, T2, fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI) and Apparent Diffusion Coefficient (ADC). We (NG and LB) reviewed the neuroimaging reports to confirm the radiological diagnosis of PRES. We also re-examined the FLAIR, DWI/and ADC sequences images to describe the anatomical distribution of the acute abnormalities.
We classified the imaging patterns based on FLAIR sequences as posterior, holohemispheric watershed, or superior frontal sulcus, as previously described [18]. We also recorded the presence and location (cortical vs. subcortical; anterior [frontal and anterior-mid temporal lobes] vs. posterior [parietal and occipital lobes or posterior temporal]; left vs. right vs. bilateral) of restricted diffusion as defined by high signal intensity on DWI with associated low signal on ADC. If DWI/ADC abnormalities were present bilaterally and in both anterior and posterior regions, they were considered diffuse.
We defined an overlap between CEEG abnormalities (PDs or NCSz) and DWI abnormalities when they occurred in the same regions (left anterior, left posterior, right anterior or right posterior).
When available, reports from follow-up MRIs were reviewed to confirm improvement or resolution of abnormalities as well as to identify permanent imaging sequelae.
Statistical Analysis
Continuous variables are presented as median (interquartile range [IQR]), and discrete variables as frequency (%). We compared the clinical and imaging characteristics and outcome of the group with PDs or NCSz during CEEG and the group without PDs or NCSz.
The Fisher exact test and the Mann–Whitney U test were used to assess differences in categorical and numerical variables, respectively. A p value < 0.05 was considered statistically significant. Statistical analysis was performed using MATLAB (MathWorks; http://www.mathworks.com).
Results
Demographics and Clinical Characteristics
We identified 42 patients, but excluded 5 due to a lack of an acute MRI. All 37 remaining cases fulfilled the clinical criteria of PRES. Their characteristics are presented in Table 1 (“all patients”).
The most common etiologies of PRES were malignant hypertension (N = 15 [41%]), acute renal failure (N = 15 [41%]) and drug toxicity (N = 12 [32%]). All patients had encephalopathy. Headache and visual disturbances occurred in 12 (32%) and 9 (24%) cases, respectively. Eleven (30%) had a focal neurological deficit. Clinical seizures prior to CEEG occurred in 31 (84%) cases, including 11 (30%) with SE. The most common seizure semiologies were generalized convulsive (21 [57%]) and focal motor (7 [19%]).
Electroencephalographic Characteristics
The time between the clinical recognition of PRES and CEEG was 0 (0–1) days.
All CEEG were abnormal with at least some degree of background EEG abnormalities (Table 1 [“all patients”]). Fourteen (38%) patients had no PDs or NCSz. Their characteristics are presented in Table 1 (“no PDs/NCSz group”). Twenty-three (62%) patients had PDs or NCSz, including 22 (59%) with PDs and 16 (43%) with NCSz. Their characteristics are presented in Table 1 (“PDs/NCSz group”). The presence of NCSz was associated with the presence of PDs (15/22 [68%] vs. 1/15 [7%]; p = 0.0002). Only 1 patient had NCSz but did not have PDs. Six (16%) patients fulfilled criteria for NCSE. PDs were most commonly lateralized (LPDs; 14/22 [61%]) or bilateral independent (BIPDs; four [17%]). One patient with LPDs also had LRDA. The onset of NCSz was lateralized in 10 cases (63%) or bilateral independent in 6 cases (38%). Both PDs and NCSz onsets predominated (17/22 [74%]) in the posterior regions.
Imaging Characteristics
The most frequent radiological pattern was the posterior pattern (24 [65%]), followed by the holohemispheric watershed pattern (8 [22%]). Cortical restricted diffusion was noted in 18 (49%) cases, with 17/23 (74%) in the PDs/NCSz group versus 1/14 (7%) in the no PDs/NCSz group (p < 0.001). Example cases are presented in Figs. 1 and 2. In those with restricted diffusion, 12/18 had a repeat MRI scan during follow-up, with 8/12 (67%) had complete resolution while 4 (33%) showed improvement (or incomplete resolution). Three (8%) patients developed ischemic stroke and irreversible injury, two of whom were in the PDs/NCSz group.
MR FLAIR (a, c, e, g) and DWI (b, d, f, h) images and 30-second EEG excerpts (i, j) from a 66-year-old male with drug-induced PRES. Two days after a bilateral lung transplant and initiation of tacrolimus, the patient started complaining of visual blurring then became stuporous. His MRI showed bilateral parieto-occipital FLAIR (arrows in a, c, e, g) and DWI (arrows in b, d, f, h) hyperintense abnormalities. Please note that DWI and FLAIR abnormalities do not strictly overlap. His CEEG showed right occipital LPDs (lines in i) and bilateral independent occipital NCSz (boxes in i and j). High and low-pass filters were set at 1 and 70 Hz, respectively. The notch filter was off
MR FLAIR (a, d), DWI (b, e) and ADC (c, f) images and 10-second EEG excerpts (g, h) from a 80-year-old female with PRES in the setting of hypertensive emergency. The patient was admitted to the emergency department with headache and elevated blood pressure (209/110 mmHg). She was confused and complained of visual blurring. Her MRI showed bilateral parieto-occipital FLAIR (arrows in a), left parieto-occipital DWI hyperintense (arrow in b) and ADC hypointense (arrow in b) abnormalities. These abnormalities had resolved on the follow-up MRI, which was performed 3 months after discharge (d–f). Her CEEG showed left occipital LPDs (lines in g) and bilateral independent occipital NCSz (boxes in h). High and low-pass filters were set at 1 and 70 Hz, respectively. The notch filter was off
Treatment
Twenty-two (59%) patients required aggressive blood pressure management. Six (16%) patients received renal replacement therapy. The causative drug was discontinued in 6 (16%) patients.
Twenty-two (59%) patients received intravenous benzodiazepine and 35 (86%) patients received conventional anti-seizure medications. Six (14%) patients required more than 2 medications and 4 (11%) required continuous anesthetic infusion for NCSz control.
Determinants of Periodic Discharges and Seizures during CEEG
There were no demographic, etiological or clinical differences between the PDs/NCSz group and the no PDs/NCSz group (Table 1), although malignant hypertension and headache were more frequent in the no PDs/NCSz group, this was not statistically significant. There was also a trend toward higher MAP values in the no PDs/NCSz group. The degree of encephalopathy on EEG was similar in both groups.
As expected, patients in the PDs/NCSz group received more anti-seizure medications (2 [1, 2] vs. 1 [1]; p = 0.003) and four required continuous anesthetic infusion, compared to none in the no PDs/NCSz group (not significant). The distribution of imaging findings was also similar in both groups, but cortical restricted diffusion was more frequent in the PDs/NCSz group (17/23 [74%] vs. 1/14 (7%); p < 0.001). The patient in the no PDs/NCSz group with restricted diffusion presented a stroke resulting in left hemiparesis. His follow-up MRI showed a persisting ischemic lesion.
Location of EEG and DWI/ADC Abnormalities
In the PDs/NCSz group, electrographic abnormalities overlapped with the pattern of cortical restricted diffusion in 10/23 (43%) cases (Figs. 1, 2). In 2 patients, the diffusion abnormalities were more widespread than the EEG patterns. One of them, with left posterior LPDs and diffuse restricted diffusion, had a generalized tonic clonic seizure before CEEG. Both his neurological exam and MRI improved at follow-up. The other patient, with right posterior LPDs and NCSz onset and bilateral posterior diffusion abnormalities, had NCSE and left posterior sporadic epileptiform discharges. His follow-up MRI showed an ischemic stroke. In three other cases, restricted diffusion was noted in the hippocampus on the same side as the EEG abnormalities. Finally, in eight cases, the electrographic abnormalities were more widespread than the diffusion abnormalities; six patients had no restricted diffusion in spite of PDs or NCSz and two had bilateral PDs or NCSz and only unilateral restricted diffusion.
Outcome
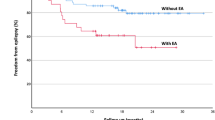
Overall, 26 (70%) patients achieved low or moderate disability (GOS = 5 or 4) at discharge and 19 (51%) were discharged home. Three patients (8%) died prior to discharge.
Patients in the PDs/NCSz group achieved a slightly worse outcome, with all 3 deaths occurring in that group and only 4 (17%) vs. 9 (64%) achieving low disability at discharge (p < 0.04 for comparisons of all outcome categories; Table 1). Nine (39%) patients in the PDs/NCSz group vs. 10 (71%) in the no PDs/NCSz group were discharged home (not significant). Twenty patients (59%) were still receiving anti-seizure medications at discharge, including 6 (43%) in the no PDs/NCSz group and 14 (61%) in the PDs/NCSz group (not significant).
Follow-up data at 6 months was available in 27 patients. No significant difference was observed between the two groups. Six (22%) patients were still receiving anti-seizure medications but none had seizures.
Discussion
We report a high prevalence of NCSz (43%), including NCSE (16%), and PDs (59%) in 37 critically ill patients with PRES undergoing CEEG. We also report an association between these CEEG findings on the one hand, and restricted diffusion on MRI and poor outcome, on the other hand. As suggested by prior case reports and series [8, 9, 11], both PDs and NCSz were mostly focal, either lateralized or bilateral independent, and were predominately in the posterior regions. None of the baseline clinical variables we investigated showed a statistically significant association with NCSz or PDs.
The etiology and radiological features in our sample are in line with most previous studies [7, 19,20,21]. However, with a higher prevalence of clinical seizures (84 vs. 70%) and encephalopathy (100 vs. 50–80%) in comparison with a general PRES population [1], our sample is more similar to a recent multicenter series of patients with PRES admitted to an ICU [19] and is not representative of the full spectrum of the syndrome [7, 20, 21]. This is likely due to one of our main inclusion criteria being the use of CEEG. Our cohort may thus be biased toward the most severe cases and overestimate the prevalence of PDs and NCSz in PRES. A similar prevalence of 10–15% of NCSE has been reported in critically ill patients with PRES receiving CEEG [8, 19].
We found an association between the presence of restricted diffusion on MRI and the presence of PDs or NCSz. The prevalence of diffusion abnormalities in our study (49%) is in the range of previous studies, albeit in the higher part of the range (1–33 and 63%) [3, 6]. Our findings indicate that NCSz or PDs may lead to the development of secondary cytotoxic edema in patients with PRES, as previously proposed by others [8], and reported in other patients with acute convulsive or NCSz and SE [22,23,24]. The reversibility of these changes, as observed in 73% of our cases as well as in most reported cases [5, 6, 25], further supports their peri-ictal rather than ischemic nature [22, 23]. This finding also has an important clinical implication: The presence of cytotoxic edema on imaging, especially in the cortex or hippocampus, in patients with PRES might be used to identify those who are at higher risk of NCSz and could benefit from CEEG.
We did not identify NCSz in all patients with diffusion abnormalities and some patients had more widespread, or too remote, diffusion abnormalities that would be expected if they were due to focal ictal activity. There are several possible, and complementary, explanations for this discrepancy. First, a minority (10–15%) [20] of patients with PRES develop irreversible ischemic injury, which might cause cytotoxic edema in the absence of PDs or NCSz. This was the case in one of our patients. Second, peri-ictal MRI abnormalities can occur in structures remote from the seizure focus. This is particularly the case with the thalamus and the hippocampus, which might display abnormalities in the setting of prolonged or frequent neocortical ictal activity, usually ipsilaterally [22, 23]. This was the case in three of our patients. Third, MRI abnormalities can persist for days after seizures and PDs have resolved [22, 23].
In our study, outcome was worse in patients with PDs or NCSz. Previous studies in PRES have underlined the benign nature of clinical seizures in PRES [9] but to our knowledge, none has systematically investigated the impact of NCSz and NCSE. Our findings are consistent with previous studies which found that the presence of NCSz and NCSE is associated with increased mortality, morbidity and long-term disability in acutely ill neurological patients [26] and might provide an explanation for the reported association between diffusion abnormalities in PRES and unfavorable clinical outcome [3].
In addition to the inclusion bias mentioned above, other limitations of our study include its retrospective design, although patients were prospectively identified. The timing between the clinical onset of PRES and CEEG or initial MRI was not standardized, although most CEEG were performed within 24 h. The study might have been underpowered due to its small sample size. This small sample also precluded us from investigating the impact of NCSz and PDs burden and of other electrographic features, such as frequency. Finally, follow-up imaging and neurological assessment were not systematically performed in all patients.
Conclusion
This study revealed a high prevalence of NCSz and PDs in critically ill patients with PRES undergoing CEEG, and an association between NCSz/PDs and both restricted diffusion and outcome. Their association with worse outcome suggests that they may contribute to neuronal injury and warrant aggressive treatment. Additionally, our results emphasize the role of CEEG to facilitate the prompt recognition and treatment of NCSz in this setting. These findings should be validated in a larger prospective cohort study.
References
Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914–25. https://doi.org/10.1016/S1474-4422(15)00111-8.
Roth C, Ferbert A. Posterior reversible encephalopathy syndrome: long-term follow-up. J Neurol Neurosurg Psychiatr. 2010;81:773–7. https://doi.org/10.1136/jnnp.2009.189647.
Schweitzer AD, Parikh NS, Askin G, Nemade A, Lyo J, Karimi S, Knobel A, Navi BB, Young RJ, Gupta A. Imaging characteristics associated with clinical outcomes in posterior reversible encephalopathy syndrome. Neuroradiology. 2017;59:379–86. https://doi.org/10.1007/s00234-017-1815-1.
Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. Am J Neuroradiol. 2002;23:1038–48.
Kastrup O, Schlamann M, Moenninghoff C, Forsting M, Goericke S. Posterior reversible encephalopathy syndrome: the spectrum of MR imaging patterns. Clin Neuroradiol. 2015;25:161–71. https://doi.org/10.1007/s00062-014-0293-7.
Moon S-N, Jeon SJ, Choi SS, Song CJ, Chung GH, Yu IK, Kim DH. Can clinical and MRI findings predict the prognosis of variant and classical type of posterior reversible encephalopathy syndrome (PRES)? Acta Radiol. 2013;54:1182–90. https://doi.org/10.1177/024185113491252.
Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85:427–32. https://doi.org/10.4065/mcp.2009.0590.
Kozak OS, Wijdicks EFM, Manno EM, Miley JT, Rabinstein AA. Status epilepticus as initial manifestation of posterior reversible encephalopathy syndrome. Neurology. 2007;69:894–7. https://doi.org/10.1212/01.wnl.0000269780.45472.16.
Kastrup O, Gerwig M, Frings M, Diener H-C. Posterior reversible encephalopathy syndrome (PRES): electroencephalographic findings and seizure patterns. J Neurol. 2012;259:1383–9. https://doi.org/10.1007/s00415-011-6362-9.
Sha Z, Moran BP, McKinney AM, Henry TR. Seizure outcomes of posterior reversible encephalopathy syndrome and correlations with electroencephalographic changes. Epilepsy Behav. EB. 2015;48:70–4. https://doi.org/10.1016/j.yebeh.2015.05.027.
Datar S, Singh T, Rabinstein AA, Fugate JE, Hocker S. Long-term risk of seizures and epilepsy in patients with posterior reversible encephalopathy syndrome. Epilepsia. 2015;56:564–8. https://doi.org/10.1111/epi.12933.
Kamiya-Matsuoka C, Tummala S. Electrographic patterns in patients with posterior reversible encephalopathy syndrome and seizures. J Neurol Sci. 2017;375:294–8. https://doi.org/10.1016/j.jns.2017.02.017.
Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–8.
Cordelli DM, Masetti R, Bernardi B, Barcia G, Gentile V, Biagi C, Prete A, Pession A, Franzoni E. Status epilepticus as a main manifestation of posterior reversible encephalopathy syndrome after pediatric hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2012;58:785–90. https://doi.org/10.1002/pbc.23344.
Ferreira FL, Bota DP, Bross A, Mélot C, Vincent J-L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–8. https://doi.org/10.1001/jama.286.14.1754.
Hirsch LJ, Laroche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, Mani R, Arif H, Jette N, Minazad Y, Kerrigan JF, Vespa P, Hantus S, Claassen J, Young GB, So E, Kaplan PW, Nuwer MR, Fountain NB, Drislane FW. American clinical neurophysiology society’s standardized critical care eeg terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. https://doi.org/10.1097/WNP.0b013e3182784729.
Beniczky S, Hirsch LJ, Kaplan PW, Pressler R, Bauer G, Aurlien H, Brøgger JC, Trinka E. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013;54(Suppl 6):28–9. https://doi.org/10.1111/epi.12270.
Bartynski WS, Boardman JF. Distinct Imaging Patterns and Lesion Distribution in Posterior Reversible Encephalopathy Syndrome. Am. J. Neuroradiol. 2007;28:1320–7. https://doi.org/10.3174/ajnr.A0549.
Legriel S, Schraub O, Azoulay E, Hantson P, Magalhaes E, Coquet I, Bretonniere C, Gilhodes O, Anguel N, Megarbane B, Benayoun L, Schnell D, Plantefeve G, Charpentier J, Argaud L, Mourvillier B, Galbois A, Chalumeau-Lemoine L, Rivoal M, Durand F, Geffroy A, Simon M, Stoclin A, Pallot J-L, Arbelot C, Nyunga M, Lesieur O, Troché G, Bruneel F, Cordoliani Y-S, Bedos J-P, Pico F. Critically III Posterior Reversible Encephalopathy Syndrome Study Group (CYPRESS), Determinants of recovery from severe posterior reversible encephalopathy syndrome. PLoS ONE. 2012;7:e44534. https://doi.org/10.1371/journal.pone.0044534.
Siebert E, Bohner G, Endres M, Liman TG. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome: does age make a difference?—A retrospective comparison between adult and pediatric patients. PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0115073.
Li Y, Gor D, Walicki D, Jenny D, Jones D, Barbour P, Castaldo J. Spectrum and Potential Pathogenesis of Reversible Posterior Leukoencephalopathy Syndrome. J Stroke Cerebrovasc Dis. 2012;21:873–82. https://doi.org/10.1016/j.jstrokecerebrovasdis.2011.05.010.
Cole AJ. Status epilepticus and periictal imaging. Epilepsia. 2004;45:72–7. https://doi.org/10.1111/j.0013-9580.2004.04014.x.
Cianfoni A, Caulo M, Cerase A, Marca GD, Falcone C, Lella GMD, Gaudino S, Edwards J, Colosimo C. Seizure-induced brain lesions: a wide spectrum of variably reversible MRI abnormalities. Eur J Radiol. 2013;82:1964–72. https://doi.org/10.1016/j.ejrad.2013.05.020.
Magnetic resonance imaging changes related to acute seizure activity—UpToDate, (n.d.). https://www-uptodate-com.ezproxy.ulb.ac.be/contents/magnetic-resonance-imaging-changes-related-to-acute-seizure-activity?source=search_result&search=peri%20ictal%20MRI&selectedTitle=9~150. Accessed August 23 2017.
Pande AR, Ando K, Ishikura R, Nagami Y, Takada Y, Wada A, Watanabe Y, Miki Y, Uchino A, Nakao N. Clinicoradiological factors influencing the reversibility of posterior reversible encephalopathy syndrome: a multicenter study. Radiat Med. 2006;24:659–68. https://doi.org/10.1007/s11604-006-0086-2.
Laccheo I, Sonmezturk H, Bhatt AB, Tomycz L, Shi Y, Ringel M, DiCarlo G, Harris D, Barwise J, Abou-Khalil B, Haas KF. Non-convulsive status epilepticus and non-convulsive seizures in neurological ICU patients. Neurocrit Care. 2015;22:202–11. https://doi.org/10.1007/s12028-014-0070-0.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
LB, BL, NR, EJG, LJH, NG: acquisition of data, analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
LB, BL, NR, and EJG have nothing to disclose. LJH has received research support from Yale University for investigator-initiated studies from Eisai and Upsher-Smith; consultation fees for advising from Ceribell, Eisai, Monteris, Neuropace, Sun Pharma, and Engage Therapeutics; royalties for authoring chapters for UpToDate-Neurology, chapters for Medlink—Neurology, and from Wiley for co-authoring the book “Atlas of EEG in Critical Care,” by Hirsch and Brenner; and honoraria for speaking from Neuropace. He spends about 25% of his clinical billable time implementing and interpreting critical care EEG studies. NG is a postdoctorate Clinical Master Specialist of the Fonds pour la Recherche Scientifique and has received research funding from le Fonds Erasme pour la Recherche Médical; royalties for authoring chapters for UpToDate-Neurology, and for authoring articles for Continuum (Neurology); and honoraria for speaking from UCB.
Ethical approval and informed consent
The study was approved by the institutional review boards of Hospital Erasme and of Yale University.
Rights and permissions
About this article
Cite this article
Bastide, L., Legros, B., Rampal, N. et al. Clinical Correlates of Periodic Discharges and Nonconvulsive Seizures in Posterior Reversible Encephalopathy Syndrome (PRES). Neurocrit Care 29, 481–490 (2018). https://doi.org/10.1007/s12028-018-0548-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-018-0548-2