Abstract
Background
To assess the acute and long-term outcomes for patients with lateralized rhythmic delta activity (LRDA) compared to patients with lateralized periodic discharges (LPDs).
Methods
A single-center retrospective study examining consecutive patients older than 10 years who had LRDA, LPDs, or both on continuous electroencephalographic (cEEG) between 12/01/2015 and 12/31/2017. Outcomes included inpatient mortality, functional outcome at follow-up, inpatient electrographic seizures, and the presence of new epilepsy at follow-up. Patients were classified into 4 groups: LRDA-only (without LPDs), LPDs-only (without LRDA), LRDA/LPDs, and control (without LRDA or LPDs).
Results
Twenty-nine patients (2.7%) were in the LRDA-only group, 76 (7%) patients were in the LPDs-only group, and 25 (2.3%) patients had both patterns (LRDA/LPDs group). 68 patients were identified as a control group. Only one patient (3%) in the LRDA-only group died during their hospitalization, compared to 21 patients (28%) in the LPDs-only group, 2 (8%) LRDA/LPDs group and 7 (10%) in the control group (p 0.003). Patients in the LPDs-only group had three times higher odds of adjusted mortality compared to the control group (p 0.05), while there was no difference in the mortality odds between the LRDA-only and control groups. Patients with LRDA-only had higher odds of good functional outcome at clinic follow-up (p 0.04). When compared to control, patients with both IIC patterns (LRDA/LPDs group) had 24.3 higher odds of acute electrographic seizures (p < 0.001), followed by patients in LPDs-only (OR 12.6, p < 0.001) and then LRDA-only (OR 9.4, p = 0.002). The odds of developing epilepsy following discharge were not increased in patients with either LRDA or LPDs (p = 0.9).
Conclusions
Patients with LRDA had superior functional outcome compared to a higher mortality for patients with LPDs. Patients with both patterns had the highest odds of acute seizures, followed by those with only LPDs and then patients with only LRDA. There was no difference in the odds of developing new epilepsy compared to control with any IIC pattern. We hypothesize different underlying mechanisms of injury leading to the observed electrographic patterns.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Continuous electroencephalographic (cEEG) monitoring is an important diagnostic tool in the management of seizures and encephalopathy in critically ill patients [1, 2]. Its adoption in intensive care units has expanded in the last decade, and recent reports suggest that its use improves overall patient outcomes [3, 4]. Interpreting cEEG in critically ill patients remains challenging, as some EEG patterns are neither clearly ictal nor clearly benign. Several rhythmic and periodic patterns including lateralized rhythmic delta activity (LRDA) and lateralized periodic discharges (LPDs) have been associated with seizures in this patient population [5,6,7]. The morphology of these patterns is well-described, and they are considered part of a spectrum between definite seizure and definite non-seizure activity known as the ictal-interictal continuum (IIC) [5, 8, 9].
LPDs have been the subject of many research studies, and have been independently associated with a high risk of acute seizure as well as overall poor outcome [10,11,12,13]. At the same time, little is known about LRDA in these contexts: Initial reports by Gaspard et al. [14] showed comparable clinical characteristics as well as a similar risk of acute seizures for both LRDA and LPDs. However, the acute and long-term outcomes, risk of developing epilepsy, and correlation with disease severity in patients with LRDA are unknown. Given the similar risk of acute seizures and the fact that several patterns on the IIC often occur together, it has been suggested that the outcome of patients with LRDA may be similar to that of patients with LPDs [5].
The objective of our study was to assess the acute and chronic outcomes for patients with these patterns, including in-hospital mortality, long-term functional outcomes, and acute and chronic seizure risks. We performed a retrospective study at our institution, comparing patients with LRDA, LPDs, or both EEG patterns to a control group of patients without lateralized rhythmic or periodic patterns on cEEG.
Methods
This was a single-center retrospective study of prospectively collected data. Our prospectively assembled clinical database of all continuous EEGs performed at the Johns Hopkins Hospital is regularly updated and coded according to the American Clinical Neurophysiology Society (ACNS) Standardized Critical Care EEG Terminology [15]. The use of de-identified data from the database in conjunction with the relevant data from the patient’s medical record has been approved by the institutional review board for research purposes. We used this database to identify consecutive patients older than 10 years who had prominent LRDA, LPDs, or both on continuous EEG between 12/01/2015 and 12/31/2017. We further identified all patients from the same period with non-specific, non-rhythmic, or periodic slow activity on continuous EEG who were otherwise matched based on etiology with similar age and sex as a second control group. While non-specific slow activity may have been present in the EEGs of the target group as well, these patients’ EEGs were notable for the absence of lateralized rhythmic or periodic patterns. LRDA and LPDs were defined according to the ACNS Standardized Critical Care EEG Terminology [15]. All continuous EEG records were reviewed at the time of their recording by a board-certified epileptologist and an epilepsy or neurophysiology fellow. Electronic medical records and the electroencephalography reports were reviewed.
Data extracted from the EEG reports included the presence of IIC patterns and their characteristics, presence of electrographic seizures, and the total number of days of cEEG recording during the hospital admission.
From the medical record, we collected demographics, prior history of epilepsy, the reason for the admission and/or presumed diagnosis underlying any EEG abnormality (taken together, as “etiology”), neurological examination at the time of cEEG, MRI findings, the maximum number of antiepileptic drugs (AEDs) during the admission, and the use of anesthetic agents.
We included the neurological examination as a marker of the severity of the clinical presentations. We divided the findings into 3 groups: Coma/stupor, encephalopathy (with moderate impairment of consciousness), and no alteration of consciousness (including patients with focal neurological deficits). For AEDs, we recorded the maximum number of agents used during the admission, while the use of anesthetic agents was collected as a binary factor. For the follow-up data, we collected hospitalization outcomes (disposition at discharge) and clinical follow-up information up to December 2018.
Primary outcomes included acute inpatient mortality, functional outcome at follow-up utilizing the modified Rankin Scale (mRS) classified as “good” (mRS 0–3) or “poor” (mRS 4–5), acute inpatient electrographic seizures, and the presence of new epilepsy at clinic follow-up.
We classified patients into 4 groups for statistical analysis: LRDA-only (without LPDs), LPDs-only (without LRDA), LRDA/LPDs, and control (non-ictal slow activity without LRDA or LPDs).
Statistical Analysis
We compared clinical characteristics and primary outcomes (mortality, acute electrographic seizures, development of epilepsy, and functional outcome) of patients in the 4 groups using a chi-square test or ANOVA as appropriate. A p value of 0.05 was considered significant for the primary analysis.
We used univariable and multivariable logistic regression models with the primary outcomes as the independent variables, and adjusted for etiology, age, sex, neurological exam and anesthetic use as markers of severity, and history of epilepsy. EEG pattern was included as a factor variable. For additional exploratory analysis to better define differences between patients with LRDA, LPDs, or both, we made additional head-to-head comparisons between the individual groups when significant differences between the four groups were found and used a Bonferroni correction to adjust significant p values.
We used Stata 15.0 (College Station, TX) for statistical analysis.
Data Availability
De-identified data can be obtained by request from any qualified investigator for the purposes of replicating procedures and results.
Results
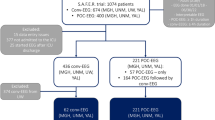
Out of the 1073 acutely ill patients monitored on cEEG over the 2-year period, 29 patients (2.7%) were in the LRDA-only group, 76 (7%) patients were in the LPDs-only group, and 25 (2.3%) patients had both IIC patterns (LRDA/LPDs group). We identified 68 patients matched for pathology with similar age and sex with nonspecific slow activity (without LRDA or LPDs) from the same time period. The median duration of cEEG was 2 days (IQR 1–4 days).
Demographics and Baseline Characteristics
There were no differences between the four groups regarding age, sex, pathology or brain MRI findings. The four groups differed with regards to prior history of epilepsy, neurological examination, maximum number of AEDs during the hospitalization, and anesthetic use. Demographics and baseline characteristics are outlined in Table 1.
Secondary exploratory analysis between the groups (using Bonferroni correction, an adjusted p value of 0.008 for significance) revealed that patients in the LRDA/LPDs and LPDs-only groups were more likely to have a prior history of epilepsy (60% and 54%, respectively, versus 10% in LRDA-only, and 31% of the control group, p < 0.001). There was no difference in the prior history of epilepsy between patients in the LRDA/LPDs and LPDs-only groups (p 0.6).
Patients in the LPDs-only group more frequently presented with a worse level consciousness when compared to the control group (p = 0.005), while there was only a trend towards a worse level of consciousness when compared to the LRDA-only (p 0.06). There were no differences in the level of consciousness between patients in the LRDA-only group compared to the control group (p = 0.84) or between patients in the LPDs-only group compared to the control group (p = 0.99). Patients in the LRDA/LPDs group had a trend towards a worse level of consciousness compared to patients in the control group (p = 0.05).
The most frequent etiology in LRDA-only patients was intracranial hemorrhage in 12 patients (41%), followed by brain tumors in 7 (24%), acute ischemic stroke in 4 (14%), CNS infectious and inflammatory disorders in 3 patients (10%), and miscellaneous (including traumatic brain injury, metabolic, genetic) in 3 patients (10%). Brain tumors and acute ischemic strokes were the most frequent etiologies in patients with LPDs-only group (Table 1).
The mean of the maximum number of AEDs used across the whole cohort was 1.9 ± 1.1. Thirty-one patients (15.7%) were on intravenous anesthetics for the treatment of either status epilepticus or elevated intracranial pressure. Across all groups, patients in the LRDA/LPDs or LPDs-only groups were on a higher mean number of AEDs compared to patients in either the LRDA-only or the control groups (p < 0.001). There was no difference in the maximum number of AEDs between patients in the LPDs-only and LRDA/LPDs groups (p = 0.8). Patients in the LPDs-only group were more likely to be on anesthetic medication when compared directly to either the LRDA-only group (p 0.005) or the control group (p < 0.001), while patients in the LRDA/LPDs group did not differ significantly from the other groups in terms anesthetics use.
Outcomes
Mortality
There were 31 (15.7%) inpatient deaths in our cohort. Only one patient (3%) in the LRDA-only and 2 patients (8%) in the LRDA/LPDs groups died during their acute hospitalization, in contrast to 21 patients (28%) of the LPDs-only group and 7 patients (10%) in the control group (p < 0.01) (Table 2). We performed a multivariable regression analysis of mortality, adjusting for age, sex, neurological examination, pathology, and anesthetic use.
There was no difference in adjusted mortality between patients in the LRDA-only group and patients in the control group (OR 0.46, 95% CI 0.05–4.35, p = 0.50, Table 3), while patients in the LPD-only group had a trend towards higher odds of adjusted mortality when compared to patients in the control group (OR 3.0, 95%CI 1.0–9.1, p = 0.05).
Upon exploratory analysis to compare the odds of mortality among the three groups (LRDA-only, LPDs-only, and LRDA/LPDs) (Table 4), patients in the LRDA-only group had a trend towards lower odds of adjusted mortality when compared to patients in the LPDs-only group (OR 0.16, 95% CI 0.02–1.4, p = 0.10). Similarly, patients in the LRDA/LPDs group had a similar trend of lower adjusted mortality odds when compared to patients in the LPDs-only group (OR 0.22, 95%CI 0.4–1.1, p = 0.07).
Acute Electrographic Seizures
About 1/3 of the cohort developed electrographic seizures during their monitoring period. The highest percentage was in the LRDA/LPDs group (60%), followed by LPDs-only (49%), LRDA-only (28%). Only 4 patients in the control group (6%) developed electrographic seizures (p < 0.001) (Table 2).
After adjusting for age, sex, prior history of epilepsy, pathology, and neurological examination, patients with both IIC patterns (LRDA/LPDs group) had 24.3 higher odds of acute in-hospital electrographic seizures compared to the control group (p < 0.001, CI 5.9–101). The odds ratios of electrographic seizures were 12.6 (p < 0.001, CI 3.8–41) and 9.4 (p = 0.002, CI 2.2–39) in the LPDs-only and LRDA-only groups, respectively, compared to control (Table 3).
Patients with any of these IIC patterns (either LRDA, LPD, or both) had 13.5 times higher odds of having acute electrographic seizures compared to the control group (p < 0.001, 95% CI 4.3–43). There was no difference in acute electrographic seizures in exploratory analysis when comparing the three groups (Table 4).
Patients with an IIC pattern (LRDA, LPDs, or both) at a frequency > 2 Hz were associated with 2.8 higher odds of having acute electrographic seizures (p 0.04, 95% CI 1.1–7.8) when compared to patients with lower frequency IIC pattern (< 2 Hz). Forty-four patients had a modifier with their IIC pattern. The presence of a modifier (+ S, + F, or + R) with any IIC pattern was associated with 2.5 higher odds of having acute electrographic seizures (p 0.03, 95% 1.1–5.8).
Outpatient Clinic Follow-up
Out of the 167 survivors, 131 (78%) patients had available follow-up in the outpatient clinic before December 2018. The median duration of follow-up for the entire cohort was 1 year (IQR 0.5–1.5). Of those who survived to discharge, 31% of LPD-only patients were lost to follow-up, compared to 11% and 13% of patients in LRDA only and control groups, (p = 0.02). There was no difference in whether a patient was lost to follow-up between the LRDA-only and the control groups (p = 0.60) or the LPDs-only and LRDA/LPDs (p = 0.70).
Functional Outcome
Good functional outcome scores on mRS (mRS 0–3) at outpatient follow-up were observed in 84% (21 patients) of the LRDA-only group, but only in 58% (22 patients) and 57% (31 patients) in the LPD-only and control groups (p = 0.07). Seven patients (47%) in the LRDA/LPDs group had a good functional outcome (Table 2).
After adjusting for covariates, patients in the LRDA-only group had higher odds of better functional outcome compared to the control group (OR 3.8, CI 1.0–13.3, p 0.04) (Table 3). There were no differences in the adjusted odds of good functional outcomes among patients in either LPDs-only or LRDA/LPDs compared to control patients.
New-Onset Epilepsy
Out of the 131 patients followed in the outpatient clinic, 45 patients were having chronic epilepsy. Sixteen patients developed new epilepsy following discharge from the hospital. There was no difference in the development of new-onset epilepsy between the 4 groups (p 0.92) (Table 2). There were no differences in both the unadjusted and the adjusted (for age, sex, etiology, and neurological examination) odds of developing new epilepsy between the groups (Table 3). Moreover, the presence of acute electrographic seizures was not associated with higher odds of developing new-onset epilepsy (p = 0.41, 95% CI 0.13–2.32) in the adjusted model.
Discussion
LRDA and LPDs are both patterns on the IIC and have been assumed to have a common pathophysiological mechanism while potentially conveying different information regarding epileptogenicity [14].
Our work is the first study to further differentiate LRDA and LPDs based on functional outcome and mortality. Importantly, we found that patients with LRDA had higher adjusted odds of a good functional outcome compared to patients with only non-rhythmic slowing, while patients with LPDs did not. Further, patients with LPDs had higher odds of mortality compared to control patients (which became a trend towards higher mortality after adjusting for age, sex, underlying etiology, neurological examination, and anesthetic use), while patients with LRDA did not. Although there were some differences in the underlying pathology of the groups, we addressed this with a multivariable regression analysis.
Both IIC patterns had significantly higher adjusted odds of in-hospital seizures than did the control group, with the highest odds in patients with both LRDA and LPDs. The long-term risk of seizures, however, was not significantly different between the groups with an IIC pattern after adjusting for all variables.
The fact that the presence of LPDs is independently associated with a higher risk of mortality has been described before [11,12,13]. We confirmed this finding in our cohort, after adjusting for underlying pathology and level of consciousness (as a surrogate marker for disease severity). No studies to date have looked at the association between LRDA and mortality. If LRDA had a similar pathophysiology as LPDs, an analogous association may be expected; however, our findings do not show this. Instead, only one patient (3%) in the LRDA-only group died, compared to 21 patients (28%) in the LPDs-only group. When LRDA and LPDs occurred concomitantly, there was a trend towards lower odds of death compared to LPDs without LRDA. There were no significant differences in the underlying brain pathology for either pattern, a finding that is in keeping with other studies [14]. Additionally, the functional outcome in the LRDA only group was significantly better than in any other group. Taken together, this suggests that LRDA is a reflection of a dynamic, functional process while LPDs are associated with static or a more permanent brain injury. The latter has been described before and LPDs are recognized as a brain injury pattern and independently associated with a poor prognosis [16, 17]. We suggest that LRDA, on the other hand, may indicate a more favorable prognosis due to its origins in a potentially reversible component of the underlying pathology.
The presence of both LRDA and LPDs predicted acute in-hospital electrographic seizures (odd ratios 9.4 vs 12.6). This is in keeping with the findings of Gaspard et al. [14] and Rodriguez Ruiz et al. [10]. These numbers also confirm that LPDs confer a higher risk of acute seizures than LRDA. We were further able to show that the presence of LRDA in addition to LPDs raises the odds ratio of acute seizures to 24.3. This supports the findings reported by Gaspard et al. [14] and confirms that both patterns likely represent different aspects of acute seizure generation [14]. At the same time, there was no difference in the odds ratio for development of incident epilepsy for either pattern compared to the control group, or when the groups were compared to each other. Further, the presence of acute electrographic seizure activity did not predict the development of epilepsy in our cohort. Therefore, the ultimate mechanism for developing chronic epilepsy is likely separate from the mechanisms underlying acute seizure generation. Certainly, multiple factors likely contribute to seizures in acutely ill patients. Prior reports concerning the risk of chronic epilepsy following acute LPDs have all been retrospective analyses and are somewhat conflicting [11, 18, 19], with some authors reporting an overall increase in the risk of chronic seizures as a result of LPDs. No literature regarding the chronic epilepsy risk as a sequela to LRDA exists before our study.
In fact, only two studies to date have directly examined the characteristics of LRDA and/or LRDA compared to LPDs. Gaspard et al. examined a cohort consisting of 558 patients, of which 27 subjects had LRDA and 49 subjects had LPDs. The authors reported significant overlap between the groups; LRDA-only or LPD-only groups were not examined separately. In our cohort, patients with LRDA were less likely to have a prior history of epilepsy compared to Gaspard et al.’s cohort (10% vs 22%) and were less likely to be stuporous or comatose (10% vs 63%), but the cohorts were otherwise similar. Rodriguez-Ruiz et al. reported on a large cohort of 4772 patients (of whom 7% of the cEEG sessions had LRDA) and examined the characteristics of IIC patterns and the risk of acute seizures.
Our study is the first to evaluate the acute and long-term outcomes of critically ill patients with LRDA. Importantly, we were able to look at patients who had only one pattern or the other, while also evaluating the effect of both patterns together on outcomes. We had an excellent follow-up in 78% of the patients with a median duration of 1 year (IQR 0.5–1.5). In addition, we adjusted for possible confounding factors including neurologic status at the time of EEG.
This study has several limitations. First, it is a retrospective single-center study, which has the potential to introduce bias for patient selection. A reporting bias can occur in a study based on chart review. Assessing the inter-rater variability was not possible, and the classification of electrographic patterns was based on the official clinical EEG read. An additional limitation is the slight difference in follow-up between the LPDs only group and the LRDA only group, which may introduce bias. However, patients with worse after-hospital outcomes may be more likely to be lost to follow-up [20], and so our finding of better outcomes in the LRDA only group is not expected to be affected by this limitation. The study is further limited by its relatively small sample size, which may explain that some of our findings did not reach statistical significance. The small sample size might also overestimate odd ratios due to the inherent mathematical properties of the logistic regression model [21].
Conclusions and Future Directions
LRDA and LPDs are both patterns on the IIC. Both have been associated with a risk of acute seizures. However, the contrasting findings of overall superior functional outcome for patients with LRDA compared to the increased mortality for LPDs suggest different underlying mechanisms of injury leading to the observed electrographic patterns. LRDA may be a reflection of a functional disturbance, while LPDs may be caused by a static injury. This hypothesis should be further explored in a larger cohort.
References
Young GB, Mantia J. Continuous EEG monitoring in the intensive care unit. Handb Clin Neurol. 2017;140:107–16.
Hirsch LJ. Continuous EEG monitoring in the intensive care unit: an overview. J Clin Neurophysiol. 2004;21:332–40.
Hill CE, Blank LJ, Thibault D, et al. Continuous EEG is associated with favorable hospitalization outcomes for critically ill patients. Neurology. 2019;92:e9–e18.
Ney JP, van der Goes DN, Nuwer MR, Nelson L, Eccher MA. Continuous and routine EEG in intensive care: utilization and outcomes, United States 2005–2009. Neurology. 2013;81:2002–8.
Johnson EL, Kaplan PW. Population of the ictal-interictal zone: the significance of periodic and rhythmic activity. Clin Neurophysiol Pract. 2017;2:107–18.
Rodriguez V, Rodden MF, LaRoche SM. Ictal-interictal continuum: a proposed treatment algorithm. Clin Neurophysiol. 2016;127:2056–64.
Claassen J. How I treat patients with EEG patterns on the ictal-interictal continuum in the neuro ICU. Neurocrit Care. 2009;11:437–44.
Kalamangalam GP, Pohlmann-Eden B. Ictal-interictal continuum. J Clin Neurophysiol. 2018;35:274–8.
Pohlmann-Eden B, Hoch DB, Cochius JI, Chiappa KH. Periodic lateralized epileptiform discharges—a critical review. J Clin Neurophysiol. 1996;13:519–30.
Rodriguez Ruiz A, Vlachy J, Lee JW, et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically Ill patients. JAMA Neurol. 2017;74:181–8.
Pedersen GL, Rasmussen SB, Gyllenborg J, Benedek K, Lauritzen M. Prognostic value of periodic electroencephalographic discharges for neurological patients with profound disturbances of consciousness. Clin Neurophysiol. 2013;124:44–51.
Claassen J, Hirsch LJ, Frontera JA, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006;4:103–12.
Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–65.
Gaspard N, Manganas L, Rampal N, Petroff OA, Hirsch LJ. Similarity of lateralized rhythmic delta activity to periodic lateralized epileptiform discharges in critically ill patients. JAMA Neurol. 2013;70:1288–95.
Hirsch LJ, LaRoche SM, Gaspard N, et al. American clinical neurophysiology society's standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27.
Witsch J, Frey HP, Schmidt JM, et al. Electroencephalographic periodic discharges and frequency-dependent brain tissue hypoxia in acute brain injury. JAMA Neurol. 2017;74:301–9.
Vespa P, Tubi M, Claassen J, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016;79:579–90.
Punia V, Bena J, Krishnan B, Newey C, Hantus S. New onset epilepsy among patients with periodic discharges on continuous electroencephalographic monitoring. Epilepsia. 2018;59:1612–20.
Punia V, Garcia CG, Hantus S. Incidence of recurrent seizures following hospital discharge in patients with LPDs (PLEDs) and nonconvulsive seizures recorded on continuous EEG in the critical care setting. Epilepsy Behav. 2015;49:250–4.
Wang GJ, Judelson DR, Goodney PP, Bertges DJ. Loss to follow-up 1 year after lower extremity peripheral vascular intervention is associated with worse survival. Vasc Med. 2019;24:332–8.
Nemes S, Jonasson JM, Genell A, Steineck G. Bias in odds ratios by logistic regression modelling and sample size. BMC Med Res Methodol. 2009;9:56.
Acknowledgements
The authors would like to thank Nirma Carballido Martinez, MSc Eng, R. EEG T., CLTM for her enormous contributions to the cEEG service at Johns Hopkins Hospital.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors was involved in study concept and design and in critical revision of the manuscript for important intellectual content. KH was involved in acquisition, analysis, or interpretation of data and in drafting of the manuscript. EJ and KH was involved in statistical analysis.
Corresponding author
Ethics declarations
Conflicts of interest
All authors report no conflict of interest.
Ethical approval
This study was approved by the institutional review board (IRB) in accordance with the declaration of Helisinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Husari, K.S., Johnson, E.L. & Ritzl, E.K. Acute and Long-Term Outcomes of Lateralized Rhythmic Delta Activity (LRDA) Versus Lateralized Periodic Discharges (LPDs) in Critically Ill Patients. Neurocrit Care 34, 201–208 (2021). https://doi.org/10.1007/s12028-020-01017-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-020-01017-y