Abstract
Background
Despite being a rare cause of stroke, spontaneous subarachnoid hemorrhage (SAH) is associated with high mortality rates. The prediction models that are currently being used on SAH patients are heterogeneous, and few address premature mortality. The aim of this study was to develop a mortality risk stratification score for SAH.
Methods
A retrospective study was carried out with 536 patients diagnosed with SAH who had been admitted to the intensive care unit (ICU) at the University Hospital Complex of A Coruña (Spain) between 2003 and 2013. A multivariate logistic regression model was developed to predict the likelihood of in-hospital mortality, adjusting it exclusively for variables present on admission. A predictive equation of in-hospital mortality was then computed based on the model’s coefficients, along with a points-based risk-scoring system. Its discrimination ability was also tested based on the area under the receiver operating characteristics curve and compared with previously developed scores.
Results
The mean age of the patients included in this study was 56.9 ± 14.1 years. Most of these patients (73.9%) had been diagnosed with aneurysmal SAH. Their median length of stay was 7 days in the ICU and 20 days in the general hospital ward, with an overall in-hospital mortality rate of 28.5%. The developed scales included the following admission variables independently associated with in-hospital mortality: coma at onset [odds ratio (OR) = 1.87; p = 0.028], Fisher scale score of 3–4 (OR = 2.27; p = 0.032), Acute Physiology and Chronic Health Evaluation II (APACHE II) score within the first 24 h (OR = 1.10; p < 0.001), and total Sequential Organ Failure Assessment (SOFA) score on day 0 (OR = 1.19; p = 0.004). Our predictive equation demonstrated better discrimination [area under the curve (AUC) = 0.835] (bootstrap-corrected AUC = 0.831) and calibration properties than those of the HAIR scale (AUC = 0.771; p ≤ 0.001) and the Functional Recovery Expected after Subarachnoid Hemorrhage scale (AUC = 0.814; p = 0.154).
Conclusions
In addition to the conventional risk factors for in-hospital mortality, in our study, mortality was associated with the presence of coma at onset of the condition, the physiological variables assessed by means of the APACHE II scale within the first 24 h, and the total SOFA score on day 0. A simple prediction model of mortality was developed with novel parameters assessed on admission, which also assessed organ failure and did not require a previous etiological diagnosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-traumatic or spontaneous subarachnoid hemorrhage (SAH) is a condition with a complex pathogenesis, which, despite representing the least frequent stroke subtype (5%) [1, 2] has devastating consequences and is associated with high morbidity and mortality rates [3, 4]. After surviving the initial hemorrhage, only 33% of these patients progress well after receiving treatment and 50% develop some type of secondary morbidity [5, 6].
Establishing a prognosis on admission is crucial in these patients and depends on the assessment of clinical variables associated with a certain degree of short- or long-term disability and the likelihood of death [7,8,9,10]. Prognostic factors with a positive impact should also be considered, such as those resulting from the advancement of both diagnostic and therapeutic radiological techniques.
Different types of prognostic scales have been applied to SAH patients, including the Glasgow Coma Scale (GCS) [11], despite not having been specifically developed for this pathology. The first prognostic indicators developed specifically for this condition were the Hunt and Hess scale (H–H) and the World Federation of Neurological Surgeons (WFNS) classification [12, 13], based on the patients’ level of consciousness and the clinical presentation on admission. These scales do not consider the failure of other systems, which may cause the risk of mortality to be underestimated. Another limitation is their ambiguous terminology, which may cause inter-observer variability [14]. Imaging tests also have prognostic implications, as they allow patients to be classified according to their bleeding pattern [9, 15,16,17]. Other relevant factors not included in traditional predictive models are the patient age, their neurological grade at admission, and the clot thickness revealed in computed tomography (CT) images [7, 18, 19].
Different scales have therefore been developed to better estimate the prognosis of SAH, such as the H–H, Age, Intraventricular hemorrhage, and Rebleed (HAIR) scale and the Functional Recovery Expected after Subarachnoid Hemorrhage (FRESH) scale [5, 20]. Most of these scales have significant limitations, such as the need to perform complex calculations or the inclusion of variables collected during the patients’ hospital stay [5, 21].
The predictive models currently used on patients with SAH are heterogeneous, and the best predictive scale is yet to be defined. Thus, without forgetting the relevance of updating and externally validating existing models, the development of new predictive models such as the one proposed in this work may have additional benefits because it allows us to explore the impact on mortality risk prediction of including the scales most often used to evaluate critical patients that were excluded from other studies. In this sense, we wanted to evaluate the role of organ failure in the prognosis of these patients. Hence, in this study we sought to develop a prognostic model for SAH that would allow the likelihood of in-hospital mortality to be for predicted based on patient admission variables with the use of simple calculations.
Methods
Study Design
This was a retrospective observational follow-up study of patients aged ≥ 15 years and diagnosed with SAH based on the criteria of the Neurocritical Care Society (NCS) [6] who were admitted to the ICU at the University Hospital Complex of A Coruña (CHUAC, Complexo Hospitalario Universitario de A Coruña) between 2003 and 2013 (n = 536). This ICU is equipped with 32 surgical beds and serves approximately 990 critical patients every year. Patients admitted to this unit are referred from the Emergency Room at the same clinic or from clinics located in the referral area. From the beginning of the study until 2009/2010, our hospital was a reference site for other health care areas in which neuro-interventionism was being developed and from which complex cases were referred to our unit.
Patients meeting the following criteria were excluded from the study: (a) a suspected diagnosis of subarachnoid hemorrhage not confirmed by imaging tests [CT/cranial magnetic resonance imaging (MRI) scan] or a lumbar puncture, (b) a post-traumatic subarachnoid hemorrhage, or (c) the existence of an aneurysm with no associated subarachnoid hemorrhage. Patients with a diagnosis of perimesencephalic SAH (PM-SAH) according to Rinkel’s criteria [15] were also excluded from the calculations used in the mortality prediction model because of the clearly differentiated prognosis of this subtype from that of aneurysmal (A-SAH) and idiopathic (I-SAH) SAH.
Parameters
Patient data were obtained by reviewing their medical records. Variables relating to their demographic characteristics (age and sex), comorbidities (assessed by Charlson index), SAH (delayed hospital arrival and symptomatology at onset), severity (GCS [11], H–H [12], WFNS [13], Fisher [18], Acute Physiology and Chronic Health Evaluation II (APACHE II) [22], and Sequential Organ Failure Assessment (SOFA) [23] scores), diagnosis (CT/MRI/arteriography), therapeutic management (embolization or surgical clipping), and complications occurring during the arteriography procedure were collected. Finally, data on the patient length of hospital stay (days), in-hospital mortality rates, and cause of death after the patients’ discharge from the hospital were also collected.
The clinical grade of the SAH on admission was assessed using the GCS, H–H, and WFNS scales. The Fisher scale was used to classify the severity of the hemorrhages revealed by initial CT scans images with a numerical value classified into low (1–2), medium (3), or high (4) grades.
Definitions
‘Coma at Onset’ was defined as a state of unconsciousness at the time of subarachnoid bleeding.
‘APACHE II in the first 24 h’ referred to the lowest value registered from the time of ICU admission until 24 h later.
‘SOFA day 0’ was defined as the lowest value registered from the time of ICU admission until 07:59 a.m. the next morning.
Therapeutic Management Protocol
All patients were initially admitted to the ICU, and then a multidisciplinary team discussed the decision-making process relating to their treatment. The therapeutic management of SAH in our unit consisted in the administration of prophylaxis for vasospasm (oral nimodipine 60 mg/4 h for 21 days), gastric stress ulcers, and deep vein thrombosis from the time of patient admission to the clinic, along with a personalized regimen of sedation and analgesia. The tests required to identify the cause of the hemorrhage were performed within the first 24–48 h in order to enable the classification of patients into 3 groups: A-SAH, I-SAH with a localized hemorrhage similar to that caused by an aneurysmal lesion, or PM-SAH (the latter was excluded due to being associated with a clearly differentiated prognosis compared to the other subtypes). The optimal treatment modality (surgery or an endovascular approach) was chosen based on the clinical condition of the patient and on the size, morphology, and location of the aneurysmal lesion. Possible neurological complications were monitored by neurological surveillance, a transcranial Doppler (TCD) ultrasound, and CT scan. When a patient was stabilized in the ICU, they were subsequently discharged to the Neurosurgery or the Neurology ward for surgery or another treatment, respectively.
Statistical Analyses
The descriptive statistics were summarized as means (and standard deviation) or as median values in the case of quantitative variables, and as frequencies (percentages) in the case of qualitative variables.
The sociodemographic and clinical characteristics of the patients who died during their stay in the hospital were compared with those of the ones who did not. Chi-squared tests or Fisher’s exact tests were used to compare categorical variables, and Student’s T test or Mann–Whitney test were used to compare the quantitative variables, as appropriate. Furthermore, the assumption of normality was checked with the Kolmogorov–Smirnov test. The univariate odds ratio (OR) and relative risk (RR) were also calculated for each variable together with their 95% confidence intervals (95% CIs).
A multivariate logistic regression model was then developed to predict in-hospital mortality. All the admission variables that appeared to be associated with the SAH outcome in the univariate analyses were considered and, only those with p values < 0.05 were used in the final model. Multicollinearity was assessed using tolerance and variance inflation factor (VIF). No evidence of multicollinearity was determined, with tolerance values above 0.10 and variance inflation factor values < 10 in all cases. None of the variables explained a variance proportion of > 0.5 of two or more of the other variables. Finally, the functional form of continuous predictors (linear vs. nonlinear relationships) was assessed using restricted cubic splines, taking the 50th percentile as a reference point.
We computed a predictive equation of in-hospital mortality with the coefficients obtained from this multivariate logistic regression model, so the risk of mortality for SAH patients is estimated based on the following equation (A):
with LP = a + b1X1 + b2X2 +··· + bpXp, being b1, b2, …., bp the regression coefficients associated to each of the variables in the final logistic model. Using the method described by Sullivan et al. [24], we also developed a points-based risk-scoring system based on the findings of the final logistic regression model. A bootstrapping method (1000 repetitions) was also used to obtain a relatively unbiased estimate of the model’s ability to distinguish between deceased patients and survivors based on the area under the receiver operating characteristics (ROC) curve. Furthermore, both the Hosmer–Lemeshow test and the Brier score were used to measure its calibration. Graphical representations of the relationship between the observed outcome frequencies and the predicted likelihoods (calibration curves) were also plotted. Finally, the discrimination ability of the proposed scores was compared with that of other published scales based on the AUCs.
Two-sided tests were also used, with p values < 0.05 being considered statistically significant. All the statistical analyses were performed using SPSS (version 24.0; IBM Corp., Armonk, NY) and R (version 2.12.2) software for Windows.
Ethical Aspects
At all times, the study was carried out respecting the ethical and legal requirements demanded by the applicable biomedical research regulations in force, and confidentiality was ensured in accordance with the provisions of the current data protection legislation. The study was approved by the Galician Clinical Research Ethics Committee (CAEIG, Comité Autonómico de Ética de Investigación de Galicia) (authorization code 2012/268).
Results
A total of 536 patients with a mean age of 56.9 ± 14.1 years, 60% of whom were women, were included in this study (Fig. 1). An aneurysm was detected in 73.9% of the patients (n = 396). Of the remaining patients, 16 (3.0%) had a PM-SAH and 90 (16.8%) had an I-SAH. The neurological severity of the condition on admission was mild in most cases, with 60.3% of the patients having a GCS of 14–15, 61.9% with a H–H grade of I–II, and 60.4% had a WFNS grade of I–II, except for the Fisher scale, which classified 50.7% of the cases as grade IV. At the time of patient admission to the ICU, the APACHE II and SOFA severity scales yielded mean scores of 11.8 ± 6.8 and 2.4 ± 2.8, respectively. The neurological complications observed during the patients’ stays in the unit were, in a decreasing order of frequency, coma (46.5%), vasospasm (35.4%), hydrocephalus (28.0%), and, finally, rebleeding (7.8%).
The mean length of stay of the patients was 9.8 ± 9.1 days (median stay = 7 days) in the ICU and 36.5 ± 90.2 days (median stay = 20 days) in the general hospital ward. In-hospital mortality was calculated at 28.5% (95% CI = 24.6–32.5%).
None of the patients with PM-SAH died during their stay in the hospital, and this condition was associated with the shortest lengths of stay both in the ICU (median length = 3.5 days) and in the general hospital ward (median length = 19.5 days). The in-hospital mortality rates and lengths of stay in the ICU for patients with A-SAH were much higher than those of the patients with I-SAH [28.5% vs. 12.2% (p = 0.001); median length of stay of 9 days vs. 4 days], with no differences being observed in the median length of stay in the general hospital ward at around 21 days.
Table 1 shows the association of different admission variables with the in-hospital mortality rates seen among our patient population calculated by means of a bivariate analysis. The multivariate analysis determined that the variables that were independently associated with an increased risk of in-hospital death were coma at onset (OR = 1.87; p = 0.028), a Fisher scale score of 3–4 (OR = 2.27; p = 0.032), the total APACHE II score within the first 24 h (OR = 1.10; p < 0.001), and the total SOFA score on day 0 (OR = 1.19; p = 0.004) (Table 2). We found that the existence of an aneurysmal lesion was not significantly associated with a higher likelihood of in-hospital death when adjusting the analysis for the remaining variables. No evidence of multicollinearity was determined, with tolerance values above 0.10 and variance inflation factor values < 10 in all cases. None of the variables explained a variance proportion of > 0.5 of two or more of the other variables.
We then developed a numerical scoring system that allowed for estimating the risk of in-hospital mortality for patients with SAH, according to equation (A), where LP = − 3.665 + 0.625 × (comma at onset = yes) + 0.817 × (Fisher scale score = 3 − 4) + 0.177 × total SOFA score on day 0 + 0.098 × APACHE II score within the first 24 h.
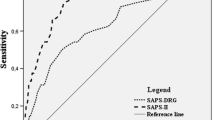
The area under the ROC curve for this equation was 0.835, and so it demonstrated good discrimination and calibration properties (Fig. 2), and the bootstrap-corrected area under the ROC curve was 0.831. These figures for patients with SAH were higher than those obtained with the HAIR (AUC = 0.771; p = 0.154), FRESH (AUC = 0.814; p < 0.001), or other neurological assessment scales (Fig. 3). The calibration graph also revealed a good concordance between the likelihood of predicted death and in-hospital mortality observed throughout the scale’s score range (corrected Brier score = 0.142).
We also developed a points-based prediction scoring system (Table 3), in which the score obtained with the regression model ranged from 0 to 10 points, with a score of 0 corresponding to the lowest risk of mortality. The area under the ROC curve for the points-based score was 0.825 (95% CI = 0.784–0.866). This score also demonstrated a good calibration (Hosmer–Lemeshow test: χ2 = 0.065; p = 0.999) (Brier’s score: B = 0.005) (Fig. 4).
Discussion
This study provides a prediction model of in-hospital mortality for HAS patients based on the clinical information available within the first 24 h of the patients’ admission to the clinic, disregarding any other in-hospital complications that could have potentially impacted mortality rates. This new model includes variables that were previously untested in this patient population, such as the total SOFA score on day 0, and other parameters that had been associated as indicators of poor long-term prognosis, such as coma at onset of the condition [25, 26], along with variables that had already been used in other predictive models [5, 20], including a Fisher scale score of 3–4 on admission and the assessment of the patients’ physiological condition based on their APACHE II score within the first 24 h. We used these variables to develop a numerical and a points-based model that demonstrated good discrimination and calibration properties for the early identification of patients with a high risk of in-hospital mortality.
In this study, in-hospital mortality was 27.8%, with the mortality rate being significantly higher among patients with A-SAH (15–42% based on the patient condition on admission) [27,28,29] and is also correlated with a greater number of complications. This in-hospital mortality rate fell within the ranges described in other studies [6, 30, 31], but was lower than the rates described over past decades, most likely owing to the recent advances made in the field of critical care that minimize the consequences of initial bleeding and secondary brain injury [1, 3, 32, 33].
Although several studies have analyzed the long-term mortality of SAH, very few address premature mortality and its contributing factors [25, 28, 29, 32, 34, 35]. The first prognostic scales of mortality used in patients with SAH were the H–H and WFNS neurological assessment scales [12, 13]. Although neither of these clinical scales were created to predict mortality, they are used as clinical indicators of severity and, therefore, as indirect prognostic markers. In addition, other authors have examined the validity of isolated physiological parameters or generic risk scales to determine the likelihood of death among critically ill patients [36, 37].
The first prognostic model used to predict in-hospital mortality described in the literature was the HAIR scale developed by Lee et al. [5] within the context of a retrospective study performed with 400 patients with SAH. This scale is based on four criteria assessed within the first 24 h of admission: severity of the initial bleeding (assessed according to the H–H scale and intraventricular hemorrhage), age, and rebleeding within the first 24 h. This model provides three risk strata that simplify the classification process: low risk (score: 0–2) associated with a 4.8% likelihood of death, moderate risk (score: 3–5) associated with a 60% likelihood of death, and high risk (score: 6–8) associated with a 71.4% likelihood of death. However, the decision to prematurely remove a patient’s life support measures constituted the main cause of death in this study. According to the authors themselves, this could be a major limitation of the HAIR score, which could underestimate mortality in patients with early care limitations and overestimate mortality in those without early do-not-resuscitate orders. Abulhasan et al. [31] recently validated the HAIR scale in a retrospective study performed with 434 patients with SAH. In their case series, they found that the primary factors associated with mortality among their sample of patients were age, H–H score, and the existence of an intracerebral hemorrhage. They also discovered that the HAIR scale adequately distinguished patients who would die from those who would survive, with an AUC of 0.89, but that it tended to overestimate the predicted mortality.
In a study performed with 279 patients with A-SAH, Maragkos et al. [38] also recently proposed using another scale to predict functional prognosis and mortality after hospital discharge based on 4 criteria: a GCS score ≤ 8 after the patients’ initial resuscitation, an age ≥ 70 years, a previous line of antiplatelet treatment, and a clot thickness in the subarachnoid space > 10 mm, with a score of 0–5. The prediction of mortality was found to be reliable with an AUC of 0.825, which was higher than that obtained for the WFNS scale, the modified Fisher scale, or Ogilvy’s scale, and it had predictive ability similar to that of the H–H scale. However, the use of this scale was limited to patients with aneurysmal lesions, which consequently conditioned its validity in patients with SAH on admission and means that it requires external validation.
The variables used in our predictive model of in-hospital mortality differ greatly from those used in previous studies, including the presence of coma at onset to assess the initial neurological severity of the condition, the assessment of the patients’ physiological condition based on the APACHE II score within the first 24 h, and the assessment of the existence of organ failure according to the total SOFA score on day 0. In our model, the presence of coma at onset had the greatest prognostic impact on the assessment of the initial neurological severity of the pathology regardless of the patients’ neurological condition on admission. Thus, our model was likely superior to the H–H scale because it simplified the scale’s calculations and reduced its subjectivity. For its part, the APACHE II scale considers the patients’ physiological condition within the first 24 h of admission, and, when assessed in our case series, proved to be a relevant prognostic factor for short-term mortality, thus improving the predictive power of our model. The inclusion of the APACHE II scale in our model could have influenced the fact that, unlike in most mortality prognosis equations [5], the age of the patients included in our study seemed to have no impact on the frequency of in-hospital mortality, because this parameter is already included within the variables assessed by this scale. The degree of organ failure assessed by the SOFA scale has become increasingly relevant in prognostic studies of mortality performed with this patient population in recent years [30, 39], although it has not yet been included in predictive models. Hence, the inclusion of this scale in predictive models used on patients with SAH is a novelty, assigning it prognostic importance (by adequately grading organ failure) similar to that attributed to its use with other patient populations.
Finally, we compared the ability of the logistic regression model developed in our study to predict in-hospital mortality with that of the severity scales used on admission (GCS, H–H, WFNS, Fisher, APACHE II, and total SOFA score on day 0) and of the predictive models developed by Lee et al. [5] and Witsch et al. [20], although the latter was developed to predict long-term prognosis. The AUC obtained for our model was significantly greater than that obtained for the severity scales, thus demonstrating its superiority over the predictive equations developed by Lee et al. and Witsch et al. Hence, when we used the HAIR scale to predict in-hospital mortality, we found that its predictive ability was not remarkably better than that of the other neurological scales (AUC = 0.77), with the FRESH scale being the closest one to our model (AUC = 0.814).
Our model has some advantages over previous ones, the main one being that it allows an individual estimation of the likelihood of in-hospital mortality to be obtained based on a limited number of factors that are easily assessable and that can be evaluated at early stages, as it happens with the HAIR score. Secondly, the inclusion of the presence of coma at onset of the condition as a variable in the model allows the assessment to be simplified so that it can be applied at the patients’ bedside and without the need for a prior diagnosis in order to establish the prognosis. Thirdly, other models do not include scales that assess the patients’ physiological condition, such as the APACHE II scale, nor do they rate the degree of organ failure with scales such as the SOFA, and consequently, our model allows patients to be more comprehensively.
Despite its advantages, our study also had some limitations. Firstly, covered a period of 10 years, meaning that the likelihood of death could have been related to changes in the treatment protocols for patients with SAH introduced during this time [40, 41]. Secondly, all the patients included in this study were treated at tertiary hospital received patients transferred from other hospitals, which could have affected the representativeness of our study. Thirdly, this study was subject to the limitations inherent to all retrospective studies, including bias caused by missing data. Moreover, the proposed model included several variables related to the altered level of consciousness. Although a multicollinearity analysis was performed, it cannot be ruled out that this issue could have influenced the associated effect sizes and maybe the associations themselves. Finally, this study only represents the clinical experience at our hospital, and so our results must be validated externally with an independent cohort. Nevertheless, despite these limitations, the scale that we developed is a reliable and useful tool that represents the experience of our hospital, can be applied without requiring a previous etiological diagnosis, and includes data from an initial radiological assessment.
Conclusions
In addition to the conventional risk factors for in-hospital mortality, in our study, mortality was associated with the presence of coma at onset of the condition, the physiological variables assessed by means of the APACHE II scale within the first 24 h, and the total SOFA score on day 0. This allowed us to develop a simple predictive model of mortality that includes parameters obtained on admission, does not require a previous etiological diagnosis, and assesses the existence of organ failure, which suggests that improving management of non-neurologic organ dysfunction could reduce the risk of in-hospital mortality among patients with SAH. Although this model demonstrated a good discrimination ability in our patient population, it still requires external validation.
References
Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology. 2010;74(19):1494–501.
van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124(Pt 2):249–78.
Ingall T, Asplund K, Mähönen M, Bonita R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke. 2000;31(5):1054–61.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–292.
Lee VH, Ouyang B, John S, Conners JJ, Garg R, Bleck TP, et al. Risk stratification for the in-hospital mortality in subarachnoid hemorrhage: the HAIR score. Neurocrit Care. 2014;21(1):14–9.
Szklener S, Melges A, Korchut A, Zaluska W, Trojanowski T, Rejdak R, et al. Predictive model for patients with poor-grade subarachnoid haemorrhage in 30-day observation: a 9-year cohort study. BMJ Open. 2015;5(6):e007795.
Jaja BN, Cusimano MD, Etminan N, Hanggi D, Hasan D, Ilodigwe D, et al. Clinical prediction models for aneurysmal subarachnoid hemorrhage: a systematic review. Neurocrit Care. 2013;18(1):143–53.
Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38(8):2315–21.
Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–17.
Josephson SA, Douglas VC, Lawton MT, English JD, Smith WS, Ko NU. Improvement in intensive care unit outcomes in patients with subarachnoid hemorrhage after initiation of neurointensivist co-management. J Neurosurg. 2010;112(3):626–30.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–4.
Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28(1):14–20.
Drake CG. Report of World Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988;68(6):985–6.
Rosen DS, Macdonald RL. Subarachnoid hemorrhage grading scales: a systematic review. Neurocrit Care. 2005;2(2):110–8.
Rinkel GJ, Wijdicks EF, Vermeulen M, Ramos LM, Tanghe HL, Hasan D, et al. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: CT and MR patterns that differ from aneurysmal rupture. AJNR Am J Neuroradiol. 1991;12(5):829–34.
van Gijn J, van Dongen KJ, Vermeulen M, Hijdra A. Perimesencephalic hemorrhage: a nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology. 1985;35(4):493–7.
Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6(1):1–9.
Lagares A, Gómez PA, Alen JF, Lobato RD, Rivas JJ, Alday R, et al. A comparison of different grading scales for predicting outcome after subarachnoid haemorrhage. Acta Neurochir (Wien). 2005;147(1):5–16 (discussion 16).
Lo BW, Fukuda H, Nishimura Y, Farrokhyar F, Thabane L, Levine MA. Systematic review of clinical prediction tools and prognostic factors in aneurysmal subarachnoid hemorrhage. Surg Neurol Int. 2015;6:135.
Witsch J, Frey HP, Patel S, Park S, Lahiri S, Schmidt JM, et al. Prognostication of long-term outcomes after subarachnoid hemorrhage: the FRESH score. Ann Neurol. 2016;80(1):46–58.
Jaja BNR, Saposnik G, Lingsma HF, Macdonald E, Thorpe KE, Mamdani M, et al. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: the SAHIT multinational cohort study. BMJ. 2018;360:j5745.
Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9(8):591–7.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10.
Sullivan LM, Massaro JM, D’Agostino RB. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23(10):1631–60.
Suwatcharangkoon S, Meyers E, Falo C, Schmidt JM, Agarwal S, Claassen J, et al. Loss of consciousness at onset of subarachnoid hemorrhage as an important marker of early brain injury. JAMA Neurol. 2016;73(1):28–35.
Hendrix P, Foreman PM, Senger S, Burkhardt BW, Harrigan MR, Fisher WS, et al. Loss of consciousness at onset of aneurysmal subarachnoid hemorrhage in good-grade patients. Neurosurg Rev. 2019. https://doi.org/10.1007/s10143-019-01142-z.
Olsen MH, Orre M, Leisner ACW, Rasmussen R, Bache S, Welling KL, et al. Delayed cerebral ischaemia in patients with aneurysmal subarachnoid haemorrhage: functional outcome and long-term mortality. Acta Anaesthesiol Scand. 2019;63:1191–9.
Sarabia R, Lagares A, Fernández-Alén JA, Arikan F, Vilalta J, Ibáñez J, et al. Idiopathic subarachnoid hemorrhage: a multicentre series of 220 patients. Neurocirugia (Astur). 2010;21(6):441–51.
Schuss P, Hadjiathanasiou A, Borger V, Wispel C, Vatter H, Güresir E. Poor-grade aneurysmal subarachnoid hemorrhage: factors influencing functional outcome—a single-center series. World Neurosurg. 2016;85:125–9.
Lantigua H, Ortega-Gutierrez S, Schmidt JM, Lee K, Badjatia N, Agarwal S, et al. Subarachnoid hemorrhage: who dies, and why? Crit Care. 2015;19:309.
Abulhasan YB, Alabdulraheem N, Simoneau G, Angle MR, Teitelbaum J. Mortality after spontaneous subarachnoid hemorrhage: causality and validation of a prediction model. World Neurosurg. 2018;112:e799–811.
Lago A, López-Cuevas R, Tembl JI, Fortea G, Górriz D, Aparici F, et al. Short- and long-term outcomes in non-aneurysmal non-perimesencephalic subarachnoid hemorrhage. Neurol Res. 2016;38(8):692–7.
Delgado-López PD, López-Martínez JL, Gero-Escapa M, Martín-Alonso J, Castaño-Blazquez M, Ossa-Echeverri S, et al. Aneurysmal subarachnoid haemorrhage: results after introducing endovascular therapy in a medium-low volume centre. Neurocirugia (Astur). 2016;27:207–19.
Lagares A, Gómez PA, Alén JF, Arikan F, Sarabia R, Horcajadas A, et al. Aneurysmal subarachnoid hemorrhage: group of study of Cerebrovascular Pathology of the Spanish Society of Neurosurgery management guideline. Neurocirugia (Astur). 2011;22(2):93–115.
von Vogelsang AC, Forsberg C, Svensson M, Wengström Y. Patients experience high levels of anxiety 2 years following aneurysmal subarachnoid hemorrhage. World Neurosurg. 2015;83(6):1090–7.
Claassen J, Vu A, Kreiter KT, Kowalski RG, Du EY, Ostapkovich N, et al. Effect of acute physiologic derangements on outcome after subarachnoid hemorrhage. Crit Care Med. 2004;32(3):832–8.
Schuiling WJ, de Weerd AW, Dennesen PJ, Algra A, Rinkel GJ. The simplified acute physiology score to predict outcome in patients with subarachnoid hemorrhage. Neurosurgery. 2005;57(2):230–6 (discussion-6).
Maragkos GA, Enriquez-Marulanda A, Salem MM, Ascanio LC, Chida K, Gupta R, et al. Proposal of a grading system for predicting discharge mortality and functional outcome in patients with aneurysmal subarachnoid hemorrhage. World Neurosurg. 2019;121:e500–10.
Basile-Filho A, Lago AF, Menegueti MG, Nicolini EA, Nunes RS, Lima SL, et al. The use of SAPS 3, SOFA, and Glasgow Coma Scale to predict mortality in patients with subarachnoid hemorrhage: a retrospective cohort study. Medicine (Baltimore). 2018;97(41):e12769.
Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711–37.
Diringer MN, Bleck TP, Claude Hemphill J, Menon D, Shutter L, Vespa P, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s multidisciplinary consensus conference. Neurocrit Care. 2011;15(2):211–40.
Funding
This study has no sources of external funding.
Author information
Authors and Affiliations
Contributions
MMF contributed to the data collection, literatura search, study design, data interpretation, writing. SP contributed to the data analysis, data interpretation, writing. RG contributed to the data interpretation, critical revision.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
This study was performed in compliance with the Helsinki Declaration and approved by the local ethics committee (code: 2012/268).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mourelo-Fariña, M., Pértega, S. & Galeiras, R. A Model for Prediction of In-Hospital Mortality in Patients with Subarachnoid Hemorrhage. Neurocrit Care 34, 508–518 (2021). https://doi.org/10.1007/s12028-020-01041-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-020-01041-y