Abstract
Background
Early prediction of increased morbidity and mortality in aneurysmal subarachnoid hemorrhage (aSAH) remains crucial to improving patient management. Most prediction models lack external validation and focus on disease-specific items without considering physiological parameters and the past medical history. The aim was to assess the validity of the established Simplified Acute Physiology Score II (SAPS-II) in an aSAH cohort for the prediction of hospital mortality and to identify additional physiological and clinical predictors.
Methods
The predictive value of SAPS-II for hospital mortality was assessed in a retrospective analysis of 263 consecutive patients with aSAH. Additional physiological and clinical parameters including the past medical history were analyzed by forward selection multivariate analysis to identify independent predictors of hospital mortality and to improve the prediction model.
Results
The SAPS-II predicted hospital mortality with an area under the curve (AUC) of 0.834 with an odds ratio (OR) of 1.097 [95 % confidence interval 1.067-1.128) for each additional point. Forward selection multivariate analysis identified the Glasgow Coma Scale score (P < 0.001), history of chronic headache (P = 0.01) and medication with anticoagulants (P = 0.04) as independent predictors of hospital mortality. Adding these parameters to the SAPS-II, the AUC increased to 0.86.
Conclusion
This study validates the predictive accuracy of SAPS-II for hospital mortality in aSAH patients. Additional parameters from the past medical history increase its predictive power. From a practical viewpoint, SAPS-II alone already represents a sufficient and powerful score to predict hospital mortality at an early time point and may help to improve patient management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is still a life-threatening disease despite the improvement of treatment strategies in the last decades [20]. While the reported mortality decreased from around 50 to 17 % in a recently published study, the early stratification of patients at risk for hospital mortality (HM) remains crucial in order to optimize patient management during the early critical disease course [1, 4].
A multitude of general and specific neurological parameters such as age, Fisher grade, World Federation of Neurological Surgeons (WFNS) grade, aneurysm size and Hunt & Hess grade are commonly accepted to identify patients with a high risk of mortality after aSAH [3, 5, 10, 13, 15, 17, 18, 21, 26–28, 31]. However, these parameters provide only a crude prediction of the clinical course; thus, improved and validated prediction models are clearly needed [13]. Additional studies focused on medical complications during the treatment of aSAH patients and identified fever, anemia and hyperglycemia as prognostic factors [34]. Only a few studies evaluated systemic physiological variables at the early stage of aSAH treatment and identified these as independent predictors of a poor outcome resulting in a new predictive model [3]. A major limitation of most predictive models is the lack of external validation as only the external validation in a new or external patient cohort proves the validity [13]. One of the most validated predictive models for HM in general is the simplified acute physiology score II (SAPS-II), which was introduced in 1993 and contains a mixture of physiologic parameters and general predictors such as age and the Glasgow Coma Scale (GCS) score [16]. Even though the SAPS-II is well established, only two studies have assessed this score for the prediction of outcome and HM in cohorts of aSAH patients with valid accuracy [22, 29]. Apart from physiologic parameters, the SAPS-II also contains parameters from the medical history such as chronic diseases. The relevance of existing disorders prior aSAH is conflicting since Mocco et al. failed to identify a significant influence of the past medical history on the clinical outcome, while others identified a history of hypertension to be an independent predictor [18, 27]. Taken together, apart from the commonly used predictors such as the Hunt & Hess or WFNS grade, age and Fisher grade, the predictive value of other physiological and clinical parameters or the past medical history differs from study to study.
The aim of this study was to assess the validity of the established SAPS-II in a modern aSAH cohort for the prediction of HM and to identify other possibly relevant physiological or clinical predictors including parameters from the past medical history. The results were further analyzed with the overall goal to improve the predictive accuracy of the SAPS-II by using additional relevant parameters available in the first 24 h after admission.
Methods
Study population and treatment
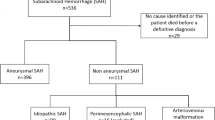
This is a retrospective analysis of 317 patients suffering from SAH admitted to our institution between November 2010 and November 2014. Fifty-four patients were excluded because of angiogram-negative perimesencephalic SAH, leaving 263 patients included in this study. No patients with arteriovenous malformation or any syndrome were included in this study. In accordance with local and institutional laws and data protection regulations, no approval by the local ethics committee was necessary as all data had been made anonymous prior to evaluation.
The treatment of the ruptured aneurysm by either intraoperative clipping or endovascular coil embolization followed an interdisciplinary discussion between the departments of neurosurgery and neuroradiology. If the clinical condition was too poor, no further treatment was performed. In two patients definite identification of the rupture site was not possible because of incomplete diagnostics due to the poor clinical condition.
All patients were treated at the intensive care unit (ICU) according to standard SAH guidelines and treatment protocols with nimodipine application, balanced fluid management aiming for normovolemia, and normo- to hypertensive arterial blood pressures. Nutrition of all patients followed a combined enteral and parenteral protocol as described before [14].
Data collection
The collection of the SAPS-II Diagnosis-Related Groups (DRG) was done semiautomatically using the “Integrated Care Manager” (ICM) by Dräger Medical Deutschland GmbH, Lübeck, Germany, which filters all data in a given 24-h period for the worst SAPS DRG score. This semiautomatically prepared SAPS-II DRG score is identical to the original SAPS-II except that the GCS is not included. After proving the validity of each semiautomatically calculated SAPS-II DRG by the authors, the first corresponding GCS points were added to the SAPS-II DRG resulting in the original SAPS-II score. The SAPS-II consists of 17 variables including age; heart rate; systolic blood pressure; body temperature; if the patient is ventilated, the partial pressure of oxygen in arterial blood/fractional inspired oxygen (PaO2 mmHg/FiO2); urinary output; serum urea level; white blood cell count; serum potassium level; serum sodium level; serum bicarbonate level; bilirubin level; GCS; autoimmune deficiency syndrome (AIDS); hematological malignancy; metastatic cancer; the type of admission (scheduled surgical, unscheduled surgical or medical) [16]. AIDS, hematological malignancy and metastatic cancer are pooled as chronic diseases as described by Le Gall et al., leaving 15 variables in the SAPS-II for further analysis [16]. All references within the result section are synonymous with an SAPS-II score of 0 representing the variable with the lowest mortality risk or the baseline characteristics. The maximum value of the SAPS-II is 154 points.
The severity of the aSAH was measured by the Hunt & Hess grade. Localization of aneurysms was categorized into two groups based on digital subtraction angiography or computer tomography angiography. The anterior circulation group contained aneurysms of the anterior cerebral, anterior communicating, internal carotid and middle cerebral artery. Posterior aneurysms included aneurysms of the basilar artery, posterior communicating artery, vertebral artery and its branches. Beyond that, we collected the Fisher grade on the initial CCT, diameter and quantity of aneurysms, the treatment of the aneurysm by either intraoperative clipping or endovascular coil embolization, the initial intracranial pressure (ICP) and the highest ICP within 24 h after admission (if measured; otherwise the patient was allocated to the reference group) and treatment with anticoagulation drugs (e.g., acetylsalicylic acid, clopidogrel or phenprocoumon) or nonsteroidal antiinflammatory drug (NASIDs; e.g., ibuprofen). In addition, the medical history of all patients was investigated for known diseases and/or medications. Initial cerebral herniation was defined as either one or both dilated pupils. Chronic headache was defined based on a medical history with either migraine or any other recurrent kind of headache such as tension headache, cluster headache, etc. All patients treated with antihypertensive drugs were defined as positive for arterial hypertension.
Data collection for 21 patients was incomplete because of no treatment of the aneurysm due to the moribund clinical condition and/or the knowledge of the patient’s provision.
Data analyses
As a first step of the statistical analysis, the influence of the SAPS-II and separately each of its 17 components on the HM in aSAH patients was calculated by univariate logistic regression. We also performed univariate logistic regressions for the additional parameters. The level of significance was set to <0.05.
The SAPS-II scores were divided into terciles to analyze the risk of HM with increasing SAPS-II as well as for continuous SAPS-II. SAPS-DRG and SAPS-II scores were used to calculate the receiver-operating curve (ROC) with the value of the area under the curve (AUC) and 95 % confidence interval (CI) HM in the univariate model.
In a second step, a multivariate logistic regression was used to identify additional parameters to improve the prediction of the SAPS-II. All parameters from the univariate logistic regression model were used as potential predictors. In order to assess the validity of this model for the prediction of mortality for the following course of the disease, the 21 patients with an incomplete data set due to poor clinical conditions at admission were excluded. IBM® SPSS® Statistics 19 (IBM Corp., Armonk, NY, USA) was used to perform all statistical analysis.
Results
A total of 263 patients were included in this study. Causes of death were therapy-refractory ICP in association with cerebral infarction in 36 patients (73.5 %), hypoxic brain damage in 5 cases (10.2 %), SAH rebleeding in 4 cases (8.2 %) and sepsis leading to multiorgan failure in 3 patients (6.1 %). One single patient (2 %) was admitted under cardiopulmonary resuscitation and died in the first hours after admission. The mean age of all patients was 54.38 ± 13.71 years; 70 % (184) were female. The GCS had a distribution with two peaks at both ends of the score (GSC 14–15 or <6). Severity of the aSAH was consistently distributed over all five Hunt & Hess grades. Most patients (68.8 %) suffered from Fisher IV aSAH.
Almost two thirds (61.2 %) of all aneurysms were treated using endovascular coil embolization, whereas in 21 (8.0 %) patients no treatment of the aneurysm was performed because of the severity of the aSAH and unfavorable outcome prognosis. All details of the baseline characteristics are presented in Table 1.
The overall HM rate in our cohort was 18.3 % (49 of 263 patients). The range of the SAPS-II was 14 to 102 points with a median of 37 points. With a higher SAPS-II (>45) the risk for HM was higher than for an SAPS-II of <30 (OR 21.812; 95 % CI 6.361–74.790; p < 0.001). Each additional sum score of SAPS-II was associated with an increased HM risk of 9.7 % (OR 1.097 [1.067–1.128]). Details of the SAPS-II results are presented in Table 2.
Univariate analysis
Heart rates <70 beats/min, systolic blood pressure >200 mmHg, serum potassium level <3 mmol/l or >5 mmol/l, serum bicarbonate level 15–19 mEq/l, PaO2 mmHg/FiO2 <100, metastatic cancer in the patient history, GCS <9 and age ≥80 years were associated with an increased HM in logistic regression (Table 3). The serum urea level, bilirubin level as well as subgroups of chronic diseases (hematologic malignancy and AIDS) are not included in Table 3 as for all patients in our cohort these levels were within the normal range and none suffered from hematologic malignancy or AIDS.
Beside the statistically significant SAPS-II parameters mentioned above, we identified the localization and diameter of the aneurysm, the highest ICP within 24 h after admission and cardiopulmonary resuscitation of the patient and initial signs of cerebral herniation to be factors associated with a higher HM rate. All other factors presented in Table 4 are not associated with an increase in HM.
Receiver-operating characteristic (ROC) analysis performed to identify the validity of the SAPS-DRG and SAPS-II in the prediction of HM SAPS-DRG resulted in an area under the curve (AUC) of 0.670 (95 % CI [0.583–0.758]) and an AUC for SAPS-II of 0.834 (95 % CI 0.771–0.896) (see Table 5 and Fig. 1).
Multivariate analysis
In order to identify independent predictors of HM a multivariate analysis was performed. Forward selection multivariate analysis demonstrated that the GCS in accordance with the distribution in SAPS-II, usage of anticoagulation drugs in the medical history, and medical history of migraines or chronic headaches were associated with a higher risk of HM (Table 6).
All identified independent predictors (Table 6) of HM were assessed for their ability to improve the prediction of the SAPS-II for patients who receive the full therapeutic management. The combination of SAPS-II with the independent predictors (intake of anticoagulation drugs and history of chronic headaches, SAPS-II-GCS already included in the SAPS-II) resulted in an improvement of prediction to an AUC of 0.860 (95 % CI [0.786–0.934]). In this subgroup of aSAH patients (n = 242), the SAPS-II as a standalone predictor decreased to 0.769 (95 % CI [0.676–0.862]), most likely because of excluding patients with incomplete data sets due to the moribund state at admission and the poor prognosis that excluded them from further therapy (Table 7 and Fig. 2).
Discussion
To the best of our knowledge, this is the largest study evaluating and demonstrating a highly predictive power of the SAPS-II in a modern series of patients suffering from aSAH. In order to assess whether the predictive value of the SAPS-II for HM can be improved, we included further clinical parameters from the initial 24 h after admission and parameters from the past medical history. The analyses revealed that the GCS, a history of migraines or chronic headaches, or the intake of anticoagulation drugs were independent predictors of mortality and raised the predictive value of the SAPS-II to a statistical level with an AUC of 0.860 (Table 7).
The prediction of mortality and outcome in patients suffering from aSAH has been the aim of several studies [3, 13, 18, 22, 27, 29, 34]. Most studies focused on a wide range of predictors such as demographic factors, WFNS and Hunt & Hess grades, vasospasm, GCS, history of arterial hypertension, radiological findings, aneurysm size or aneurysm location [3, 5, 10, 15, 17, 18, 21, 26–28, 31]. However only well-established predictors such as age and the Fisher, WFNS and Hunt & Hess grades seem to be valid and reliable across studies, whereas laboratory findings, clinical results and items of the medical history remained inconclusive in the majority of the studies [13]. Another weakness of newly developed scores is the lack of sufficient external validation [3, 19]. Given these conflicting results of multiple studies for aSAH, it seems reasonable to validate the general but well-established SAPS-II as a basic score for aSAH [16]. The validation of the power of the SAPS-II was proven for traumatic brain injury and stroke patients [12, 24]. Only two studies analyzed the SAPS-II in aSAH patients as a predictor of mortality and outcome and demonstrated a predictive value of the SAPS-II with an AUC of 0.85 (n = 136) for death or dependence and an AUC of 0.82 (n = 159) for HM [20, 26]. These results are in line with our study demonstrating an AUC of 0.83 for HM and confirm the highly predictive power of the SAPS-II in our cohort of 263 patients with aSAH.
Even though the HM rate decreased over this time because of the improvement of patient management in the ICU, the SAPS-II retained its predictive value for HM as demonstrated by our results [1, 4]. In German ICUs the daily documentation of the SAPS-II or SAPS-DRG is recommended for all ICU patients by the German Institute of Medical Documentation and Information (www.dimdi.de). The SAPS-II/SAPS-DRG is used to calculate the severity of patient’s diseases and to class the patient correctly within the diagnosis-related groups (DRG) for accounting with the patient’s insurance or benefactor.
The high relevance of the GCS on HM is in line with previous studies and becomes apparent when comparing the AUC of SAPS-II (0.834) with the SAPS-DRG (0.670), which does not contain the GCS and is clearly lower than that of the SAPS-II [22, 29]. GCS was also an independent prognostic parameter in some studies, while others identified the H&H or WFNS as independent predictors, which in major parts are interrelated with the GCS [3, 5, 10, 13, 15, 17, 18, 21, 26–28, 31]. Some studies found age to be a significant predictor of outcome, whereas others did not find a similar correlation [3, 5, 10, 15, 17, 18, 26–28, 31]. In light of these conflicting results, in our study cohort the overall age analysis failed to reach the level of significance, but the age groups 70–74 (OR 6.500 [95 % CI 1.140–37.046]) and ≥80 years (OR 7.429 [95 % CI 1.121–49.244]) were significantly associated with a higher rate of mortality (Table 3). Furthermore, the finding that systolic blood pressure and serum urea levels were independent prognostic markers is not supported by our data analysis of a significantly larger cohort of patients with aSAH [22, 29].
Chronic headache including migraines was an independent prognostic marker for HM in the analyzed subpopulation of our study. Migraine was identified to be a prognostic marker for rupture of cerebral aneurysms, and one study demonstrated that migraines are associated with a greater risk of delayed cerebral ischemia (DCI) [7, 33]. The pathogenic links between migraines and angiographic vasospasm have not been clearly unraveled, but it was demonstrated that vasoconstriction of the basal cerebral arteries occurs in migraine patients [2]. Frontera et al. demonstrated that DCI has an impact on death or severe disability in aSAH patients [8]. Therefore, our results support the hypothesis that migraines are associated with an unfavorable outcome after aSAH, although due to the design of our study and our focus on parameters that are available within 24 h of admission we did not include data on DCI in our analysis.
Medication with anticoagulation drugs prior to aSAH is still a controversial aspect in the prognosis with regard to the HM. Use of the specific medication acetylsalicylic acid prior to aSAH was not associated with an increase of HM, while another study that focused on any kind of anticoagulation drug found an association with death and poor outcome [25, 30]. This is in line with our results demonstrating that prior medication with any anticoagulation drug is associated with a significantly higher risk of HM during the disease course in the subgroup of patients that is not moribund at admission and therefore eligible to receive the full treatment. On the other hand, we failed to detect an association with mortality when initially moribund patients were included in the analysis, suggesting that the intake of anticoagulation drugs has no impact on the presenting clinical grade, similar to the data of Gross et al. [11] It seems reasonable to speculate that other parameters are outweighing the possible negative effects of anticoagulation drugs on initial moribund patients. Nevertheless, the true impact of the quite heterogeneous group of anticoagulation drugs is poorly understood, which is underlined by the multitude of conflicting results [9, 11, 32].
Limitations of our study are the natural given characteristics of a retrospective analysis such as incomplete data and treatment bias. To reduce most kinds of bias we focused only on parameters that are clearly identifiable within 24 h of admission and do not require any special skills or experiences such as the presence of acute hydrocephalus or development of DCI. Another limitation might be a selection bias of the parameters we investigated as we did not include the body mass index (BMI) or obesity, for example, as most studies found no correlation between BMI and outcome in SAH, while another prospective study observed a correlation between obesity and the occurrence of vasospasm [6, 23]. Additionally, all aSAHs with no further treatment were excluded from the analysis of additional parameters to prevent selection and treatment bias in the further analysis.
Conclusion
This study clearly demonstrates that the SAPS-II is a valid and practical tool for HM in an aSAH cohort more than 20 years after the introduction of this score. Our findings reveal the GCS is one of the most powerful predictors of HM. Excluding initially moribund patients, we identified that still the GCS, a history of chronic headaches or anticoagulation drug medication was associated with an increase in HM. Including these independent predictors in the SAPS-II, we achieved a higher predictive power. Despite this increase of predictive power in our model, from a practical viewpoint, this study suggests that the SAPS-II alone, which already includes the GCS, represents a powerful score for the sufficient prediction of HM after aSAH. The SAPS-II is easily applicable since it can be semiautomatically calculated in ICUs within seconds, does not require any specific skills and is associated with a low proneness to error.
References
Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A (1994) Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 25:1342–1347
Call GK, Fleming MC, Sealfon S, Levine H, Kistler JP, Fisher CM (1988) Reversible cerebral segmental vasoconstriction. Stroke 19:1159–1170
Claassen J, Vu A, Kreiter KT, Kowalski RG, Du EY, Ostapkovich N, Fitzsimmons BF, Connolly ES, Mayer SA (2004) Effect of acute physiologic dearangments on outcome after subarachnoid hemorrhage. Crit Care 32:832–838
Czorlich P, Ricklefs F, Reitz M, Vettorazzi E, Abboud T, Regelsberger J, Westphal M, Schmidt NO (2015) Impact of intraventricular hemorrhage measured by Graeb and LeRoux score on case fatality risk and chronic hydrocephalus in aneurysmal subarachnoid hemorrhage. Acta Neurochirur 157:409–415
de Toledo P, Rios PM, Ledezma A, Sanchis A, Alen JF, Lagares A (2009) Predicting the outcome of patients with subarachnoid hemorrhage using machine learning techniques. IEEE Trans Inf Technol Biomed 13:794–801
Dhandapani SS, Kapoor A, Gaudihalli S, Dhandapani M, Mukherjee KK, Gupta SK (2015) A prospective study of trends in anthropometric nutritional indices and their impact on patients with subarachnoid hemorrhage. Neurol India 63:532–537
Dreier JP, Kremer C, Lammers G, Lohmann F, Hansen HC, Valdueza JM (2007) Migraine and delayed cerebral ischaemic neurological deficit after subarachnoid haemorrhage in women: a case-control study. Eur J Neurol 14:1363–1368
Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, Connolly ES, Mayer SA (2009) Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke 40:1963–1968
Garbe E, Kreisel SH, Behr S (2013) Risk of subarachnoid hemorrhage and early case fatality associated with outpatient antithrombotic drug use. Stroke 44:2422–2426
Germanson TP, Lanzino G, Kongable GL, Torner JC, Kassell NF (1998) Risk classification after aneurysmal subarachnoid hemorrhage. Surg Neurol 49:155–163
Gross BA, Rosalind Lai PM, Frerichs KU, Du R (2014) Aspirin and aneurysmal subarachnoid hemorrhage. World Neurosurg 82:1127–1130
Handschu R, Haslbeck M, Hartmann A, Fellgiebel A, Kolominsky-Rabas P, Schneider D, Berrouschot J, Erbguth F, Reulbach U (2005) Mortality prediction in critical care for acute stroke: severity of illness-score or coma-scale? J Neurol 252:1249–1254
Jaja BN, Cusimano MD, Etminan N, Hanggi D, Hasan D, Ilodigwe D, Lantigua H, Le Roux P, Lo B, Louffat-Olivares A, Mayer S, Molyneux A, Quinn A, Schweizer TA, Schenk T, Spears J, Todd M, Torner J, Vergouwen MD, Wong GK, Singh J, Macdonald RL (2013) Clinical prediction models for aneurysmal subarachnoid hemorrhage: a systemic review. Neurocrit Care 18:143–153
Kreymann KG, de Heer G, Felbinger T, Kluge S, Nierhaus A, Suchner U, Meier RF (2007) Nutrition of critically ill patients in intensive care. Internist (Berl) 48:1084–1092, Article in German
Lagares A, Gómez PA, Alen JF, Lobato RD, Rivas JJ, Alday R, Campollo J, de la Camara AG (2005) A comparison of different grading scales for predicting outcome after subarachnoid haemorrhage. Acta Neurochirur 147:5–16
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS-II) based on a European/North American multicenter study. JAMA 270:2957–2963
Le Roux PD, Elliott JP, Newell DW, Grady MS, Winn HR (1996) Predicting outcome in poor-grade patients with subarachnoid hemorrhage: a retrospective review of 159 aggressively managed cases. J Neurosurg 85:39–49
Mocco J, Ransom ER, Komotar RJ, Schmidt JM, Sciacca RR, Mayer SA, Connolly ES Jr (2006) Preoperative prediction of long-term outcome in poor-grade aneurysmal subarachnoid hemorrhage. Neurosurgery 59:529–538
Naidech AM, Drescher J, Tamul J, Shaibani A, Batjer HH, Alberts MJ (2006) Acute physiological derangement is associated with early radiographic cerebral infarction after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 77:1340–1344
Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ (2009) Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and origin: a meta-analysis. Lancet Neurol 8:635–642
Ogilvy CS, Quinones-Hinojosa A (1998) Surgical treatment of vertebral and posterior inferior cerebellar artery aneurysms. Neurosurg Clin N Am 9:851–860
Park SK, Chun HJ, Kim DW, Im TH, Hong HJ, Yi HJ (2009) Acute physiology and chronic health evaluation II and simplified acute physiology score II in predicting hospital mortality of neurosurgical intensive care unit patients. J Korean Med Sci 24:420–426
Platz J, Güresir E, Schuss P, Konczalla J, Seifert V, Vatter H (2013) The impact of the body mass index on outcome after subarachnoid hemorrhage: is there an obesity paradox in SAH? A retrospective analysis. Neurosurgery 73:201–208
Raj R, Skrifvars M, Bendel S, Selander T, Kivisaari R, Siironen J, Reinikainen M (2014) Predicting six-month mortality of patients with traumatic brain injury: usefulness of common intensive care severity scores. Crit Care 18:R60. doi:10.1186/cc13814
Rinkel GJ, Prins NE, Algra A (1997) Outcome on aneurysmal subarachnoid hemorrhage in patients on anticoagulant treatment. Stroke 28:6–9
Risselada R, Lingsma HF, Bauer-Mehren A, Friedrich CM, Molyneux AJ, Kerr RS, Yarnold J, Sneade M, Steyerberg EW, Sturkenboom MC (2010) Prediction of 60 day case-fatality after aneurysmal subarachnoid haemorrhage: results from the International Subarachnoid Aneurysm Trial (ISAT). Eur J Epidemiol 25:261–266
Rosen DS, Macdonald RL (2004) Grading of subarachnoid hemorrhage: modification of the world federation of neurological societies scale on the basis of data for a large series of patients. Neurosurgery 54:566–575
Salary M, Quigley MR, Wilberger JE Jr (2007) Relation among aneurysm size, amount of subarachnoid blood, and clinical outcome. J Neurosurg 107:13–17
Schuilling WJ, de Weerd AW, Dennesen PJ, Algra A, Rinkel GJ (2005) The simplified acute physiology score to predict outcome in patients with subarachnoid hemorrhage. Neurosurgery 57:230–236
Toussaint LG 3rd, Friedman JA, Wijdicks EF, Piepgras DG, Pichelmann MA, McIver JI, McClelland RL, Nichols DA, Meyer FB, Atkinson JL (2004) Influence of aspirin on outcome following aneurysmal subarachnoid hemorrhage. J Neurosurg 101:921–925
Turck N, Vutskits L, Sanchez-Pena P, Robin X, Hainard A, Gex-Fabry M, Fouda C, Bassem H, Mueller M, Lisacek F, Puybasset L, Sanchez JC (2010) A multiparameter panel method for outcome prediction following aneurysmal subarachnoid hemorrhage. Intensive Care Med 36:107–115
van den Bergh WM, MASH Study Group, Algra A, Dorhout Mees SM, van Kooten F, Dirven CM, van Gijn J, Vermeulen M, Rinkel GJ (2006) Randomized controlled trial of acetylsalicylic acid in aneurysmal subarachnoid hemorrhage: the MASH Study. Stroke 37:2326–2330
Vlak MH, Rinkel GJ, Greebe P, Algra A (2013) Risk of rupture of an intracranial aneurysm based on patient characteristics: a case-control study. Stroke 44:1256–1259
Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, Parra A, Connolly ES, Mayer SA (2006) Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med 34:617–623
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/disclosures
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Rights and permissions
About this article
Cite this article
Czorlich, P., Sauvigny, T., Ricklefs, F. et al. The simplified acute physiology score II to predict hospital mortality in aneurysmal subarachnoid hemorrhage. Acta Neurochir 157, 2051–2059 (2015). https://doi.org/10.1007/s00701-015-2605-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-015-2605-3