Abstract
Sustained intracranial hypertension and acute brain herniation are “brain codes,” signifying catastrophic neurological events that require immediate recognition and treatment to prevent irreversible injury and death. As in cardiac arrest, a brain code mandates the organized implementation of a stepwise management algorithm. The goal of this emergency neurological life support protocol is to implement an evidence-based, standardized approach to the evaluation and management of patients with intracranial hypertension and/or herniation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
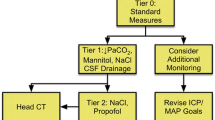
The ENLS-suggested algorithm for the initial management of intracranial hypertension or herniation is shown in Fig. 1.
The sum of intracranial contents—brain, blood, and cerebrospinal fluid (CSF)—represents a fixed volume determined by the invariant constraints of the cranial vault [1, 2]. Relative volumes of these contents will change to accommodate an acutely developing, space-occupying mass; however, this compensation is lost once a critical volume change has occurred, as demonstrated by the inflection point of the pressure–volume relationship (Fig. 2). Intracranial hypertension and cerebral herniation are “brain codes”—life-threatening neurological emergencies indicating that intracranial compliance adaptive mechanisms have been overwhelmed.
Although frequently linked, elevations of intracranial pressure (ICP) and brain herniation can occur independently. Intracranial hypertension is defined as a sustained (>5 min) elevation of ICP above 20 mmHg [3]. Detection requires invasive monitoring, but certain clinical and physiological signs may suggest elevated ICP prior to instrumentation. Herniation syndromes result from intracranial compartmental pressure gradients leading to parenchymal tissue shifts that compress or displace the brainstem, cranial nerves, or cerebral vasculature. Ischemia or infarction from vascular compression may cause edema and further deterioration in compliance.
The etiologies of brain codes are classified anatomically as extra-axial, focal, or diffuse intraparenchymal processes (Table 1). In the emergent setting of a brain code, resuscitative measures are pursued even if the etiological mechanism has not been fully characterized.
Presentation
Clinically, symptoms of increased ICP include headache, nausea, and vomiting, or altered mental status with physical signs of hypertension, bradycardia, and irregular respirations or apnea (Cushing’s triad), although the concurrence of these three signs is less frequent [4]. Common sites for herniation are the cingulum (subfalcine herniation), medial temporal lobe (uncal herniation), and inferior cerebellum (tonsillar herniation).
The cardinal signs of transtentorial (uncal) herniation are an acute loss of consciousness associated with ipsilateral pupillary dilation and contralateral hemiparesis, resulting, respectively, from compression or displacement of ascending arousal pathways, oculomotor nerve (III), and corticospinal tract [5, 6]. In a subset of patients, herniation-associated shift of the midbrain compresses the contralateral anterior cerebral peduncle (crus cerebri) against the tentorium, resulting in hemiparesis that is ipsilateral to the lesion (Kernohan’s false localizing sign) [7]. Transtentorial herniation may cause ipsilateral cerebral infarction due to occlusion of the posterior cerebral artery.
Neuroimaging
In the emergent setting of a brain code, a cranial computed tomography (CT) scan should be obtained to identify a process that may require surgical intervention. Initial resuscitative measures and stabilization, including airway interventions, circulatory and ventilatory support, and initial hyperosmolar therapy, must be initiated prior to transport to the radiology suite. Cranial CT is preferred over magnetic resonance imaging (MRI) due to availability and speed of imaging. In the majority of cases, CT will identify the underlying process (see Table 1), although MRI may subsequently be needed for characterization. MRI should only be sought if the imminent risk of an additional brain code has been addressed by medical and/or surgical intervention.
ICP Monitoring
ICP monitors are invasive and are of several different types, including intraventricular catheters as well as intraparenchymal, subdural, and epidural devices. The decision to proceed with ICP monitoring is determined by the underlying process and the likelihood of its progression. In traumatic brain injury (TBI), neurosurgical guidelines recommended placement of an ICP monitor in patients with severe TBI who are comatose after resuscitation [Glasgow Coma Scale (GCS) of 3–8] and have either (1) abnormalities on cranial CT scan or (2) meet at least two of the following three criteria: age >40 years; systolic blood pressure <90 mmHg; or abnormal posturing [8]. Indications for ICP monitoring are less well established in non-traumatic coma. However, a recent international multidisciplinary consensus conference recommended that ICP and CPP should be monitored in patients at risk for ICP elevation based on clinical and/or imaging features to guide medical and surgical interventions [9].
Cerebral perfusion pressure (CPP), used as a surrogate for global cerebral blood flow (CBF), is approximated by the equation:
CPP = Mean arterial pressure (MAP) – ICP.
In patients with TBI, available data support maintaining CPP >50–60 mmHg in adults to prevent cerebral ischemia [3]. Efforts to augment CPP greater than 60 mmHg may elevate the risk of systemic complications, including acute respiratory distress syndrome [10]. CPP targets for patients with non-traumatic intracranial hypertension have not been adequately studied.
Tier Zero
Brain code resuscitation begins with an assessment of circulation, airway patency, and ventilation. The head of the bed should be elevated to >30° and the head kept midline to facilitate cerebral venous drainage [11, 12]. Stimuli such as tracheal suctioning, that may elevate ICP, should be minimized. If hyperthermia is present, measures should be taken to normalize body and brain temperature. Only iso- or hyperosmotic fluids should be used as intravenous solutions. If hyponatremia is present, steps should be initiated for correction. High-dose corticosteroid therapy is initiated for vasogenic edema resulting from brain tumors, abscesses, or non-infectious neuroinflammatory conditions [13, 14]. If the brain has not yet been imaged, a non-contrast head CT scan should be performed when the patient can be transported safely.
Tier One
For acute elevations in ICP, hyperosmolar therapy with either mannitol or hypertonic saline (HTS) has shown equivalent efficacy in lowering of ICP [15–18]. Mannitol is administered as 0.5–1 g/kg intravenous (IV) bolus through a peripheral intravenous line and may be repeated every 4–6 h if serum osmolality is monitored [19]; no therapeutic benefit is appreciable with osmolality >320 mOsm/kg. HTS is available in concentrations from 2 to 23.4 % and can be administered as a bolus alone or in addition to mannitol. HTS concentrations boluses ≥7.5 % should be given via a central venous catheter; when using concentrations lower than this, peripheral lines may be used, but the infusion should be in a large vessel, and the IV site should be carefully monitored for infiltration. Since two pre-hospital trials using 7.5 and 3 % NaCl solutions via peripheral catheters had no negative effects, in the brain code setting these treatments should not be withheld simply because central access is yet be available [20, 21]. Bolus 23.4 % NaCl has been associated with ICP reduction and reversal of transtentorial herniation [22]. When infusing HTS, target serum sodium concentration should be determined and serum sodium level checked every 4–6 h. When acute obstructive hydrocephalus is present as determined by neuroimaging, an external ventricular drainage (EVD) system should be placed emergently. If an EVD system is already in place, drain 5–10 mL of CSF [23] for acute rises in ICP. As a temporizing measure, a brief course (<2 h) of hyperventilation to a PaCO2 of 30–35 mmHg may be considered, while definitive treatment is provided [24, 25]. If ICP is not controlled, and/or clinical signs of herniation do not resolve with Tier One interventions, review decompressive surgical options [26, 27]. If surgery is not appropriate or not undertaken, move to Tier Two. If ICP is controlled with Tier One interventions, consider repeating the head CT scan to rule out new processes.
Head CT
After Tier One or Tier Two (below), if brain imaging has not yet been performed, a head CT scan should be performed to determine the cause of herniation or intracranial hypertension, for the reasons explained in the neuroimaging section above.
Tier Two
If Tier One interventions have failed to control ICP, Tier Two should be engaged. If hyperosmolar therapy with HTS has been administered, serum sodium goals should be increased, although sodium levels >160 mmol/L are unlikely to provide additional benefit. The target for serum sodium is controversial and it depends on pathophysiological state. If the patient is continuing to have increased ICP (and needs to advance to Tier Two), one needs to maintain a gradient between brain and serum in order to promote the egress of water from the brain. Once the ICP has stabilized, one needs to maintain sodium at the current high level until the brain edema process is waning. Sedation should be increased to aid in ICP management. Propofol has been shown to reduce CMRO2 and CBF volume and, consequently, ICP [28]. It is administered as a bolus of 1–3 mg/kg and may be continued as an infusion (titrate to maximum 200 µg/kg/min) in ventilated patients. Propofol, especially when given as a bolus dose, is associated with circulatory depression, which should be corrected with IV fluids and/or vasopressors to maintain CPP goals. A small subset of patients receiving propofol may develop a propofol infusion syndrome characterized by metabolic acidosis, cardiac dysfunction, rhabdomyolysis, and hypertriglyceridemia, often with a fatal outcome [29, 30]. Propofol infusion syndrome is more likely to develop at doses >100 mcg/kg/min administered for >48 h; so if propofol is increased to these extreme ranges (200 µg/kg/min), it should only be done temporarily, while other measures are put in place.
If ICP is not responsive to Tier Two interventions, rescue decompressive surgery should be considered. If the patient is ineligible for surgery, Tier Three (below) should be engaged.
Decompression
Surgery is considered for brain code patients who have failed medical management. Decompressive surgical interventions for the management of brain code include (a) placement of a ventricular drain, (b) evacuation of extra-axial lesion (e.g., epidural hematoma), (c) resection of intracerebral lesion (e.g., lobar hemorrhage), (d) removal brain parenchyma (e.g., cerebellar mass), and (d) uni- or bilateral craniectomies.
Selected patients with rapid neurological deterioration from focal space-occupying lesions may benefit from surgical decompression. This includes patients with brain tumors, brain abscesses, and parenchymal hemorrhages, particularly when the hemorrhages are lobar [31] or cerebellar [32, 33] in location. Decompressive craniectomy may also be considered in patients with diffuse brain swelling associated with TBI [34–37]; stroke with brain edema, the process in which hemicraniectomy has been most extensively studied [38, 39]; meningoencephalitis; or non-infectious neuroinflammatory conditions (e.g. acute demyelinating encephalomyelitis).
Tier Three
Tier Three measures represent the most aggressive level of management and carry the highest risk of adverse effects. Rigorous randomized prospective studies are lacking, and recommendations are driven by consensus. This tier includes administration of pentobarbital (bolus 10 mg/kg over 30 min–2 h, then 5 mg/kg/h × 3 h; maintenance infusion of 1–4 mg/kg/h) titrated to ICP goal. Some patients may not tolerate pentobarbital at these doses because of hypotension and arterial vasopressors may be necessary. The electroencephalogram (EEG) should be continuously monitored and pentobarbital titrated either to ICP or to EEG burst suppression of 5–20 s. The pentobarbital infusion is continued for 24–96 h, while the underlying processes driving ICP are treated [40–42]. Pentobarbital is associated with respiratory depression, cardiovascular instability, immune suppression, and paralytic ileus; neurological examination is limited to an assessment of pupillary reactivity. Pentobarbital plasma clearance may take days after discontinuation of infusion; however, it redistributes out of the CNS more rapidly.
Moderate hypothermia (target core temperature 32–34 °C) is associated with a predictable reduction in ICP. It may be induced with external cooling devices or with IV infusion of cooled fluids [43–48]. Hypothermia may be associated with shivering, cardiac arrhythmias, sepsis, and electrolyte disturbances and protocols for induction, maintenance, and rewarming should be used to optimally manage these complications.
Hyperventilation to achieve mild to moderate hypocapnia (PaCO2 25–35 mmHg) and cerebrovascular constriction may be considered in selected patients who have failed other interventions in the acute period. Prolonging hyperventilation for more than 6 h is unlikely to be beneficial and may cause or exacerbate ischemic injury [25]. Hence, hyperventilation should ideally be accomplished in conjunction with a cerebral oxygenation monitor (e.g. jugular venous oximetry and brain tissue oxygen probe), in order to detect cerebral ischemia.
Consider Additional Monitoring
Patients who have had or are at risk for a brain code may benefit from additional neuromonitoring, including jugular venous oximetry, brain tissue oxygenation, and cerebral microdialysis. Treatment based on ICP and CPP overlooks significant information on the physiologic and metabolic state of the injured brain. Moreover, assumptions regarding CPP may not hold if CBF autoregulation is impaired. Complementary neuromonitoring techniques should be considered to optimize medical management in selected patients with severe brain injury.
Studies using brain tissue oxygen sensors indicate that significant parenchymal hypoxia may occur even when ICP and CPP are normal [49, 50]. Cerebral microdialysis measures brain interstitial lactate, pyruvate, glucose, and glutamate, indicators of cerebral metabolic activity whose levels may be altered independently of ICP and CPP [51]. Dynamic indices of cerebral autoregulation express the correlation between a systemic hemodynamic parameter (arterial blood pressure or CPP) and an intracranial physiological parameter such as ICP (PRx), transcranial Doppler-derived CBF velocity (Mx), or brain tissue PO2 (Orx). High degrees of correlation suggest a failure of autoregulation and an increased risk of injury due to hypo or hyperperfusion [52, 53].
Revise ICP/MAP Goals
Depending on the specific circumstances and the invasive or noninvasive monitoring that are available, the standard goals of MAP and ICP should be re-considered and customized to the patient. For example, a patient who is awake, without symptoms, and in whom ICP is in excess of 20 mmHg, or a CPP below 50 mmHg, may not require any intervention.
Pre-hospital
Recognition of a brain code in the pre-hospital setting is important because life-saving therapies can be initiated prior to arrival in the Emergency Department. Clinical signs of herniation may be apparent and include unilateral dilated pupil, loss of consciousness, posturing, as well as hypertension and bradycardia. Resuscitation begins with the management of circulation, airway patency, and ventilation. If capnography is available after intubation, a goal of end-tidal CO2 of 30–35 mmHg should be targeted. The head of the bed should be elevated to facilitate cerebral venous drainage; although these patients are typically backboarded, it may be possible to place a rolled blanket or towel beneath the board during transport to elevate the head. Hyperventilation can be initiated using BVM ventilation. Pre-hospital notification is also important so that resources are available upon arrival.
Pediatric Considerations
Management for intracranial hypertension in the pediatric population should follow analogous tiers of treatment and indications for monitoring as in adults. Importantly, the presence of an open fontanel in infants does not preclude the development of intracranial hypertension and cerebral herniation [54]. In children with TBI <5 years old, CPP should be maintained >40 mmHg, whereas in children 6–17 years of age a target of >50 mmHg is appropriate [55, 56]. Establishment of optimal CPP may require advanced neuromonitoring (i.e., brain tissue oxygen tension) due to the lack of well-defined age-dependent CPP thresholds and the risk of abnormal cerebrovascular autoregulation [57].
Hyperosmolar therapy with either mannitol or 3 % HTS may be administered for ICP elevations >20 mmHg or for suspected herniation. Mannitol bolus dose is 0.5–1 g/kg. Mannitol can be administered every 4 h as needed for ICP >20 mmHg as long as the osmolar gap is less than 20 mOsm. A Foley catheter should be placed in children receiving mannitol to prevent bladder overdistention due to mannitol-induced diuresis. Such diuresis should be anticipated and additional boluses of normal saline provided to prevent hypotension.
Hypertonic 3 % saline may be given as a bolus dose of 5–10 mL/kg for acute elevations in ICP or signs of brain herniation. HTS may then be administered as a continuous infusion at 0.5–1.5 mL/kg/h [55]. The expected serum sodium rise is 1 mEq/L for every 1 mL/kg bolus dose given and 1 mEq/L/h for every 1 mL/kg/h continuous infusion. Levels of serum sodium >160–165 mEq/L are unlikely to provide additional benefit in reducing intracranial hypertension. Compared to mannitol, HTS has the added benefit of improved hemodynamic stability, an important consideration given the deleterious effect of hypotension in patients with acute brain injury.
Brain herniation is potentially reversible with appropriate and timely therapy. Reversal of transtentorial herniation has been observed in 50–75 % of adult patients with either TBI [58] or with supratentorial mass lesions [59]. Long-term outcomes after successful treatment for herniation may be more favorable in children than in adults [58].
A special category of children at risk for increased ICP and brain herniation are children with diabetic ketoacidosis (DKA). Approximately 0.5–1 % of children with DKA will have severe cerebral edema, which carries a mortality rate of 20–25 % [60, 61]. Factors thought to be associated with increased risk of neurologic complications in pediatric DKA are young age, duration and severity of symptoms, low pCO2, overly aggressive fluid resuscitation, administration of hypotonic fluids, administration of sodium bicarbonate, and decreases in serum glucose >100 mg/dL/h [61, 62]. Hence, initial resuscitation should be administered with caution. Boluses of sodium bicarbonate must be avoided. Mental status should be checked at least hourly. Children with DKA will often become somnolent with initiation of treatment, possibly due to subtler cerebral edema, but they should remain arousable with stimulation. Children who are unarousable, those with papilledema and those with warning signs of cerebral herniation (Cushing’s triad) should receive hyperosmolar therapy and be hyperventilated to prevent further deterioration. HTS may be preferred to mannitol, since in DKA osmolality is already high due to elevated glucose concentrations and mannitol-induced diuresis may aggravate dehydration.
References
Monro A. Observations on the structure and functions of the nervous system. Edinburgh: Creech & Johnson; 1783.
Kellie G. Appearances observed in the dissection of two individuals; death from cold and congestion of the brain. Trans Med-Chir Soc Edinburgh. 1824;1:84.
Brain Trauma F, American Association of Neurological S, Congress of Neurological S, et al. Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotrauma. 2007;24(1):S55–8.
Cushing H. Concerning a definite regulatory mechanism of the vasomotor centre which controls blood pressure during cerebral compression. Bull Johns Hopkins Hosp. 1901;126:289–92.
Meyer A. Herniation of the brain. Arch Neurol Psychiatry. 1920;4:387–400.
Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med. 1986;314:953–8.
Kernohan JW, Woltman HW. Incisura of the crus due to contralateral brain tumour. Arch Neurol Psychiatry. 1929;21:274–87.
Brain Trauma F, American Association of Neurological S, Congress of Neurological S, et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(1):S37–44.
LeRoux P, Menon DK, Citerio G, et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1189–209.
Contant CF, Valadka AB, Gopinath SP, Hannay HJ, Robertson CS. Adult respiratory distress syndrome: a complication of induced hypertension after severe head injury. J Neurosurg. 2001;95:560–8.
Feldman Z, Kanter MJ, Robertson CS, et al. Effect of head elevation on intracranial pressure, cerebral perfusion pressure, and cerebral blood flow in head-injured patients. J Neurosurg. 1992;76:207–11.
Ng I, Lim J, Wong HB. Effects of head posture on cerebral hemodynamics: its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery. 2004;54:593–7.
Galicich JH, French LA, Melby JC. Use of dexamethasone in treatment of cerebral edema associated with brain tumors. J Lancet. 1961;81:46–53.
Quartey GR, Johnston JA, Rozdilsky B. Decadron in the treatment of cerebral abscess: an experimental study. J Neurosurg. 1976;45:301–10.
Qureshi A, Wilson D, Traystman R. Treatment of elevated intracranial pressure in experimental intracerebral hemorrhage: comparison between mannitol and hypertonic saline. Neurosurgery. 1999;44(5):1055–63.
Francony G, Fauvage B, et al. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med. 2008;36(3):795–800.
Battison C, Andrews PJ, Graham C, et al. Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury. Crit Car Med. 2005;33:196–202.
Ichai C, Armando G, Orban JC, et al. Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intensive Care Med. 2009;35:471–9.
Francony G, Fauvage B, Falcon D, et al. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med. 2008;36:795–800.
Bulger EM, May S, Brasel KJ, et al. Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. JAMA. 2012;304:1455–64.
Dubick MA, Wade CE. A review of the efficacy and safety of 7.5% NaCl/6% dextran 70 in experimental animals and in humans. J Trauma. 1994;36:323–30.
Koenig MA, Bryan M, Lewin JL 3rd, Mirski MA, Geocadin RG, Stevens RD. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70:1023–9.
Kerr EM, Marion D, Sereika MS, et al. The effect of cerebrospinal fluid drainage on cerebral perfusion in traumatic brain injured adults. J Neurosurg Anesthesiol. 2000;12:324–33.
Coles JP, Minhas PS, Fryer TD, et al. Effect of hyperventilation on cerebral blood flow in traumatic head injury: clinical relevance and monitoring correlates. Crit Care Med. 2002;30:1950–9.
Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75:731–9.
Eberle BM, Schnuriger B, Inaba K, Gruen JP, Demetriades D, Belzberg H. Decompressive craniectomy: surgical control of traumatic intracranial hypertension may improve outcome. Injury. 2010;41:894–8.
Johnson RD, Maartens NF, Teddy PJ. Decompressive craniectomy for malignant middle cerebral artery infarction: evidence and controversies. J Clin Neurosci. 2011;18:1018–22.
Kelly DF, Goodale DB, Williams J, et al. Propofol in the treatment of moderate and severe head injury: a randomized, prospective double-blinded pilot trial. J Neurosurg. 1999;90:1042–52.
Roberts RJ, Barletta JF, Fong JJ, et al. Incidence of propofol-related infusion syndrome in critically ill adults: a prospective, multicenter study. Crit Care. 2009;13:R169.
Fong JJ, Sylvia L, Ruthazer R, Schumaker G, Kcomt M, Devlin JW. Predictors of mortality in patients with suspected propofol infusion syndrome. Crit Care Med. 2008;36:2281–7.
Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–97.
Pfefferkorn T, Eppinger U, Linn J, et al. Long-term outcome after suboccipital decompressive craniectomy for malignant cerebellar infarction. Stroke. 2009;40:3045–50.
Raco A, Caroli E, Isidori A, Salvati M. Management of acute cerebellar infarction: one institution’s experience. Neurosurgery. 2003;53:1061–5.
Jiang JY, Xu W, Li WP, et al. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: a multicenter, prospective, randomized controlled study. J Neurotrauma. 2005;22:623–8.
Olivecrona M, Rodling-Wahlstrom M, Naredi S, Koskinen LO. Effective ICP reduction by decompressive craniectomy in patients with severe traumatic brain injury treated by an ICP-targeted therapy. J Neurotrauma. 2007;24:927–35.
Taylor A, Butt W, Rosenfeld J, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst. 2001;17:154–62.
Cooper DJ, Rosenfeld JV, Murray L, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493–502.
Hofmeijer J, Kappelle LJ, Algra A, et al. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009;8:326–33.
Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–22.
Brain Trauma Foundation. The use of barbiturates in the control of intracranial hypertension. J Neurotrauma. 1996;13:711–4.
Eisenberg HM, Frankowski RF, Contant CF, Marshall LF, Walker MD. High-dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J Neurosurg. 1988;69:15–23.
Perez-Barcena J, Llompart-Pou JA, Homar J, et al. Pentobarbital versus thiopental in the treatment of refractory intracranial hypertension in patients with traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12:R112.
Jiang J, Yu M, Zhu C. Effect of long-term mild hypothermia therapy in patients with severe traumatic brain injury: 1-year follow-up review of 87 cases. J Neurosurg. 2000;93:546–9.
Liu WG, Qiu WS, Zhang Y, Wang WM, Lu F, Yang XF. Effects of selective brain cooling in patients with severe traumatic brain injury: a preliminary study. J Int Med Res. 2006;34:58–64.
Marion DW, Obrist WD, Carlier PM, Penrod LE, Darby JM. The use of moderate therapeutic hypothermia for patients with severe head injuries: a preliminary report. J Neurosurg. 1993;79:354–62.
Marion DW, Penrod LE, Kelsey SF, et al. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336:540–6.
Qiu W, Zhang Y, Sheng H, et al. Effects of therapeutic mild hypothermia on patients with severe traumatic brain injury after craniotomy. J Crit Care. 2007;22:229–35.
Shiozaki T, Sugimoto H, Taneda M, et al. Effect of mild hypothermia on uncontrollable intracranial hypertension after severe head injury. J Neurosurg. 1993;79:363–8.
Chang JJ, Youn TS, Benson D, et al. Physiologic and functional outcome correlates of brain tissue hypoxia in traumatic brain injury. Crit Care Med. 2009;37:283–90.
Longhi L, Pagan F, Valeriani V, et al. Monitoring brain tissue oxygen tension in brain-injured patients reveals hypoxic episodes in normal-appearing and in peri-focal tissue. Intensive Care Med. 2007;33:2136–42.
Chen HI, Stiefel MF, Oddo M, et al. Detection of cerebral compromise with multimodality monitoring in patients with subarachnoid hemorrhage. Neurosurgery. 2011;69:53–63.
Schmidt B, Czosnyka M, Raabe A, et al. Adaptive noninvasive assessment of intracranial pressure and cerebral autoregulation. Stroke. 2003;34:84–9.
Brady K, Joshi B, Zweifel C, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010;41:1951–6.
Khoshyomn S, Tranmer BI. Diagnosis and management of pediatric closed head injury. Semin Pediatr Surg. 2004;13:80–6.
Kochanek P, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents-second edition. Pediatr Crit Care Med. 2012;13(1):s1–84.
Allen BB, Chiu YL, Gerber LM, Ghajar J, Greenfield JP. Age-specific cerebral perfusion pressure thresholds and survival in children and adolescents with severe traumatic brain injury. Pediatr Crit Care Med. 2014;15(1):62–70.
Udomphorn Y, Armstead WM, Vavilala MS. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr Neurol. 2008;38:225–34.
Skoglund TS, Nellgârd B. Long-time outcome after transient transtentorial herniation in patients with traumatic brain injury. Acta Anaesthesiol Scand. 2005;49(3):337–40.
Koenig MA, Bryan M, Lewin JL 3rd, Mirski MA, Geocadin RG, Stevens RD. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70(13):1023–9.
Edge J, Hawkins M, Winter D, Dunger D. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Arch Dis Child. 2001;85:16–22.
Glaser N, Barnett P, McCaslin I, et al. Risk factors for cerebral edema in children with diabetic ketoacidosis. N Engl J Med. 2001;344:264–9.
Sperling MA, Weinzimer SA, Tamborlane WV. Chapter 10: diabetes mellitus. In: Sperling MA, editor. Pediatric endocrinology. 3rd ed. Philadelphia: Saunders Elsevier; 2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stevens, R.D., Shoykhet, M. & Cadena, R. Emergency Neurological Life Support: Intracranial Hypertension and Herniation. Neurocrit Care 23 (Suppl 2), 76–82 (2015). https://doi.org/10.1007/s12028-015-0168-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-015-0168-z