Abstract
Objectives
Traumatic brain injury (TBI) is still a major cause of mortality and morbidity. Recent trials have failed to demonstrate a beneficial outcome from therapeutic treatments such as corticosteroids, hypothermia and hypertonic saline. We investigated the effect of a new hyperosmolar solution based on sodium lactate in controlling raised intracranial pressure (ICP).
Design and setting
Prospective open randomized study in an adult ICU.
Patients
Thirty-four patients with isolated severe TBI (Glasgow Coma Scale ≤ 8) and intracranial hypertension were allocated to receive equally hyperosmolar and isovolumic therapy, consisting of either mannitol or sodium lactate. Rescue therapy by crossover to the alternative treatment was indicated when ICP could not be controlled. The primary endpoint was efficacy in lowering ICP after 4 h, with a secondary endpoint of the percentage of successfully treated episodes of intracranial hypertension. The analysis was performed with both intention-to-treat and actual treatments provided.
Measurements and results
Compared to mannitol, the effect of the lactate solution on ICP was significantly more pronounced (7 vs. 4 mmHg, P = 0.016), more prolonged (fourth-hour-ICP decrease: −5.9 ± 1 vs. −3.2 ± 0.9 mmHg, P = 0.009) and more frequently successful (90.4 vs. 70.4%, P = 0.053).
Conclusion
Acute infusion of a sodium lactate-based hyperosmolar solution is effective in treating intracranial hypertension following traumatic brain injury. This effect is significantly more pronounced than that of an equivalent osmotic load of mannitol. Additionally, in this specific group of patients, long-term outcome was better in terms of GOS in those receiving as compared to mannitol. Larger trials are warranted to confirm our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) remains a highly detrimental illness with fatal outcomes ranging from 20–50% and severe neurological disability affecting 35–50% [1–4]. Three recent multicenter trials investigating the effect of prehospital management with hypertonic sodium chloride [5], corticosteroids [6] or hypothermia during the acute phase [7] all proved negative on outcome. Several treatments including drainage of cerebrospinal fluid [8], hypothermia [7, 9] may lower intracranial pressure as does osmotherapy. Nevertheless, the infusion of bolus(es) of mannitol represents the first treatment currently recommended to decrease cerebral edema, since its efficacy has been demonstrated over many years [10–12]. Only a few randomized controlled trials have evaluated the effect of mannitol on intracranial hypertension [10, 13]. None showed much difference between treatments, and the effect of mannitol on neurological outcome is still open to debate [14]. Side effects such as dehydration, hypotension and renal failure may limit its use [11], and its transitory action may be accompanied by a rebound effect [15]. The principal mechanism of mannitol is believed to be osmotic, leading to brain dehydration and a decrease in ICP, thus improving cerebral perfusion. Although this mechanism of osmotic dehydration of the swollen brain may occur, mannitol lowers ICP through several other mechanisms, including increased CSF absorption, rheology (induction of blood dilution decreasing viscosity), and perfusion pressure changes leading to vasoconstriction and decreased cerebral blood volume [12, 16, 17].
In recent years, hypertonic sodium chloride has been introduced in the treatment of brain edemas in animal studies [18] and in humans [19, 20]. In a prospective study in patients with TBI [21], saline/dextran mixture was judged to be superior to mannitol in decreasing ICP; however, contradictory results have recently been shown [12].
Contrary to popular belief, lactate is now recognized as a key inter-cellular or inter-organ metabolite between glycolysis and oxidative phosphorylation that can be both produced and utilized by the brain under pathological conditions [22, 23]. Animal and human studies show that lactate can prevent the neurological effect of hypoglycemia [24, 25], indicating that systemic lactate can be metabolized by the brain. Experimental data on rat hippocampi submitted to an ischemia reperfusion injury found that lactate was a better substrate than glucose [26]. Hypertonic sodium lactate also produced a significantly better cognitive function in rats 10–15 days post-TBI when compared to a hypertonic sodium chloride solution [27, 28]. In a porcine model of TBI, hyperosmolar sodium lactate was shown to decrease ICP and to increase cerebral blood flow by arteriolar vasodilation [29, 30].
We therefore hypothesized that hypertonic sodium lactate may combine the advantages of lactate as a preferred fuel for the brain under the condition of ischemia-reperfusion injury (as in the early phase of severe TBI), together with arteriolar cerebral vasodilation and anti-edematous effect. Consequently, we undertook a prospective randomized controlled study of patients suffering from severe TBI. The study endpoint was to compare the effect of a bolus of an equally hyperosmolar, isovolumic infusion of either sodium lactate or mannitol on the evolution of ICP during episodes of intracranial hypertension. We have also compared (1) the efficacy (percentage of failure) of both treatments on intracranial pressure (ICP), and (2) the effects of these two treatments on 1-year neurological outcomes assessed by the Glasgow Outcome Score.
Materials and methods
Patients
Approval was obtained from the Nice Hospital Ethical Research Committee. All patients presenting with an acute, isolated, severe head injury were eligible for enrolment. Patients were included within the first 8 h after injury if they had either an initial prehospital Glasgow Coma Score of ≤8, or a rapid worsening in neurological status before admission. Patients younger than 18 years, older than 65 years and pregnant women were excluded, as were patients requiring (1) a neurosurgical intervention, (2) with polytrauma, (3) with bilateral fixed dilated pupils, (4) with a prolonged prehospital episode of hypoxemia or arterial hypotension, (6) with rhinorrhea, (7) with initial hypernatremia (>155 mmol/L), (8) with penetrating head injury, and (9) patients with prehospital barbiturate, steroids or osmotherapy administration.
General head injury management is described in detail in the electronic supplement material. Any increase in ICP > 25 mmHg which persisted for more than 5 min in the absence of noxious stimulations was considered to be an episode of intracranial hypertension requiring osmotherapy.
Study protocol
Following approval from the next-of-kin, suitable patients were randomly allocated to receive either treatment by using sealed envelopes to a ratio of 5:5 in blocks of 10. Sodium lactate solution (half-molar sodium lactate) was specifically designed to have a similar osmolarity to 20% mannitol, as assessed by ∆ cryoscopic determination (1,100 mosm/L for LAC and 1,160 mosm/L for MAN). The lactate solution contained Na 504 mmol/L, K 4 mmol/L, Ca 1.36 mmol/L, Cl 6.74 mmol/L and lactate 504.1 mmol/L. Osmotic treatment consisted of an acute infusion of 1.5 ml/kg of either mannitol (20%, i.e., 0.3 g/kg) or lactate over 15 min. Fifteen minutes after the end of infusion, treatment was considered to be successful if ICP decreased by >5 mmHg or reached a value of ≤20 mmHg. In case of failure, a second treatment was immediately prescribed in a crossover fashion, i.e., lactate after mannitol or vice versa. Hence, the raised ICP episode was treated with either (1) mannitol alone, (2) lactate alone or (3) both mannitol and lactate, depending on the efficacy of the first treatment. In the case of frequent recurrent episodes in one patient, the study protocol considered only the first three episodes which occurred during the first 48 h. Further episodes were treated by standard osmotherapy, i.e., mannitol, in both groups.
Data collection
Neurological status was scored before inclusion in the prehospital period using the Glasgow Coma Scale [31]. Brain damage was evaluated by CT scan performed immediately after admission and stratified according to the Marshall score [32]. Long-term outcome was assessed 12 months after trauma by physician-conducted blind phone interviews. The questionnaire was based on the five-category Glasgow Outcome Score (GOS): G = good recovery, M = moderate disability, S = severe disability, V = vegetative state, D = death [31].
Temperature, heart rate, mean arterial pressure, urine output, ICP, CPP, SvJO2, laboratory values and doses of selected medications were recorded during the entire course of ICP monitoring. Osmotherapy was evaluated on data collected before (baseline) and 15 min after the end of infusion, i.e., 30 min after baseline measurements. When rescue therapy was necessary, the baseline data measured before the second treatment corresponded to those obtained after the end of the previous infusion. Arterial samples were collected at the same time for assessing glucose, lactate, plasma osmolality, hemoglobin, hematocrit, sodium, chloride and blood gases. Simultaneously, venous blood samples were drawn to measure glucose and lactate in order to calculate arteriojugular venous differences and SvJO2.
Study endpoints
The study endpoint was to evaluate the efficacy of both acute osmotherapies (mannitol or lactate) on ICP at the fourth hour following the commencement of infusion. In addition, we also considered (1) the percentage of successfully treated episodes between both acute osmotherapy treatments, and (2) the 1-year neurological status.
Sample size determination and statistical analysis
The maximal effect of mannitol mainly occurs during the first 2 h after administration (20% decrease of ICP); a more prolonged action is not well established. Our aim was to examine the longer-term effects of LAC in comparison to MAN at the fourth hour. This aim was based on the literature as well as on our own data with mannitol; we therefore hypothesized a 20 ± 14% ICP decrease at the 4th hour with LAC, compared to 10 ± 14% with mannitol [16]. Given this hypothesis, we calculated that a total number of 64 episodes (i.e., 32 in each group) would be required to reach a statistical power of 80% at a significance level of 0.05. Assuming (1) that outcomes (ICP decrease) of the different episodes are independent, (2) an average number of 1.5 intracranial hypertensive episodes per enrolled patient and (3) a probability of failure of the mannitol therapy of 20% (a value hypothesized to be identical with lactate), we required two groups of 17 patients, aiming to investigate at least 32 episodes per group.
The specific design of this study led us to analyze the results in different ways. Firstly, the efficacy of treatment by mannitol or lactate was assessed by comparing the ICP decrease at the fourth hour following acute infusion as planned by the randomization (“intention-to-treat analysis”: MAN vs. LAC). In addition, we also analyzed the effect of the actual treatments administered on ICP decrease by comparing the three groups: i.e., if successful, either the initially randomized treatment (mannitol or lactate) or the rescue therapy (mannitol/lactate) was used when deemed necessary. Similarly, the 1-year neurological outcomes were evaluated by both intention-to-treat analysis and by comparing the actual treatments administered.
When distribution was normal, statistical analysis was performed when necessary by the following parametric tests: univariate t test, two-way ANOVA for repeated measures, followed by post-hoc analysis (unpaired student’s t test), as indicated in Tables and Figures. When distribution was not normal, non-parametric tests were used: Mann–Whitney rank sum test, Kruskall-Wallis test, and univariate sign test, also as indicated. Categorical parameters were compared using chi-square or Fisher’s exact test.
Results
Of the 132 patients assessed for eligibility, 96 were ineligible as they did not meet the inclusion criteria or no informed consent was obtained from their next-of-kin (see appendix electronic material Figure I suppl.). One patient died rapidly and another was excluded due to failed SvJO2 monitoring. Thirty-four patients were thus eligible and randomized, with 17 in each group (MAN or LAC). These two groups were subsequently divided according to the actual treatment received: either as initially randomized or as crossover rescue treatment. Nine patients received only mannitol, 12 received only lactate and 13 received both mannitol and lactate.
Initial clinical status and patient management
As shown in Table 1, the two randomized groups (MAN and LAC) were comparable for age, gender, initial Glasgow score and initial brain damage, as evaluated by the Marshall score performed on the CT scan [32]. The average time to ICU admission was longer in the LAC group in comparison to MAN: 195 (142–450) versus 105 (60–300) min, P = 0.059. Occurrence of unilateral pupillary widening (25 vs. 24%) or central diabetes insipidus (29 vs. 18%) was identical in the two groups as well as prothrombin time (median 69 vs. 62%) and activated Kaolin time (median: 33 vs. 34 s) for LAC and MAN respectively, NS. The characteristics of the treatments applied to these patients during the episodes of intracranial hypertension are also shown in Table 1. During the period of data collection (4 h following osmotherapy infusion), all patients received norepinephrine to maintain cerebral perfusion pressure in a same manner. Tidal volume remained unchanged and end-tidal CO2 was maintained between 35 and 40 mmHg by modifying the respiratory frequency. In the MAN group, 8 of the 17 required rescue therapy with lactate, while in the LAC group, 5 of the 17 required rescue treatment with mannitol. Fifty-eight episodes were treated in all patients during the first 48 h of the study: 27 in MAN and 31 in LAC. Of these, 11 required rescue treatment, leading to a total of 69 treatments (30 MAN and 39 LAC). The percentage of episodes requiring rescue treatment was higher in MAN compared to LAC (29.6 vs. 9.6%, P = 0.053). Neurosedation as well as length of stay in ICU was comparable in both groups. No difference regarding patients’ characteristics, initial severity (prehospital GCS and Marshall score), length of stay in the ICU or neurological outcome was found regarding the need or not for rescue therapy (data not shown).
Effect of treatments on ICP and CPP
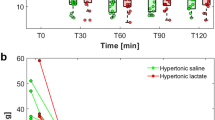
The effect of osmotherapy on ICP is shown in Fig. 1. ICP expressed by the difference from baseline (delta) decreased in the two groups following osmotherapy (time effect, P < 0.0001, Fig. 1a). However, significant differences were observed among groups: LAC was significantly lower than MAN (group effect P = 0.016). Moreover, the interaction between time and group effects was also significant (P = 0.0049), indicating a more prolonged and more pronounced change: at the fourth hour the decrease in ICP was −5.9 ± 1 versus −3.2 ± 0.9 mmHg for LAC and MAN respectively, P = 0.009. When considering the actual treatment administered (lactate alone, mannitol or lactate/mannitol when rescue therapy was necessary, Fig. 1b), we found that the three groups were different (P = 0.032). Interestingly, at the fourth hour, the effect of lactate alone was significantly more pronounced compared to both mannitol alone (P = 0.051) and lactate/mannitol (P = 0.005), while mannitol versus lactate/mannitol were not different (P = 0.67). When ICP is expressed as absolute values (median ± 25–75th percentiles, Fig. 1c), the decrease in the three groups following osmotherapy was significant (P < 0.0001), as were differences among groups (P < 0.001): lactate alone versus mannitol alone (P = 0.0061), lactate alone versus lactate/mannitol (P < 0.0001) and mannitol alone versus lactate/mannitol (P < 0.0001). Hence, there was a more pronounced decrease in ICP with lactate alone compared to the two other treatments: mannitol alone and mannitol plus sodium lactate.
Intracranial pressure changes during the first 4 h of treatment of hypertensive episodes by osmotherapy. Data are expressed as delta from T0 (mean ± SEM) (a, b) and as absolute values (median ± 25–75th percentiles) (c). a Changes in ICP from baseline (delta from baseline, mm Hg) following the treatments of episodes of intracranial hypertension (n = 58) were divided into two groups according to the initial randomization (intend-to-treat analysis). Filled circles lactate, n = 30; open circles mannitol, n = 28. Inter-group comparison by ANOVA for repeated measures: group effect P < 0.016; time effect P < 0.0001; interaction time-group effect P = 0.0049. Post-hoc intergroup analysis (student’s t test): 30 min P = 0.064; 60 min P = 0.008; 120 min P = 0.006; 180 min P = 0.001; 240 min P = 0.009. b Changes in ICP from baseline (delta from baseline, mm Hg) following the treatments of episodes of intracranial hypertension (n = 58) were divided into three groups according to the actual treatment received (i.e. initial randomized treatment followed or not by alternative crossover therapy: see text for explanation). Filled squares lactate alone, n = 25; open squares mannitol alone, n = 18; dashed squares mannitol/lactate, n = 15. Comparison between the three groups was assessed by ANOVA for repeated measures: group effect P = 0.032; time effect P < 0.0001; interaction time-group P = 0.007. Post-hoc analysis (unpaired student’s t test): mannitol alone versus lactate alone 180 min P = 0.004; 240 min P = 0.051; lactate alone versus lactate/mannitol 60 min P = 0.029; 120 min P = 0.025; 180 min P = 0.009; 240 min P = 0.005; mannitol alone versus mannitol/lactate no significant difference at any time. c ICP evolution (absolute values, mm Hg) following the treatments of episodes of intracranial hypertension (n = 58) were divided into three groups according to the actual treatment received (i.e. initial randomized treatment followed or not by alternative crossover therapy: see text for explanation). Filled squares lactate alone, n = 25; open squares mannitol alone, n = 18; dashed squares mannitol/lactate, n = 15. Comparison between the three groups was assessed by Kruskall-Wallis test, intergroup comparison P = <0.0001: lactate alone versus mannitol alone P = 0.0061; lactate alone versus lactate/mannitol P < 0.0001, mannitol alone versus lactate/mannitol P < 0.0001
As shown in Fig. 2, CPP increased in both groups (time effect P < 0.0001, Fig. 2a), and the difference between the two groups was close to significant (group effect P = 0.057). We found similar results when considering the actual treatments administered, i.e., when comparing the three groups lactate alone, mannitol alone and lactate/mannitol (time effect P < 0.0001, group effect P = 0.18, Fig. 2b).
Cerebral perfusion pressure changes during the first 4 h of treatment of hypertensive episodes by osmotherapy. Data are expressed as mean ± SEM. a Cerebral perfusion pressure during the treatments of intracranial hypertensive episodes. Episodes of HICP were divided into two groups according to the initial randomization (intend-to-treat analysis). Filled circles: lactate n = 30; open circles: mannitol, n = 28. Inter-group comparison by ANOVA for repeated measures: group effect P = 0.057; time effect P < 0.0001; interaction time-group effect P = 0.0001. b Cerebral perfusion pressure during the treatments of intracranial hypertensive episodes. Episodes of HICP were divided into three groups according to the actual treatment received (i.e. initial randomized treatment followed or not by alternative crossover therapy: see text for explanation). Filled squares lactate alone, n = 25; open squares mannitol alone, n = 18; dashed squares mannitol/lactate, n = 15. Comparison between the three groups was assessed by ANOVA for repeated measures: group effect P = 0.18; time effect P = 0.001; interaction time-group P < 0.0001
Urine output collected every hour (see Figure II suppl.) peaked at the first hour in the mannitol group and at the second hour in lactate group, however, no statistical differences were observed.
Metabolic, oxygenation and hemodynamic effects of osmotherapy
Basal metabolic (glucose, lactate, osmolality, sodium, chloride, pH, PaCO2, PaO2, total CO2) parameters were not different between the two treatments (Table 2). Neither plasma glucose nor lactate was affected by mannitol, while lactate infusion significantly increased glucose (+3.8 ± 1.3%, P < 0.01) and lactate (+48.6 ± 6.1%, P < 0.001), the difference between the groups being significant (P < 0.0001 and P = 0.04 for lactate and glucose respectively). Mannitol infusion was responsible for a moderate but significant decrease in both sodium and chloride, while after lactate infusion, only chloride was significantly affected. Lactate infusion moderately increased arterial pH (+0.5 ± 0.1%, P < 0.001). PaO2 and PaCO2 were not significantly affected by the treatments, while total CO2 increased following lactate administration (+5.1 ± 1.1%, P < 0.001). Plasma osmolality significantly rose following mannitol (+3.06 ± 0.67 mosm/L, P < 0.001), but not lactate (+0.66 ± 0.95 mosm/L, P = 0.491) infusion, the difference being significant (P = 0.046, (Table 2). SvJO2 significantly increased only after lactate administration (+2.48 ± 0.5%, P < 0.0001; Table 2). A positive and significant (P < 0.0001) arteriovenous difference in glucose, which was not affected by osmotherapy, was found after both treatments (data not shown), indicating similar brain glucose consumption. Lactate arteriovenous differences across the brain showed no significant effect in either treatment.
Neurological status was assessed after 1 year of evolution using the Glasgow Outcome, data are presented in the electronic material supplement.
Discussion
This study aimed to investigate the efficacy and safety of a specifically designed solution enriched with half-molar sodium lactate in comparison with mannitol in the treatment of episodes of intracranial hypertension in severe TBI. Our purpose was to compare the efficacy of a new treatment with the most standard recommended treatment; therefore, mannitol was taken as a control since it remains the reference osmotherapy treatment in the guidelines [4, 10, 11, 16, 17]. We report here that hyperosmolar lactate solution is safe and more effective than mannitol in achieving a prolonged decrease in intracranial pressure during acute episodes of intracranial hypertension (study endpoint). In this study we assumed independent outcomes (ICP decrease) of all different episodes considered (58), however considering the first episode only (i.e., 34 episodes, 17 in each group) lead to a similar conclusion, without assuming independency between episodes (see Table I suppl.). In addition, a better neurological status after 1 year was noted in the LAC group as compared to MAN (Fig. III suppl), but the sample size of this study was not powered for this purpose.
Patients were similar for the various initial criteria, which are important prognosis factors [2, 4, 32, 33]. Time before admission to the ICU [34] was slightly longer in the LAC group (P = 0.059). Failure to lower ICP was observed in 19% of all treated episodes; however, this occurred less often with lactate when compared to mannitol (P < 0.051, Table 1).
Metabolic parameters were comparable before acute infusion. Lactate infusion significantly increased plasma glucose (P < 0.05) and lactate (P < 0.001). However, the gluconeogenic effect of lactate is limited (3.8% or 0.22 mmol/L), and probably cannot be considered to be harmful to the brain [35]. The increase in lactate concentration is moderate (0.9 mM), indicating an active metabolism as already reported [36, 37].
No effect on gas exchange was observed in the two groups. The modest alkalinizing effect of lactate infusion [38] is negligible (less than 0.01 pH units) with no relevant effect on cerebral perfusion [39]. The significant increase in SvJO2 following lactate infusion, in the absence of any change in PaO2, indicates either a decrease in cerebral oxygen consumption, or an increase in cerebral blood flow. We failed to find any difference between lactate or mannitol groups in the following parameters known to accompany a decrease in brain oxygen consumption: identical patient sedation, no difference in body temperature, comparable brain glucose uptake (from AV difference) and lactate consumption (no lactate release). Furthermore, CPP increase was more pronounced after lactate. Lactate infusion could be responsible for a decrease in cerebral vascular resistance leading to increased cerebral blood flow as already suggested in experimental studies [29, 30].
Increased plasma osmolality upon mannitol infusion (1%, i.e., 3.06 mosm/kg, Table 2) is the expected consequence of the osmotic load resulting from hyperosmolar mannitol infusion (1160 mosm/kg). Interestingly, equivalent hyperosmolar sodium lactate infusion did not affect plasma osmolality (0.66 ± 0.95 mosm/kg, P = 0.491). Although these solutions are equally hyperosmolar, sodium lactate solution is less hypertonic since monocarboxylate carriers allow lactate to cross the cellular plasma membrane [40]. However, despite being less hypertonic than mannitol, lactate solution induces a stronger effect on ICP. The lactate solution used in this study contains metabolized (lactate) and non-metabolized (inorganic) ions. Lactate is rapidly metabolized, but non-organic ions are not, thus creating an imbalance between positive and negative charges. A net anion efflux from intracellular space must therefore compensate for the excess of extracellular positive charges due to sodium in order to maintain electroneutrality. Chloride, the principal intracellular inorganic anion, is responsible for a substantial part of intracellular tonicity, hence the net efflux of chloride is probably accompanied by a net flux of water. This hypothesis fits with the reported data in Table 2, showing an increase in sodium accompanied by no change in chloride concentrations in the LAC group, while both sodium and chloride decrease in the MAN group. Recent data on Na+, K+ and Cl− co-exchange support a significant role of chloride balance on cell volume regulation, especially in cerebral ischemia reperfusion [41]. In the light of this, a change in cell volume could result from the combination of increased extracellular tonicity and decreased intracellular tonicity. This hypothetical mechanism might explain why infusion of the sodium lactate solution induces a stronger effect on high ICP than mannitol, while changes in plasma osmolality were hardly detectable. The imbalance between intra- and extracellular tonicity due to mannitol disappears after its excretion into the urine, and water can then re-enter intracellular space. However, when exogenous sodium and chloride are eliminated into the urine, intracellular chloride loss persists, and there is no thermodynamic force to drive water back into cellular compartments.
A defect in cellular energy status is a major cause of cellular dysfunction leading to cellular swelling. Lactate is a suitable source of energy for the brain, and evidence from previous literature supports brain lactate metabolism from either local [24, 42, 43] or systemic [24, 27, 28] origins, thanks to monocarboxylate carriers in the BBB [44]. Sodium lactate is probably working not only as a hypertonic agent, but also as a metabolic fuel for the brain.
This study has several methodological limitations: it is a monocentric study with ethical constraints leading to the proposal of an early rescue therapy when treatment was judged to be ineffective after 15 min. Because of the specific metabolic effects of the two treatments (e.g., on plasma lactate and pH), it was not possible to conduct a double-blind study but only a prospective randomized open-label study. However, all data were analyzed in a blind manner including the 1-year neurological outcome evaluation.
In conclusion, hyperosmolar sodium-lactate solution appears to be an interesting alternative in the treatment of episodes of cranial hypertension in TBI patients. This solution is more effective on ICP than the reference treatment mannitol. However, a further multicenter study is warranted to confirm this finding.
References
Bulger EM, Nathens AB, Rivara FP, Moore M, MacKenzie EJ, Jurkovich GJ (2002) Management of severe head injury: institutional variations in care and effect on outcome. Crit Care Med 30:1870–1876
Jiang JY, Gao GY, Li WP, Yu MK, Zhu C (2002) Early indicators of prognosis in 846 cases of severe traumatic brain injury. J Neurotrauma 19:869–874
Stocchetti N, Penny KI, Dearden M, Braakman R, Cohadon F, Iannotti F, Lapierre F, Karimi A, Maas A Jr, Murray GD, Ohman J, Persson L, Servadei F, Teasdale GM, Trojanowski T, Unterberg A (2001) Intensive care management of head-injured patients in Europe: a survey from the European brain injury consortium. Intensive Care Med 27:400–406
Stocchetti N, Rossi S, Buzzi F, Mattioli C, Paparella A, Colombo A (1999) Intracranial hypertension in head injury: management and results. Intensive Care Med 25:371–376
Cooper DJ, Myles PS, McDermott FT, Murray LJ, Laidlaw J, Cooper G, Tremayne AB, Bernard SS, Ponsford J (2004) Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. Jama 291:1350–1357
Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, Cottingham R, Svoboda P, Brayley N, Mazairac G, Laloe V, Munoz-Sanchez A, Arango M, Hartzenberg B, Khamis H, Yutthakasemsunt S, Komolafe E, Olldashi F, Yadav Y, Murillo-Cabezas F, Shakur H, Edwards P (2004) Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet 364:1321–1328
Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR Jr, Muizelaar JP, Wagner FC Jr, Marion DW, Luerssen TG, Chesnut RM, Schwartz M (2001) Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med 344:556–563
Polderman KH, Tjong Tjin Joe R, Peerdeman SM, Vandertop WP, Girbes AR (2002) Effects of therapeutic hypothermia on intracranial pressure and outcome in patients with severe head injury. Intensive Care Med 28:1563–1573
Qiu W, Zhang Y, Sheng H, Zhang J, Wang W, Liu W, Chen K, Zhou J, Xu Z (2007) Effects of therapeutic mild hypothermia on patients with severe traumatic brain injury after craniotomy. J Crit Care 22:229–235
Schierhout G, Roberts I (2000) Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev CD001049
Marik PE, Varon J, Trask T (2002) Management of head trauma. Chest 122:699–711
Francony G, Fauvage B, Falcon D, Canet C, Dilou H, Lavagne P, Jacquot C, Payen JF (2008) Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med 36(3):795–800
Schwartz ML, Tator CH, Rowed DW, Reid SR, Meguro K, Andrews DF (1984) The University of Toronto head injury treatment study: a prospective, randomized comparison of pentobarbital and mannitol. Can J Neurol Sci 11:434–440
Sorani MD, Manley GT (2008) Dose-response relationship of mannitol and intracranial pressure: a metaanalysis. J Neurosurg 108:80–87
McManus ML, Soriano SG (1998) Rebound swelling of astroglial cells exposed to hypertonic mannitol. Anesthesiology 88:1586–1591
Mendelow AD, Teasdale GM, Russell T, Flood J, Patterson J, Murray GD (1985) Effect of mannitol on cerebral blood flow and cerebral perfusion pressure in human head injury. J Neurosurg 63:43–48
Paczynski RP (1997) Osmotherapy. Basic concepts and controversies. Crit Care Clin 13:105–129
Berger S, Schurer L, Hartl R, Deisbock T, Dautermann C, Murr R, Messmer K, Baethmann A (1994) 7.2% NaCl/10% dextran 60 versus 20% mannitol for treatment of intracranial hypertension. Acta Neurochir Suppl (Wien) 60:494–498
Doyle JA, Davis DP, Hoyt DB (2001) The use of hypertonic saline in the treatment of traumatic brain injury. J Trauma 50:367–383
Qureshi AI, Suarez JI, Bhardwaj A, Mirski M, Schnitzer MS, Hanley DF, Ulatowski JA (1998) Use of hypertonic (3%) saline/acetate infusion in the treatment of cerebral edema: Effect on intracranial pressure and lateral displacement of the brain. Crit Care Med 26:440–446
Battison C, Andrews PJ, Graham C, Petty T (2005) Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury. Crit Care Med 33:196–202 (discussion 257–198)
Schurr A (2002) Lactate, glucose and energy metabolism in the ischemic brain (Review). Int J Mol Med 10:131–136
Schurr A, Payne RS, Miller JJ, Rigor BM (1997) Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: further in vitro validation. J Neurochem 69:423–426
Maran A, Cranston I, Lomas J, Macdonald I, Amiel SA (1994) Protection by lactate of cerebral function during hypoglycaemia. Lancet 343:16–20
King P, Kong MF, Parkin H, MacDonald IA, Barber C, Tattersall RB (1998) Intravenous lactate prevents cerebral dysfunction during hypoglycaemia in insulin-dependent diabetes mellitus. Clin Sci (Lond) 94:157–163
Schurr A, Payne RS, Miller JJ, Tseng MT (2001) Preischemic hyperglycemia-aggravated damage: evidence that lactate utilization is beneficial and glucose-induced corticosterone release is detrimental. J Neurosci Res 66:782–789
Rice AC, Zsoldos R, Chen T, Wilson MS, Alessandri B, Hamm RJ, Bullock MR (2002) Lactate administration attenuates cognitive deficits following traumatic brain injury. Brain Res 928:156–159
Holloway R, Zhou Z, Harvey HB, Levasseur JE, Rice AC, Sun D, Hamm RJ, Bullock MR (2007) Effect of lactate therapy upon cognitive deficits after traumatic brain injury in the rat. Acta Neurochir (Wien) 149:919–927 (discussion 927)
Shackford SR, Schmoker JD, Zhuang J (1994) The effect of hypertonic resuscitation on pial arteriolar tone after brain injury and shock. J Trauma 37:899–908
Shackford SR, Zhuang J, Schmoker J (1992) Intravenous fluid tonicity: effect on intracranial pressure, cerebral blood flow, and cerebral oxygen delivery in focal brain injury. J Neurosurg 76:91–98
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1:480–484
Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, Jane JA, Luerssen TG, Marmarou A, Foulkes MA (1992) The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma 9 Suppl 1:S287–S292
Marmarou A (1994) Traumatic brain edema: an overview. Acta Neurochir Suppl (Wien) 60:421–424
Stocchetti N (2001) Risk prevention, avoidable deaths and mortality-morbidity reduction in head injury. Eur J Emerg Med 8:215–219
Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ (2005) Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology 64:1348–1353
Chiolero R, Tappy L, Gillet M, Revelly JP, Roth H, Cayeux C, Schneiter P, Leverve X (1999) Effect of major hepatectomy on glucose and lactate metabolism. Ann Surg 229:505–513
Mustafa I, Roth H, Hanafiah A, Hakim T, Anwar M, Siregar E, Leverve XM (2003) Effect of cardiopulmonary bypass on lactate metabolism. Intensive Care Med 29:1279–1285
Mustafa I, Leverve XM (2002) Metabolic and hemodynamic effects of hypertonic solutions: sodium-lactate versus sodium chloride infusion in postoperative patients. Shock 18:306–310
Stocchetti N, Maas AI, Chieregato A, van der Plas AA (2005) Hyperventilation in head injury: a review. Chest 127:1812–1827
Halestrap AP, Price NT (1999) The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 343 Pt 2:281–299
Chen H, Sun D (2005) The role of Na-K-Cl co-transporter in cerebral ischemia. Neurol Res 27:280–286
Cater HL, Benham CD, Sundstrom LE (2001) Neuroprotective role of monocarboxylate transport during glucose deprivation in slice cultures of rat hippocampus. J Physiol 531:459–466
Schurr A (2006) Lactate: the ultimate cerebral oxidative energy substrate? J Cereb Blood Flow Metab 26:142–152
Pellerin L, Bergersen LH, Halestrap AP, Pierre K (2005) Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res 79:55–64
Acknowledgments
The study has been sponsored by Innogene Kalbiotech Pte. Ltd. 24 Raffles Place 27-06 Clifford Center, Singapore, 04862. The authors are most grateful to Dr. Rikrik Ilyas for a constant support, to Professor Nino Stocchetti for helpful comments and suggestions, to Professor Mervyn Singer for stimulating discussions and careful review of the manuscript and to Mr. Gareth Butt for the English corrections to this paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ichai, C., Armando, G., Orban, JC. et al. Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intensive Care Med 35, 471–479 (2009). https://doi.org/10.1007/s00134-008-1283-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1283-5