Abstract
This trial aimed to determine the possible therapeutic and immunomodulatory effects of vitamin D3 in patients with knee OA. In this open-label clinical trial, symptoms were assessed over 3 months in patients with primary knee OA receiving oral vitamin D3 4000 IU/day. Clinical response was evaluated at baseline and 3 months using WOMAC subscores and VAS. Serum levels of cytokines IL-1β, TNF-α, IL-13, IL-17, IL-33, IL-4, and IL-10 were determined by ELISA method. Eighty patients with knee OA were included. All 80 completed the study; the median 25(OH)D3 level was 23.1 ng/ml at baseline and increased by 12.3 ng/ml after treatment. Vitamin D3 after 3 months of supplementation induced a significant reduction in VAS pain and WOMAC subscores. Using OMERACT-OARSI criteria, 86.7% of patients treated with vitamin D3 responded to treatment. At the end of 3 months, systemic values of IL-1β (p < 0.01), IL-23 (p < 0.01), and IL-33 (p < 0.01) were significantly increased, values of TNF-α (p < 0.01), IL-13 (p < 0.01), and IL-17 (p < 0.01) were significantly decreased, while value of IL-4 was not significantly changed. No adverse events were detected. Treatment with vitamin D is associated with improvement in pain, as well as stiffness and physical function. Vitamin D supplementation increased systemic values of IL-33. Our results indicate that vitamin D3 supplementation may be used as a novel therapeutic in knee OA. Future studies are needed to investigate a potential role of IL-33 in the pathogenesis of knee OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the exact pathogenesis of osteoarthritis (OA) remain unclear [1], evidence suggests that proinflammatory cytokines, specifically interleukin 1-beta (IL-1β) and tumor necrosis factor alpha (TNF-α) are key mediators in progression of cartilage degradation. These cytokines compromise the balance between catabolic and anabolic processes of the major cell type of cartilage tissue, chondrocytes, and lead to tissue failure [2]. Accumulating evidence has supported a crucial role of synovial inflammation in knee OA (KOA) pathogenesis [3]. This low-grade inflammation is triggered and maintained by complex activation of the innate immune system [1].
To present, OA does not have a cure, and treatment is related to symptom relief [4, 5]. Vitamin D deficiency is common in patients with OA. Vitamin D through the vitamin D receptors fulfills various biological roles in cartilage, bone, and muscle and may have significant protective effect on these tissues in OA [6]. 1,25(OH)2D3 has the potential to influence innate and adaptive immune system [7]. Some studies have demonstrated increased concentration of proinflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-15, IL-17, and IL-18 in the serum or synovial fluid of OA patients [8, 9]. Vitamin D deficiency is associated with chronic inflammation and the increased secretion of interleukin (IL)-33 [10]. IL-33 is a member of the IL-1 family of cytokines [11]. It is constitutive expressed by endothelial and epithelial cells [12]. IL-33 is usually released by damaged cells, and it facilitates immune response [11]. Its role in KOA is still unclear. In vitro and in vivo studies reported inhibitory effect of 1,25(OH)2D3 on production of proinflammatory and facilitating effect on production of anti-inflammatory cytokines in innate and adaptive immune systems cells [13].
The role of vitamin D deficiency in the pathogenesis of OA is poorly understood [14]. Low levels of vitamin D are associated with cartilage degeneration, radiographic progression, and incidence of OA [10, 15]. Moreover, vitamin D deficiency is related to pain sensitization, severity of pain and disability in KOA [16]. Pain exacerbation in KOA is linked with elevated serum levels of CRP, TNF-α and IL-6 [17]. However, data on the effect of vitamin D supplementation on proinflammatory cytokines in patients with OA of the knee are lacking.
The aim of the present study was to evaluate the possible therapeutic and immunomodulatory effect of vitamin D3 in KOA treatment. To the best of our knowledge, serum levels of IL-33 have not been evaluated in KOA patients; accordingly, this is the first report regarding the status of IL-33 and vitamin D3 in KOA patients.
Methods
Study design
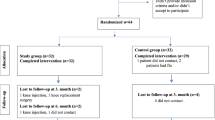
We conducted a 3-month open-label trial. In total, 80 patients with KOA visiting the Clinic for Orthopedics and traumatology of University Clinical Center, Kragujevac, were enrolled. The study was conducted from October 2020 to March 2021. The study protocol was approved by the Ethics Committee of University Clinical Center Kragujevac (reference number 01/17–4317), and all patients provided written informed consent. Inclusion criteria were the following: clinical and radiographic diagnosis of knee OA met American College of Rheumatology (ACR) criteria and Kellgren-Lawrence (K-L) grading of 2 or 3, age ≥ 45 years, constant pain defined as a minimal mean score of 40 mm on the visual analogue scale (VAS) for global pain (0–100 mm range for each), daily pain during the month before study enrollment, and no knee deformity or ankyloses. Patients were excluded if they had severe radiographic knee OA, grade 4 according to K-L score, secondary causes of OA, rheumatoid, psoriatic or septic arthritis, acute respiratory infections based on clinical symptoms and laboratory parameters, systemic disease like lupus or cancer, diseases at risk of hypercalcemia (such as primary hyperparathyroidism, sarcoidosis), severe cardiac, renal or liver impairment, any condition possibly affecting oral drug absorption (for example, gastrectomy or malabsorption syndromes), significant trauma to knees, including knee surgery, arthroscopy or significant injury to ligaments or menisci of the knee within one preceding the study, history of taking drugs that may affect bone metabolism (bisphosphonates, hormone replacement therapy, calcitonin) within 2 years, therapy with anticoagulants, parathyroid hormone, corticosteroids, non-steroidal anti-inflammatory drugs, thiazide diuretics, previous use of vitamin D supplements or intra-articular steroid injection within 3 months, and hypersensitivity to vitamin D.
Randomization and blinding
Participants were randomly assigned, based on a computer-generated random numbers list, to one of the two groups, either the vitamin D supplementation or without supplementation group at a ratio of 3:1. Investigators and participants were blinded to treatment allocation, and study treatments were identical in appearance.
Interventions
Participants in the treatment group were given the vitamin D3 (cholecalciferol) solution (Vigantol®, 20,000 IU /ml, oral drops, solution; INN:Cholecalciferol; Merck KGaA, Darmstadt, Germany) 4000 IU/day for the 3 months. Participants in the control group did not receive the vitamin D3. Moreover, all the patients were told not to receive other interventions such as exercise or lifestyle modification. During the study period, patients were allowed to take acetaminophen 500 mg tablets up to 2 g/day (500 mg four times daily) as rescue medication, except during the 48 h before clinical evaluation. We recorded the following demographic and clinical characteristics at baseline: age, sex, plasma 25(OH)D3 levels, duration of knee pain, knee circumference, and body mass index (kg/m2). Radiographic severity was evaluated through anteroposterior knee X-ray examination in the standing position and graded on the K-L scale [18].
Outcomes
In clinical trials with OA patients, improvement in symptoms was commonly assessed using the analysis of mean changes in the Western Ontario and McMaster Universities OA Index (WOMAC) and/or a 100-mm visual analogue scale (VAS). Categorization of individual response to treatment in OA clinical trials was done by established responder criteria. These involve the mean change in the WOMAC subscores [19] and the OMERACT-Osteoarthritis Research Society International (OMERACT-OARSI) responder criteria [20]. We used a modified OMERACT-OARSI criteria without patient global assessment. OMERACT-OARSI responders were participants who had a knee improvement in pain or function ≥ 50% and an absolute change in pain or function ≥ 20 points, by the mean WOMAC pain or the mean WOMAC function subscore. Another group of responders in this study was achieved improvement in both the mean WOMAC pain and the mean WOMAC function subscore ≥ 20% and an absolute change ≥ 10 points. Efficacy of vitamin D was evaluated as the proportion of responders (%) at 3 months in the intervention group. A 20–30% result is expected in KOA when evaluated by the OMERACT-OARSI criteria [20].
Measurement of cytokines levels in sera
At baseline and 3 months after, fasting morning venous blood was collected and centrifuged, with serum samples stored at − 80 °C before use. Serum levels of cytokines (IL-1β, TNF-α, IL-4, IL-13, IL-17, IL-23, IL-33) were determined by ELISA method, as described previously [21]. We used the commercially available ELISA tests following the instructions of the manufacturer (R&D Systems, Minneapolis, Minn, USA).
Statistical analysis
Data were analyzed using IBM SPSS Statistics version 22 (SPSS, Inc., Chicago, IL, USA). Kolmogorov–Smirnov or Shapiro–Wilk test was used in order that evaluate normality of data distribution. Student’s t test was used to test the time differences between groups (treatment vs. control). The chi-squared test was used to explore the proportion of OMERACT-OARSI responders within each group. Correlation between variables was tested by Spearman’s rank correlation coefficient. Data are summarized as mean ± standard deviation (SD). A p value less than 0.05 for differences and correlations was considered to be statistically significant.
Results
Baseline characteristics, such as, sex, age, BMI, duration of disease, and outcome parameters, are reported in Table 1. No adverse events were detected during the study.
Effect of vitamin D3 supplementation on plasma 25(OH)D3 level
Among 60 participants in the vitamin D supplementation group, the median 25(OH)D level at baseline was 23.1 ± 9.1 ng/ml. After 4000 IU of vitamin D3 supplementation per day for 3 months, there was a statistically significant increase in mean serum 25(OH)D level to 35.4 ± 14.6 ng/mL (p < 0.0005).
Vitamin D3 supplementation reduced pain intensity, stiffness, and improved physical functioning
In KOA patients treated with the 4000 IU vitamin D3, we found that VAS pain and WOMAC pain scores decreased significantly after 3 months of treatment. Consistently, WOMAC stiffness and function scores improved after vitamin D3 supplementation (Table 2).
There were differences at 3 months between treatment groups in the WOMAC pain score, with a decrease of 39.5% in the vitamin D supplementation group and an increase of 43.7% in the without supplementation group; WOMAC stiffness score, with an increase of 24% in the vitamin D group, compared with a decrease of 60% in the without supplementation group (p˂0.0005); WOMAC function score, with a decrease of 42.2% in the vitamin D group compared with an increase of 45.1% in the without supplementation group (p˂0.0005); and VAS, with a decrease of 44.2% in the vitamin D group versus an increase of 32.5% in the without supplementation group (p˂0.0005).
Correlation between vitamin D3 concentration and clinical parameters of disease severity
Analyses of correlation between vitamin D3 concentration and clinical parameters of KOA were made. The results showed absence of correlation between vitamin D3 concentration and knee circumference, WOMAC pain score, WOMAC stiffness score, VAS pain score, at baseline as well as in group without supplementation (data not shown), but moderate negative correlation between vitamin D3 concentration and knee circumference (r = − 0.237; p = 0.048), WOMAC pain score (r = − 0.353; p = 0.006), WOMAC stiffness score (r = − 0.348; p = 0.006), and VAS pain score (r = − 0.311; p = 0. 016), after vitamin D3 treatment (Table 3).
Vitamin D3 supplementation altered cytokine profile
Serum cytokines of interest were measured in KOA patients prior and after vitamin supplementation. Systemic values of IL-1β (p < 0.01), IL-23 (p < 0.01), and IL-33 (p < 0.01) were significantly increased, values of TNF-α (p < 0.01), IL-13 (p < 0.01), and IL-17 (p < 0.01) were significantly decreased, while value of IL-4 was not significantly changed, after vitamin D3 treatment (Fig. 1). The absence of vitamin D3 supplementation increased systemic values of IL-1β (p < 0.01), while it did not significantly alter concentration of other cytokines of interest (Fig. 1).
Systemic cytokine profile before and after treatment with/without vitamin D3. IL-1β, TNF-α, IL-4, IL-13, IL-17, IL-23, and IL-33 were measured by ELISA in the sera of patients with KOA prior and after treatment with/without vitamin D3. Statistical significance was tested by Mann–Whitney rank sum test
Increased ratios of IL-33 with proinflammatory cytokines after vitamin D3 supplementation
Analyses of the ratios of IL-33 with IL-1β, TNF-α, IL-4, IL-13, IL-23, and IL-17 were done. All analyzed ratios IL-33/IL-1β (p < 0.01), IL-33/TNF-α (p < 0.01), IL-33/IL-4 (p < 0.01), IL-33/IL-13 (p < 0.01), IL-33/IL-23 (p < 0.01), and IL-33/IL-17 (p < 0.01) were significantly higher after vitamin D3 treatment (Fig. 2). Additionally, IL-33/IL-4 (p < 0.01) and IL-33/IL-23 (p < 0.01) ratios were significantly higher after placebo treatment (Fig. 2).
Ratio of IL-33 and proinflammatory cytokines before and after treatment with/without vitamin D3. IL-1β, TNF-α, IL-4, IL-13, IL-17, IL-23, and IL-33 were measured by ELISA in the sera of KAO patients prior and after treatment with/without vitamin D3. Ratios of IL-33/IL-1β, IL-33/TNF-α, IL-33/IL-4, IL-33/IL-13, IL-33/IL-23, and IL-33/IL-17 were evaluated for each patient, separately. Statistical significance was tested by Mann–Whitney rank sum test
IL-33 negatively correlates with clinical parameters of disease, after vitamin D3 supplementation
Further, we have tested the correlation of the serum level of IL-33 with the clinical parameters of KOA, prior and after vitamin D3/placebo supplementation. The results showed absence of correlation between IL-33 concentration and all tested clinical parameters at baseline as well as after placebo treatment (data not shown), but negative correlation between IL-33 concentration and knee circumference (r = − 0.512; p = 0.001), WOMAC pain score (r = − 0.487; p = 0.001), WOMAC stiffness score (r = − 0.358; p = 0.006), VAS pain score (r = − 0.541; p = 0. 016), after vitamin D3 treatment. Finally, we found negative inter-correlation between IL-33 and vitamin D3 concentration (r = − 0.244; p = 0. 029) prior, but positive inter-correlation between them after vitamin supplementation (r = 0.282; p = 0. 011).
Discussion
KOA is condition that cause disability and vitamin D deficiency among with KOA patients leading to disability worsening and deterioration of functional ability [22]. We conducted an open-label study to assess the efficacy of 4000 IU oral vitamin D3 daily for 3 months on pain, stiffness, function, and circulating inflammatory markers in KOA patients. Using OMERACT-OARSI established criteria, 86.7% of patients treated with vitamin D responded to treatment, with clinically meaningful improvements in pain and function. We found that clinical outcomes displayed a minimal clinical significant improvement as per OARSI recommendation of 20%.
Data from observational studies revealed that vitamin D supplementation may reduce pain and disability in KOA [16]. However, there is inconsistent evidence from randomized clinical trials [23]. Sanghi et al. showed in patients with KOA and low vitamin D level that vitamin D3 supplementation at daily dosage of 60,000 IU for 10 days and followed by monthly dosage of 60,000 IU for 1 year was significantly associated with improvement of knee pain and function [24]. Jin et al. also have assessed the influence of cholecalciferol supplementation at monthly dosage of 500,000 IU for 24 months in KOA patients with vitamin D deficiency. Post hoc analysis revealed significant reduction of knee pain in the vitamin D group as compared with the placebo group. Moreover, the improvement in KOA symptom measured by the WOMAC pain and WOMAC function score was larger in patients who received vitamin D [25]. The results of our study are in line with previous randomized clinical trials, and 4000 IU vitamin D3 supplementation improves pain and disability in KOA patients.
On the contrary to our results, two randomized double-blind, placebo-controlled trials demonstrated that supplementation of vitamin D with a low daily dose had no beneficial effect on symptoms in KOA [26, 27]. We found that the arthritic symptoms were significantly improved in the vitamin D supplemental group; however, in this study, we used high‐dose of the vitamin D. In a study by McAlindon et al., majority (51%) of KOA patients in the vitamin D supplemental group had advanced KOA with K-L grades of 3 and 4 [27]. Selection of patients with early KOA is preferable because structural abnormalities at earlier stages are likely more reversible and possibly more responsive to vitamin D treatment as compared with patients with advanced KOA. This discrepancy may be addressed to factors such as vitamin D dosages, severity of KOA, age, baseline serum vitamin D level, and obesity. The influence of these factors can undermine the therapeutic effect of vitamin D supplementation. These conflicting findings could be a result of the fact that KOA is a highly heterogeneous disease with multiple etiologies.
A pooled analysis of the four studies showed significant decreased in WOMAC pain and stiffness scores, but not in WOMAC function score after vitamin D treatment at dosage more than 2000 IU/day, in contrast with our results. [28, 29]. A meta-analysis of 1599 KOA patients demonstrated similar results with our study. The vitamin D supplementation in KOA patients was significantly linked to reduction in WOMAC subscores [30]. Another meta‐analysis of nineteen placebo‐controlled clinical trials found that decreased in pain score was greater in vitamin D group [22].
It is believed that immunopathology is the main mechanism in the genesis and progression of KOA [9]. The osteochondral destruction, which is directly related to the severity of the disease, is due to an intense immune response [31]. There is sample evidence that both, innate and acquired immunity participate in immunopathogenesis of OA [11]. Our results revealed that vitamin D3 supplementation inhibits type 1 and type 17 immune responses in KAO patients, as evidenced by decreased systemic values of TNF-α, IL-13, and IL-17 (Fig. 1). In line with our findings, recent studies have suggested the association of vitamin D3 supplementation with similar immune system alteration in OA patients [32]. In vitro, these effects result in decreased production of proinflammatory markers such as: TNF-α, interferon gamma (IFN-γ), IL-2, IL-12, IL-17, and IL-21 but with increased production of anti-inflammatory cytokines such as IL-10 [7]. Vitamin D affects T cell responses indirectly, by inhibiting antigen presenting cells (downregulation of its antigen presentation function), and directly, by inhibiting the production of IL-2, IL-17, and IL-21 and by stimulating IL-4 and IL-10 production [8, 33]. Interestingly, we found increment of proinflammatory innate immunity cytokines IL-1β and IL-23 after vitamin D3 supplementation (Fig. 1). Still, their "mates in acquired immunity (TNF-α and IL-17) were decreased after the same treatment, indicating a potentially different impact of vitamin D3 supplementation on innate and acquired immunity in KOA patients. Treatment without vitamin D3 was also followed by increment of IL-1β (Fig. 1), implicating vitamin D3 independent alteration of this cytokine.
Presented data implicate that vitamin D3 supplementation inhibits development of proinflammatory type 1 and type 17 immune responses in KAO patients. Same effect of vitamin D3, as inductor of T cells differentiation towards a more tolerogenic state with an induction of T helper-2 (Th2) cells and regulatory T cells (T regs) and as down regulator of proinflammatory Th1 and Th17 cells, was established previously [33]. In our study, the representative Type 2 cytokine IL-4 was slightly increased after vitamin D3 supplementation; however, this alteration did not reach statistical significance (Fig. 1). Other cytokine IL-33, previously established as type 2 cytokine [11], was significantly increased in sera of vitamin D3 treated KAO patients, supporting the phenomenon that vitamin D3 supplementation facilitates type 2 while inhibiting type 1 and type 17 immune responses in KAO patients. Increment of IL-1β and IL-33 can be explained by their common affiliation to IL-1 cytokine family and fact that IL-1β and IL-33 both use same receptor IL-1R3 (IL-1RAcP) and subsequently activate same signaling axis [12, 34]. It appears that vitamin D3 supplementation did not inhibit IL-1β production or its inhibition was outweighed by the facilitating effect of IL-33 on IL-1β [35, 36].
IL-33 concentration is significantly increased in sera of vitamin D3 treated KOA patients (Fig. 1). The exact role of this pleiotropic cytokine in the biology of OA in not fully understood. It has been shown that IL-33 polarizes naïve T cells toward type 2 phenotype, to produce IL-4, IL-5, and IL-13 [11, 37]. Our previous studies revealed that IL-33 facilitates differentiation toward type 2 and suppress type 1 and 17 immune responses in experimental tumor models [38, 39]. It appears that vitamin D3 and IL-33 acts in the same manner, when it comes to immunomodulatory properties [40]. As systemic concentration of both increases, it implicates on their synergistic role in KOA patients. Finding of positive inter-correlation between IL-33 and vitamin D3 concentration after vitamin D supplementation supports previous statement. Further, significantly increased values of IL-33/IL-1β, IL-33/TNF-α, IL-33/IL-4, IL-33/IL-13, IL-33/IL-23, and IL-33/IL-17 ratios in vitamin D3 treated KAO patients (Fig. 2) point on predominance of IL-33 over proinflammatory cytokines after vitamin D3 supplementation. This finding additionally suggests a relationship between IL-33 and vitamin D3 treatment. Vitamin D and the IL-33/ST2 signaling pathways are closely involved in bone remodeling in psoriatic arthritis (PsoA) [41]. Polarization of immune response toward type 2 is promoted by IL-33 and vitamin D, which are stimulators of both regulatory and Th2 cells. It is likely that vitamin D and IL-33 share some signal pathways, as they share immunological functions [42]. Moreover, vitamin D acts through vitamin D receptors, which are expressed on different types of cells, including immunocompetent cells that can produce IL-33 [43]. A study by He Z et al. showed increased expression of IL-33 and its receptor ST2 in OA patients, and that use of monoclonal antibodies against IL-33 and ST2 attenuates both OA and pain in experimental model of OA (41). This apparent contradiction in role of IL-33 in arthritis could be explained by its pleiotropic function. IL-33, depending on the type of tissue and cytokine milieu, can have different effects in arthritis.
Conclusion
Our findings indicate that KOA patients may have benefit from vitamin D3 supplementation at dosage 4000 IU/day. Using OMERACT-OARSI criteria, 86.7% of patients treated with vitamin D responded to treatment, with clinically meaningful improvements in knee pain, stiffness, and function. Vitamin D supplementation also diminished type 1 and 17 immune response and increased systemic values of IL-33. Predominance of IL-33 over pro-inflammatory mediators in patients KOA may present a mechanism for limiting the inflammatory process and subsequent tissue damage and points to IL-33 as an important regulator of immune response interplay in KOA patients after vitamin D treatment. Future studies are needed to investigate the exact mechanism underlying the effect of IL-33 in biology of KOA.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to [individual privacy could be compromised] but are available from the corresponding author on reasonable request.
References
Orlowsky EW, Kraus VB. The role of innate immunity in osteoarthritis: when our first line of defense goes on the offensive. J Rheumatol. 2015;42(3):363–71. https://doi.org/10.3899/jrheum.140382.
Rainbow R, Ren W, Zeng L. Inflammation and joint tissue interactions in oa: implications for potential therapeutic approaches. Arthritis. 2012;2012:741582. https://doi.org/10.1155/2012/741582.
Wang Y, Teichtahl AJ, Pelletier JP, et al. Knee effusion volume assessed by magnetic resonance imaging and progression of knee osteoarthritis: data from the Osteoarthritis Initiative. Rheumatology (Oxford). 2019;58(2):246–53. https://doi.org/10.1093/rheumatology/key274.
Wang K, Xu J, Hunter DJ, Ding C. Investigational drugs for the treatment of osteoarthritis. Expert Opin Invest Drugs. 2015;24:1539–56. https://doi.org/10.1517/13543784.2015.1091880.
Wang XB, Zhao FC, Yi LH, et al. MicroRNA-21-5p as a novel therapeutic target for osteoarthritis. Rheumatology (Oxford). 2019;58(8):1485–97. https://doi.org/10.1093/rheumatology/kez102.
Cao Y, Winzenberg T, Nguo K, Lin J, Jones G, Ding C. Association between serum levels of 25-hydroxyvitamin D and osteoarthritis: a systematic review. Rheumatology (Oxford). 2013;52(7):1323–34. https://doi.org/10.1093/rheumatology/ket132.
Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881–6. https://doi.org/10.2310/JIM.0b013e31821b8755.
Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. https://doi.org/10.1038/nrrheum.2010.196.
Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. https://doi.org/10.1155/2014/561459.
Rai V, Radwan MM, Agrawal DK. IL-33, IL-37, and vitamin D interaction mediate immunomodulation of inflammation in degenerating cartilage. Antibodies. 2021;10(4):41. https://doi.org/10.3390/antib10040041.
Pavlović S, Zdravković N, Radosavljević G, et al. Interleukin-33/ST2: a new signaling pathway in immunity and immunopathology. Vojnosanit Pregl. 2012;69(1):69–77. https://doi.org/10.2298/vsp1201069p.
Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;28:8–27. https://doi.org/10.1111/imr.12621.
Shipton EA, Shipton EE. Vitamin D and pain: vitamin D and its role in the aetiology and maintenance of chronic pain states and associated comorbidities. Pain Res Treat. 2015;2015:904–67. https://doi.org/10.1155/2015/904967.
Alkan G, Akgol G. Do vitamin D levels affect the clinical prognoses of patients with knee osteoarthritis? J Back Musculoskelet Rehabil. 2017;30(4):897–901. https://doi.org/10.3233/BMR-160589.
Zhang FF, Driban JB, Lo GH, et al. Vitamin D deficiency is associated with progression of knee osteoarthritis. J Nutr. 2014;144(12):2002–8. https://doi.org/10.3945/jn.114.193227.
Glover TL, Horgas AL, Fillinbim RB, Goodin BR. Vitamin D status and pain sensitization in knee osteoarthritis: a critical review of the literature. Pain Manag. 2015;5:447–53. https://doi.org/10.2217/pmt.15.43.
Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis. 2013;72(4):535–40. https://doi.org/10.1136/annrheumdis-2011-201047.
Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16(4):494–502. https://doi.org/10.1136/ard.16.4.494.
Bellamy N, Bell MJ, Goldsmith CH, et al. Evaluation of WOMAC 20, 50, 70 response criteria in patients treated with hylan G-F 20 for knee osteoarthritis. Ann Rheum Dis. 2005;64:881–5. https://doi.org/10.1136/ard.2004.026443.
Pham T, Van Der Heijde D, Altman RD, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr Cartil. 2004;12:389–99. https://doi.org/10.1016/j.joca.2004.02.001.
Jovanovic M, et al. Metabolic syndrome attenuates ulcerative colitis: correlation with interleukin-10 and galectin-3 expression. World J Gastroenterol. 2019;25:6465–82. https://doi.org/10.3748/wjg.v25.i43.6465.
Wu Z, Malihi Z, Stewart AW, Lawes CM, Scragg R. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415–27.
Hussain S, Singh A, Akhtar M, et al. Vitamin D supplementation for the management of knee osteoarthritis: a systematic review of randomized controlled trials. Rheumatol Int. 2017;37:1489–98. https://doi.org/10.1007/s00296-017-3719-0.
Sanghi D, Mishra A, Sharma AC, et al. Does vitamin D improve osteoarthritis of the knee: a randomized controlled pilot trial. Clin Orthop Relat Res. 2013;471:3556–62. https://doi.org/10.1007/s11999-013-3201-6.
Jin X, Jones G, Cicuttini F, et al. Effect of vitamin D supplementation on tibial cartilage volume and knee pain among patients with symptomatic knee osteoarthritis: a randomized clinical trial. JAMA. 2016;315(10):1005–13. https://doi.org/10.1001/jama.2016.1961.
Arden NK, Cro S, Sheard S, et al. The effect of vitamin D supplementation on knee osteoarthritis, the video study: a randomised controlled trial. Osteoarthr Cartil. 2016;24:1858–66. https://doi.org/10.1016/j.joca.2016.05.020.
Mc Alindon T, Lavalley M, Schneider E, et al. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis. JAMA. 2013;309:155–62. https://doi.org/10.1001/jama.2012.164487.
Gao XR, Chen YS, Deng W. The effect of vitamin D supplementation on knee osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2017;46:14–20. https://doi.org/10.1016/j.ijsu.2017.08.010.
Diao N, Yang B, Yu F. Effect of vitamin D supplementation on knee osteoarthritis: a systematic review and meta-analysis of randomized clinical trials. Clin Biochem. 2017;50:1312–6. https://doi.org/10.1016/j.clinbiochem.2017.09.001.
Zhao ZX, He Y, Peng LH, et al. Does vitamin D improve symptomatic and structural outcomes in knee osteoarthritis? A systematic review and meta-analysis. Aging Clin Exp Res. 2021;33(9):2393–403. https://doi.org/10.1007/s40520-020-01778-8.
Tateiwa D, Yoshikawa H, Kaito T. Cartilage and bone destruction in arthritis: pathogenesis and treatment strategy: a literature review. Cells. 2019;8(8):818. https://doi.org/10.3390/cells8080818.
Glover TL, Horgas AL, Fillingim RB, Goodin BR. Vitamin D status and pain sensitization in knee osteoarthritis: a critical review of the literature. Pain Manag. 2015;5(6):447–53. https://doi.org/10.2217/pmt.15.43.
Martens P, Gysemans C, Verstuyf A, Mathieu C. Vitamin D’s effect on immune function. Nutrients. 2020;12:1248. https://doi.org/10.3390/nu12051248.
Milosavljevic MZ, Jovanovic IP, Pejnovic NN, et al. Deletion of IL-33R attenuates VEGF expression and enhances necrosis in mammary carcinoma. Oncotarget. 2016;7(14):18106–15.
Griesenauer B, Paczesny S. ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol. 2017;8:475. https://doi.org/10.3389/fimmu.2017.00475.
Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33- activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. 2011;41(6):1675–86. https://doi.org/10.1002/eji.201041033.
Jovanovic I, Pejnovic N, Radosavljevic G, Arsenijevic N, Lukic ML. IL-33/ST2 axis in innate and acquired immunity to tumors. OncoImmunology. 2012;1(2):229–31. https://doi.org/10.4161/onci.1.2.18131.
Jovanovic I, Radosavljevic G, Mitrovic M, et al. ST2 deletion enhances innate and acquired immunity to murine mammary carcinoma. Eur J Immunol. 2011;41(7):1902–12. https://doi.org/10.1002/eji.201141417.
Jovanovic I, Pejnovic N, Radosavljevic G, et al. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoural accumulation of immunosuppressive and innate lymphoid cells. Int J Cancer. 2014;134(7):1669–16682. https://doi.org/10.1002/ijc.28481.
He Z, Song Y, Yi Y, et al. Blockade of IL-33 signalling attenuates osteoarthritis. Clin Transl Immunol. 2020;9(10):e1185. https://doi.org/10.1002/cti2.1187.
De Martinis M, Ginaldi L, Sirufo MM, et al. IL-33/vitamin D crosstalk in psoriasis-associated osteoporosis. Front Immunol. 2021;11:604055. https://doi.org/10.3389/fimmu.2020.604055.
Sirufo MM, Ginaldi L, Martinis DE, M,. The IL-33/ST2 axis and vitamin D as a possible emerging therapeutic target in osteoarthritis. Rheumatology (Oxford). 2021;60(8):e300. https://doi.org/10.1093/rheumatology/keab292.
Annalora AJ, Jozic M, Marcus CB, Iversen PL. Alternative splicing of the vitamin D receptor modulates target gene expression and promotes ligand in dependent functions. Toxicol Appl Pharmacol. 2019;364:55–67. https://doi.org/10.1016/j.taap.2018.12.009.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ana Divjak, Mirjana Veselinovic, and Ivan Jovanovic. The first draft of the manuscript was written by Aleksandar Matic, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University Clinical Center, Kragujevac (Date 23.10.2017. / No 01/17–4317).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Divjak, A., Jovanovic, I., Matic, A. et al. The influence of vitamin D supplementation on the expression of mediators of inflammation in knee osteoarthritis. Immunol Res 71, 442–450 (2023). https://doi.org/10.1007/s12026-022-09354-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-022-09354-0