Abstract
The purpose of this study was to investigate the therapeutic and the chemical effects of balneological treatment (peloidotherapy + hydrotherapy), and its effects on serum levels of interleukin-1beta (IL-1β), tumor necrosis factor-alpha (TNF-α), and insulin-like growth factor–1 (IGF-1) in patients with knee osteoarthritis (OA). Sixty-four (64) knee OA patients were randomly divided into study and control groups. Balneological treatment, consisting of hydrotherapy, and peloidotherapy were given to both groups. Unlike the study group, in the control group, the peloid was applied over a stretch film cover, preventing any contact between the skin and peloid. Clinical outcome measures of the study were pain degree, patient’s and investigator’s global assessment on visual analog scale (VAS-pain, VAS-PGA, VAS-IGA), and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (pain, stiffness, and physical function). Patients were evaluated at baseline, post-treatment (after 10th session), and 3 and 6 months after treatment. Blood samples were taken at baseline, post- treatment, and 6 months after treatment for analysis of IL-1β, TNF-α, and IGF-1 serum levels. When compared with the baseline, VAS measurements decreased significantly in almost all evaluation periods in both groups, and no difference was observed between the groups. In the study group, WOMAC scores showed significant improvement in all assessments. In the control group, pain and physical function subscores of WOMAC significantly decreased at post-treatment and 3 months after treatment. In group comparison, pain and stiffness subscores showed a significant difference in favor of the study group at 6 months after treatment. No clinically significant improvement was seen in levels of IL-1β and IGF-1 in both groups during the whole assessment period. Because of TNF-α kit failure, we could not evaluate the measurements. In conclusion, balneological treatment is an effective treatment option to improve the pain and functional capacity of patients with knee OA. The application of peloid by contact with the skin is superior in the long-term period, which means that in addition to the thermal effect, the chemical content of peloid can also contribute to the therapeutic effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is one of the most common chronic health problems and can lead to pain and disability as well as depression, sleep disturbances, cardiovascular disease, and death (Vina and Kwoh 2018). In a recent study, the pooled global prevalence of knee OA was reported 22.9% in those aged ≥ 40 (Cui et al. 2020). The main characteristics of OA are disruption of articular cartilage, subchondral bone remodeling, inflammation of the synovium, ligament and menisci injury, and periarticular muscle weakness (Güngen et al. 2016). Despite OA being classified as a non-inflammatory joint disease, it is widely accepted that synovial inflammation and innate immune responses are implicated in the pathogenesis (Scanzello and Goldring 2012). Studies have shown that disease-associated molecular patterns (DAMPs) such as cartilage extracellular matrix breakdown products, intracellular alarmins, plasma proteins, and joint crystals have an important role in the pathogenesis of OA (Sokolove and Lepus 2013). DAMPs released after joint trauma and/or overuse induce the expression of inflammation-related mediators and catabolic proteases via triggering intercellular signaling pathways (Sokolove and Lepus 2013; Griffin and Scanzello 2019). Proinflammatory cytokines such as IL-1β and TNF-α are among the mediators secreted in OA, and they promote the inflammatory cascade by stimulating the release of many other mediators along with them (Chow and Chin 2020). In vitro studies have shown that IL-1β leads to impairment by inhibiting the production of cartilage matrix molecules like aggrecan and collagen type II and IX (Sokolove and Lepus 2013). Moreover, it has been reported that inflammatory mediators produced in the articular tissues can be detected outside the joint, in the systemic circulation of OA patients (Ortega et al. 2017). IL-1β and TNF-α were found to be higher in blood samples of OA patients compared to healthy subjects (Gálvez et al. 2017). Insulin-like growth factor-1 (IGF-1) is one of the growth factors defined in cartilage homeostasis and is suggested to be responsible for inadequate repair in the development of OA (Malemud 2010). In many studies, it has been shown experimentally that it protects the cartilage matrix damaged by inflammatory cytokines against degradation (Weimer et al. 2012). OA occurs as a result of the imbalance of all these anabolic and catabolic processes (Mueller and Tuan 2011).
The purpose of treatment approaches in knee OA is to increase the quality of life and functional capacity by reducing pain (Sarsan et al. 2012). Balneological treatment is one of the non-pharmacological treatment options recommended for OA in the European League Against Rheumatism (EULAR) guideline (Karagülle et al. 2007). Peloidotherapy, balneotherapy, and hydrotherapy are the most widely used balneological methods in thermal medicine (Bender et al. 2005). Vasodilation caused by thermal stimulation increases vasomotion, metabolism, and elasticity of connective tissue, resulting in relaxation in muscle tissue and reduction in pain (Sarsan et al. 2012). In addition, thermal stress provokes the release of adrenocorticotropic hormone (ACTH), cortisol, prolactin, and growth hormone without altering the circadian rhythm (Fioravanti et al. 2017). The thermal stimulus affects the hypothalamus-pituitary-adrenal axis, and the released corticosteroids and endorphins reduce edema, inflammation, and pain threshold (Fioravanti et al. 2017). Multiple studies have shown that balneological treatment improves pain level and functional conditions in various musculoskeletal diseases such as fibromyalgia, rheumatoid arthritis, and low back pain (Karagülle and Karagülle 2004). Although the researches are heterogeneous, considering the anti-inflammatory, antioxidant, and chondroprotective effects of balneological treatment, it has been shown that it can be effective as a complementary approach in various rheumatic diseases (Cheleschi et al. 2021).
Peloids (common name is mud) are therapeutic substances that are consist of organic and/or inorganic substances formed over many years by chemical and physical processes and provide heat transfer by conduction (Özkuk et al. 2017). The heat absorbed by the peloid is stored for a long time and released slowly, thereby providing a prolonged heat effect (Odabasi et al. 2008). The beneficial effects of peloid are generally considered as a consequence of the thermal effect; however, it is suggested that the chemical composition of the peloid is also involved in its therapeutic action (Odabasi et al. 2008). In vitro studies, it has been observed that mud extracts can pass through the skin in sufficient amounts and can cause biological influence (Beer et al. 2003). However, the role of the transdermal permeation of chemical substances is not clearly explained (Odabasi et al. 2008).
As a result of many studies, after balneological treatment, a decrease in serum levels of proinflammatory molecules such as TNF-α, IL-1β, prostaglandin E2 (PGE2), leukotriene B4 ( LTB4), and C-reactive protein (CRP) and an increase in IGF-1 have been shown (Maccarone et al. 2021). This study aims to evaluate the effect of balneological treatment and chemical content of peloid on clinical symptoms and serum levels of IL-1β, TNF-α, and IGF-1 in knee OA patients.
Material and methods
Study design
The study was planned as a prospective, randomized-controlled, parallel 1:1, single-blinded design. The study protocol was approved by the Clinical Research Ethics Committee of the institution and funded by the Scientific Research Projects Coordination Unit of Istanbul University, Project number: TTU-2018-30161
Subjects
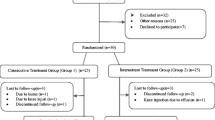
A total of 339 patients who applied to the Department of Medical Ecology and Hydroclimatology with the complaint of knee pain were evaluated for eligibility by scanning the patient files. Sixty-four (64) patients met the criteria and agreed to participate in the study (Figure 1). Each subject was given information about the study.
Inclusion criteria
-
40–70 years of age
-
Diagnosed with knee OA according to American College of Rheumatology classification criteria
-
Grade 2 or more according to the Kellgren-Lawrence score
-
Accept to sign their written consent
Exclusion criteria
The patients were excluded if they have a previous history of knee surgery, decompensated organ failure, treatment for oncological diseases, systemic inflammatory diseases, infectious diseases, intra-articular injection within the last 6 months; physical therapy or balneological treatment within the last 1 year, and change in the drug routine within last 2 months.
Intervention
All patients were given bath therapy as immersion at 38 °C hydrotherapy pool (tap water) for 20 min. After that, peloid was heated to 43 °C in the microwave oven and performed to both knees for 20 min. Unlike the study group in the control group, peloid was applied over an impermeable stretch film. After peloid application, both knees were wrapped with a stretch cover and towel to decrease the heat loss. Patients were allowed to take only oral paracetamol if they need (maximum 2 g/day). The same treatment was performed during a total of 10 sessions for 2 weeks (not given at the weekends). We considered patients who received 8 out of 10 sessions to have completed the treatment.
Peloid used in the study was a mixture of sepiolite clay and natural mineral water obtained from Tuzla Spa Resort, Istanbul (total mineralization of 3406 mg/L). The mineral water is defined as saline water because it contains high concentrations of sodium chloride (total mineralization 4145 mg/L).
Side effects
We evaluated the side effects based on the patient statements. Except for local temporary skin irritation, no side effect was reported that would affect the therapy course.
Outcome measures
Patients were evaluated with questionnaire forms at baseline, post-treatment, and 3 and 6 months after treatment. The pain Visual Analog Scale (VAS-pain), patient’s global assessment (VAS-PGA), investigator’s global assessment (VAS-IGA), and WOMAC (The Western Ontario and McMaster Universities Osteoarthritis Index) were used for clinical evaluations. The VAS is a 100-mm-long line which is a widely used method for evaluating the degree of pain. Subjects were asked to mark a point between 0 (no pain) and 100 (intolerable pain). Lower scores indicate better patient status in terms of pain and global assessment (Price et al. 1983). Pain, stiffness, and physical function were evaluated by WOMAC. There is a 5-point Likert scale of 0 to 4 for each item, 0 indicates nothing and 4 indicates severe. For interpretation, the scores of the items in each domain are added to obtain subscores. The highest subscore for pain (PS) is 20, for stiffness (SS) is 8, for function (PFS) is 68, and higher scores indicate worse pain, stiffness, or functional limitation(Bellamy et al. 1988).
Randomization and blinding
Randomization was made by using a computer-generated random number list. The physician who conducted the study (TA), allocated the intervention groups according to the number list and was aware of the assigned groups. Baseline assessments and outcome evaluations were made by a physician (BA) who was blinded to the patient groups. All meetings were done face-to-face, and questions were asked one by one. Patients were not blinded to the intervention regimens, and they were warned not to tell about their regimens. We were careful not to take different groups together in the same room. An independent blinded statistician performed the analysis of the study results.
Biochemical markers
Blood samples were collected at baseline, after treatment, and 6 months after treatment to detect serum levels of IL-1β, TNF-α, and IGF-1. They were kept waiting at room temperature for 15 min and then centrifuged at 3500 rpm for 10 min. The isolated serum samples were stored at −80 °C until assay. Serum IL-1β and TNF-α were measured by a commercial Diaclone ELISA (France) kit; serum IGF-1 levels were measured by a commercial LDN ELISA (Germany) kit. All samples were analyzed on the same day with the same kit to avoid inter-assay variations.
Statistical analysis
The data were analyzed by using the Statistical Package for the Social Sciences (SPSS) for Windows v21.0 (IBM Corp Released 2012) software based on per-protocol analysis. Sample size calculation was performed using G* Power 3.0.10 program with 32 patients for each group (α = 0.05, effect size = 0.8, and power (1 − β) = 0.88). The normal distribution of the data was checked by using Shapiro-Wilk test. The data mostly showed abnormal distribution. If the data was normally distributed, paired sample t-test to determine the changes within each group and independent sample t-test for comparisons between the groups were used. When a normal distribution was not found, the Wilcoxon signed-rank test for compare differences within each group and Mann Whitney U test for comparisons between the groups were used. Pearson chi-square test was used for demographical variables. The outcomes and the variables of the change (baseline value minus follow-up value) were presented as median (25th–75th percentile). In intergroup comparisons, p < 0.05 were accepted as significant. The significance value of intragroup pairwise comparisons was corrected by Bonferroni method. p < 0.008 for clinical findings and p < 0.0167 for laboratory findings were considered significant.
Results
Flow diagram
Sixty-four patients who agreed to sign written informed consent and met the study criteria were given the treatment. Three patients in the control group were excluded because of missing treatment sessions. One did not communicate, and two patients had flu. Four patients did not come to follow-up in the 3rd month. All patients in the study group completed the treatment. Three patients were excluded because they no longer met the study criteria during the follow-up period (2 knee injections, 1 knee replacement surgery). One patient did not contact us in the 6th month.
Baseline
The mean age of patients was 60.84 ± 7.76 years in the study group and 59.09 ± 9.15 years in the control group. Thirty-one (%96.9) of patients were female, and 1 (%3.1) were male in both groups.
The mean BMI measures were 30.34 ± 4.33 and 31.25 ± 5.15 in the study and control groups, respectively. Three (%9.4) of patients were graded as Kellgren Lawrence (KL) stage II, 15 (%46.9) of patients as stage III, and 14 (%43.8) of patients as stage IV in the study group. There were 3 (%9.4) KL stage II patients, 19 (%59.4) KL stage III, and 10 (%31.3) KL stage IV patients in the control group. There are no significant differences between the two groups regarding age (p = 0.412), sex (p = 1.0), body mass indices (BMI) (p = 0.444), and Kellgren–Lawrence (KL) scoring (p = 0.566) (Table 1).
The results and statistical comparisons of biochemical and functional parameters for baseline, post-treatment, and 3 and 6 months after the treatment in the study and control groups were shown in Table 2. Both groups did not show any difference in terms of any of the baseline measures evaluated for this study VAS-pain, VAS-PGA, VAS-IGA, WOMAC-PS, WOMAC-SS, WOMAC-PFS, IL-1β, and IGF-1 levels at baseline (p > 0.05).
Outcomes
Except for the 3rd-month evaluation of VAS-PGA in the study group, all VAS measurements showed a significant improvement in both groups (p < 0.008). Between the groups, no significant difference was detected in any evaluation period (p > 0.05).
All assessments of WOMAC scores decreased significantly in the study group (p < 0.008). The control group showed a significant improvement in WOMAC pain and physical function subscores at the post-treatment and 3 months after treatment (p < 0.008), but no significant improvement was seen after 6 months (p > 0.008). Between the groups, only the 6th-month assessment of pain and stiffness subscores showed a significant difference in favor of the study group (p < 0.05).
There was no significant change in IL-1β in both groups during the entire follow-up period (p > 0.167). IGF-1 levels did not increase in both groups, and the statistically significant decrease seen in the control group was clinically insignificant (Table 3). There was no significant difference between the groups in any evaluation period (p > 0.05). We could not evaluate the samples due to the failure of the TNF-α kit.
Discussion
The mechanisms of balneological treatment in reducing the symptoms of OA and many other rheumatic diseases have not been fully elucidated yet (Fioravanti et al. 2017). The main factors that play a role in the formation of the total effect are mechanical, thermal, and chemical mechanisms (Gutenbrunner et al. 2010). Hydrostatic pressure and temperature of the water can be effective in increasing the joint range of motion and reducing the severity of pain and muscle spasms (Tenti et al. 2014). In our trial, the improvement in symptoms over time in both the study and control groups may be due to the thermal/physical properties of the peloid and hydrotherapy. At the same time, the results showed a better clinical outcome for patients treated with direct peloid application. These findings are consistent with the previous study results. In a study on this subject, Güngen et al. (2012) compared mudpack and hot pack treatment in knee OA patients. In both treatment groups, pain scores measured by VAS at rest, at night, during the activity were significantly lower at the end of the treatment and 3rd-month follow-up compared to baseline. There was no difference between the groups. Similarly, in our study, both groups showed statistically significant improvement in almost all VAS scores. The thermal effect of balneological treatment increases the tensile strength and range of motion of collagen-rich tissues and reduces muscle spasms and pain, creating a feeling of comfort in the person (Fioravanti et al. 2011). It also stimulates the release of molecules with analgesic effects, such as beta-endorphins, from the skin (Fioravanti et al. 2017).
In a study, Odabasi et al. (2008) used mud pack therapy in knee OA patients. In the study group, mud was applied directly to the skin; in the control group, mud was performed over an impermeable nylon pack. After 3 weeks of treatment, patients were followed 24 weeks with 4-week intervals. VAS-pain, VAS-PGA, and VAS-IGA scores in the study group showed better improvement compared to the control group during the whole post-intervention follow-ups. In the study, researchers defined the term of minimal clinically important improvement (MCII) for interpreting clinical meaningful results (−40.8% for pain, −39.0% for VAS-PGA and VAS-IGA). This limitation likely overshadowed the improvement scores in the control group. In another study, Gyarmati et al. (2017) gave mud pack therapy to a group of hand OA patients to investigate the chemical effect of mud. One group received mud therapy directly on the hands; the other group was applied over nylon gloves. Both groups showed better improvement during the follow-up period. Three parameters showed better improvement at the 4th-month follow-up in favor of the directly applied group. Similarly, in our study, two parameters (pain and stiffness) showed better long-term improvement in the study group when comparing the control group. Flusser et al. (2002) treated knee OA patients on 15 sessions of mud therapy. The treatment group received mud, and the control group received mineral-depleted mud. During the follow-up period, only the treatment group showed a significant decrease in pain. In our study and the studies mentioned, the cutaneous application of the peloid showed superiority in long-term periods in some clinical outcomes. This strengthens the idea that apart from the thermal effect, some chemical mechanisms may also play a role in the effectiveness of peloid.
Peloids are formed by a combination of organic and inorganic materials. Humic substances (HS), which are formed as a result of the separation of organic substances into their components, are found in various soils, swamps, and sea sediments in nature. Various studies have revealed that HS has antiviral, anti-inflammatory, and immune-modulatory effects (Gyarmati et al. 2017). An in vitro study on humic acids, the humic acid content of sea mud obtained as a result of tidal currents in the Boryeong region of Korea showed that HS has anti-inflammatory effects on human keratinocytes (Kim et al. 2010). In an experimental study on this subject, Beer et al. (2000) showed that fulvic and ulmic acids in peat extract stimulate spontaneous muscle activity of smooth muscle cells acting on α2-adreno and D2-dopamine receptors. Although various studies have shown that the organic and mineral content have effects on the skin tissue, it is still a matter of curiosity whether it affects deeper tissues. Tateo et al. (2009) used the modified Franz-type diffusion cells to indicate the passage of chemical agents from thermal mud to adjacent tissue. As a result of the calculation made by assuming the whole-body application for 20 min, it has been argued that a significant supply amount of sodium, chlorine, calcium, and essential minerals can pass through the skin.
Cozzi et al. (2004) showed that 10 days of thermal mud and water treatment produced an anti-inflammatory effect by reducing circulating TNF-a and IL-1β levels in Freund’s adjuvant-induced arthritis in rats. Ortega et al. (2017) evaluated the levels of IL-1β and TNF-α before and after 21 sessions of whole-body peloid and spa therapy and observed that these cytokines decreased significantly after treatment. There may be some reasons why there was no significant change at the IL-1β level in our study. In the mentioned studies, the mud has a high sulfoglycolipid (SGL) content. SGLs are substances with anti-inflammatory properties and are produced after a 6–7 months “maturation” period during which thermophilic microorganisms colonize (Gomes 2018; Centini et al. 2015). In our study, the peloid is rich in inorganic substances predominantly sodium chloride, magnesium, and calcium. In addition, the peloid was applied locally to the knees instead of the whole body.
In a study, Sarsan et al. (2012) compared mud pack and hot pack treatment in knee OA and the patients were followed post-treatment and 3 and 6 months after treatment. There was no significant difference in levels of TNF-α and IL-6 in intra-group evaluations and between-group comparisons. A significant increase in IGF-1 was seen only in the mud pack group at 3rd-month follow-up, and there was a significant difference between the groups in favor of the mud pack group. In our study group, no significant increase in IGF-1 was detected after treatment and 6 months after treatment, and there was no difference between the groups. Although mud was applied locally in both studies, it is noteworthy that, unlike our study, mud with high organic content was used in the aforementioned study.
In our study, the participants are mostly obese (mean BMI > 30). A survey of the studies investigating the relationship between obesity and OA has not been able to come to a definitive conclusion that overweight is a causal factor (Aspden 2011). In addition, obesity is thought to be mostly associated with increased pro-inflammatory cytokines (IL-1β, TNF-α, IL-6) and abnormal levels of hormones and growth factors, higher bone mineral density, and other metabolic mediators (Wang and He 2018). Even though the effect of the link between obesity and metabolic factors on the development of OA cannot be fully explained (Pottie et al. 2006), it may have an impact on the results of our study.
Limitations
A small number of male patients and involving mostly obese subjects are limitations of this study in terms of generalizability. The absence of a control group without treatment is one of the weaknesses of this study. Also, the control group did not blind to the treatment. Using a demineralized type of peloid in the control group, which has the same properties as the peloid applied to the study group in terms of appearance and heat conduction, will help for obtaining more specific results. If we wash the minerals in the peloid, this will change the heat retention and transmission capacity of the peloid. In this case, the question of what the application temperature and time should be for the demineralized peloid arises. It will be useful for future studies to establish mineral amount-heat capacity measurements of peloid to be obtained with laboratory analyses for this subject. Due to the limited study budget, we were unable to evaluate laboratory parameters 3 months after treatment. Because of the limited data on long-term biomarker evaluation in the literature, we chose to perform analysis 6 months after treatment.
Conclusion
Our study demonstrates that balneological treatment has beneficial effects on knee OA. Peloidotherapy is an effective method for improving the patient’s status whether applied directly or not. Clinical results show that the chemical properties of the peloid may play a role in this effect. Studies with more patients and precise methods are required for investigating the effects of this treatment on biomarkers of OA.
References
Aspden RM (2011) Obesity punches above its weight in osteoarthritis. Nat Rev Rheumatol 7(1):65–68. https://doi.org/10.1038/nrrheum.2010.123
Beer AM, Lukanov J, Sagorchev P (2000) The influence of fulvic and ulmic acids from peat on the spontaneous contractile activity of smooth muscles. Phytomedicine 7:407–415. https://doi.org/10.1016/S0944-7113(00)80062-8
Beer AM, Junginger HE, Lukanov J, Sagorchev P (2003) Evaluation of the permeation of peat substances through human skin in vitro. Int J Pharm 253:169–175. https://doi.org/10.1016/S0378-5173(02)00706-8
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15(12):1833–1840
Bender T, Karagülle Z, Bálint GP, Chr G, Bálint PV, Sukenik S (2005) Hydrotherapy, balneotherapy, and spa treatment in pain management. Rheumatol Int 25:220–224. https://doi.org/10.1007/s00296-004-0487-4
Centini M, Tredici MR, Biondi N, Buonocore A, Maffei Facino R, Anselmi C (2015) Thermal mud maturation: organic matter and biological activity. Int J Cosmet Sci 37(3):339–347. https://doi.org/10.1111/ics.12204
Cheleschi S, Tenti S, Seccafco I, Gálvez I, Fioravanti A, Ortega E (2021) Balneotherapy year in review 2021: focus on the mechanisms of action of balneotherapy in rheumatic diseases. Environ Sci Pollut Res 29:8054–8073. https://doi.org/10.1007/s11356-021-17780-0
Chow YY, Chin KY (2020) The role of inflammation in the pathogenesis of osteoarthritis. Mediators Inflamm 2020 19 pages. https://doi.org/10.1155/2020/8293921
Cozzi F, Carrara M, Sfriso P, Todesco S, Cima L (2004) Anti-inflammatory effect of mud-bath applications on adjuvant arthritis in rats. Clin Exp Rheumatol 22:763–766
Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H (2020) Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. E Clin Med 29:100587. https://doi.org/10.1016/j.eclinm.2020.100587
Fioravanti A, Cantarini L, Guidelli GM, Galeazzi M (2011) Mechanisms of action of spa therapies in rheumatic diseases: what scientific evidence is there? Rheumatol Int 31(1):1–8. https://doi.org/10.1007/s00296-010-1628-6
Fioravanti A, Karagülle M, Bender T, Karagülle MZ (2017) Balneotherapy in osteoarthritis: facts, fiction, and gaps in knowledge. Eur J Integr Med 9:148–150. https://doi.org/10.1016/j.eujim.2017.01.001
Flusser D, Abu-Shakra M, Friger M, Codish S, Sukenik S (2002) Therapy with mud compresses for knee osteoarthritis: comparison of natural mud preparations with mineral-depleted mud. J Clin Rheumatol 8:197–203. https://doi.org/10.1097/01.RHU.0000022542.38402.A9
Gálvez I, Torres-Piles S, Hinchado MD, Álvarez-Barrientos A, Torralbo- Jiménez P, Guerrero J, Martín-Cordero L, Ortega E (2017) Immune-neuroendocrine dysregulation in patients with osteoarthritis: a revision and a pilot study. Endocr Metab Immune Disord Drug Targets 17(1):78–85. https://doi.org/10.2174/1871530317666170320113613
Gomes CSF (2018) Healing and edible clays: a review of basic concepts, benefits and risks. Environ Geochem Health 40(5):1739–1765. https://doi.org/10.1007/s10653-016-9903-4
Griffin TM, Scanzello CR (2019) Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clin Exp Rheumatol 37(Suppl 120):57–63
Güngen G, Ardic F, Fındıkoğlu G, Rota S (2012) The effect of mud pack therapy on serum YKL-40 and hsCRP levels in patients with knee osteoarthritis. Rheumatol Int 32:1235–1244. https://doi.org/10.1007/s00296-010-1727-4
Güngen GO, Ardic F, Findikoglu G, Rota S (2016) Effect of mud compress therapy on cartilage destruction detected by CTX-II in patients with knee osteoarthritis. J Back Musculoskelet Rehabil 29(3):429–438. https://doi.org/10.3233/BMR-150629
Gutenbrunner C, Bender T, Cantista P, Karagülle MZ (2010) A proposal for a worldwide definition of health resort medicine, balneology, medical hydrology and climatology. Int J Biometeorol 54(5):495–507. https://doi.org/10.1007/s00484-010-0321-5
Gyarmati N, Kulisch Á, Németh A, Bergmann A, Horváth J, Mándó Z, Matán Á, Szakál E, Sasné Péter T, Szántó D, Bender T (2017) Evaluation of the effect of Hévíz Mud in patients with hand osteoarthritis: a randomized, controlled, single-blind follow-up study. Isr Med Assoc J 19(3):177–182
Karagülle MZ, Karagülle M (2004) Balneotherapy and spa therapy of rheumatic diseases in Turkey: a systematic review. Forschende Komplementarmed Klass Naturheilkund 11(1):33–41. https://doi.org/10.1159/000077194
Karagülle M, Karagülle MZ, Karagülle O, Dönmez A, Turan M (2007) A 10-day course of SPA therapy is beneficial for people with severe knee osteoarthritis. A 24-week randomized, controlled pilot study. Clin Rheumatol 26(12):2063–2071. https://doi.org/10.1007/s10067-007-0618-x
Kim JH, Lee J, Lee HB, Shin JH, Kim EK (2010) Water-retentive and anti-inflammatory properties of organic and inorganic substances from Korean sea mud. Nat Prod Commun 5:395–398. https://doi.org/10.1177/1934578X1000500311
Maccarone MC, Magro G, Solimene U, Scanu A, Masiero S (2021) From in vitro research to real life studies: an extensive narrative review of the effects of balneotherapy on human immune response. Sport Sci Health 17:817–835. https://doi.org/10.1007/s11332-021-00778-z
Malemud CJ (2010) Anticytokine therapy for osteoarthritis. Drugs Aging 27(2):95–115. https://doi.org/10.2165/11319950-000000000-00000
Mueller MB, Tuan RS (2011) Anabolic/catabolic balance in pathogenesis of osteoarthritis: identifying molecular targets. PM&R 3:S3–S11. https://doi.org/10.1016/j.pmrj.2011.05.009
Odabasi E, Turan M, Erdem H, Tekbas F (2008) Does mud pack treatment have any chemical effect? A randomized controlled clinical study. J Altern Complement Med 14:559–565. https://doi.org/10.1089/acm.2008.0003
Ortega E, Gálvez I, Hinchado MD, Guerrero J, Martín-Cordero L, Torres- Piles S (2017) Anti-inflammatory effect as a mechanism of effectiveness underlying the clinical benefits of pelotherapy in osteoarthritis patients: regulation of the altered inflammatory and stress feedback response. Int J Biometeorol 61(10):1–9. https://doi.org/10.1007/s00484-017-1361-x
Özkuk K, Gürdal H, Karagülle M, Barut Y, ErÖksüz R, Karagülle MZ (2017) Balneological outpatient treatment for patients with knee osteoarthritis; an effective non-drug therapy option in daily routine? Int J Biometeorol 61:719–728. https://doi.org/10.1007/s00484-016-1250-8
Pottie P, Presle N, Terlain B, Netter P, Mainard D, Berenbaum F (2006) Obesity and osteoarthritis: more complex than predicted! Ann Rheum Dis 65(11):1403–1405. https://doi.org/10.1136/ard.2006.061994
Price DD, McGrath PA, Rafii A, Buckingham B (1983) The validation of visual analog scales as ratio scale measures for chronic and experimental pain. Pain 17:45–56
Sarsan A, Akkaya N, Ozgen M, Yildiz N, Atalay NS, Ardic F (2012) Comparing the efficacy of mature mud pack and hot pack treatments for knee osteoarthritis. J Back Musculoskelet Rehabil 25:193–199. https://doi.org/10.3233/bmr-2012-0327
Scanzello C, Goldring S (2012) The role of synovitis in osteoarthritis pathogenesis. Bone 51:249–257. https://doi.org/10.1016/j.bone.2012.02.012
Sokolove J, Lepus CM (2013) Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskel Dis 5(2):77–94. https://doi.org/10.1177/1759720X12467868
Tateo F, Ravaglioli A, Andreoli C, Bonina F, Coiro V, Degetto S, Giaretta A, Menconi Orsini A, Puglia C, Summa V (2009) The in-vitro percutaneous migration of chemical elements from a thermal mud for healing use. Appl Clay Sci 44(1-2):83–94. https://doi.org/10.1016/j.clay.2009.02.004
Tenti S, Fioravanti A, Guidelli GM, Pascarelli NA, Cheleschi S (2014) New evidence on mechanisms of action of spa therapy in rheumatic diseases. CELLMED 4(1):31–38
Vina ER, Kwoh CK (2018) Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol 30(2):160–167. https://doi.org/10.1097/BOR.0000000000000479
Wang T, He C (2018) Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev 44:38–50. https://doi.org/10.1016/j.cytogfr.2018.10.002
Weimer A, Madry H, Venkatesan JK et al (2012) Benefits of recombinant adeno-associated virus (rAAV)-mediated insulinlike growth factor I (IGF-I) overexpression for the long-term reconstruction of human osteoarthritic cartilage by modulation of the IGF-I axis. Mol Med 18(3):346–358. https://doi.org/10.2119/molmed.2011.00371
Funding
Scientific Research Projects Coordination Unit of Istanbul University (BAP), Project number: TTU-2018-30161
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were by the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Adıgüzel, T., Arslan, B., Gürdal, H. et al. Evaluation of the therapeutic and the chemical effects of balneological treatment on clinical and laboratory parameters in knee osteoarthritis: a randomized, controlled, single-blinded trial. Int J Biometeorol 66, 1257–1265 (2022). https://doi.org/10.1007/s00484-022-02274-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-022-02274-6