Abstract
Objective
To provide evidence on the effects of vitamin D supplementation on knee osteoarthritis (KOA) and new targets for clinical prevention and treatment of KOA.
Method
The PubMed, Embase, Web of science, Wanfang, CNKI and SinoMed databases were retrieved to investigate the effects of vitamin D supplementation on patients with KOA. The search time was from databases establishment to 15 November 2020. RevMan5.3 software was used for meta-analysis. The results were expressed as standardized mean difference (SMD) with 95% confidence interval (CI) or weighted mean difference (WMD) with 95% confidence interval (CI).
Results
A total of 1599 patients with osteoarthritis of the knee were included in the study, which involved six articles. The results of the meta-analysis showed that vitamin D supplementation is statistically significant for WOMAC score (SMD = − 0.67, 95% CI − 1.23 to − 0.12) in patients with KOA, including WOMAC pain score (SMD = − 0.32, 95% CI − 0.63 to − 0.02), function score (SMD = − 0.34, 95% CI − 0.60 to − 0.08) and stiffness score (SMD = − 0.13, 95% CI − 0.26 to − 0.01). In subgroup analysis, vitamin D supplementation less than 2000 IU was statistically significant for the reduction of stiffness score (SMD = − 0.22, 95% CI − 0.40 to − 0.04). Vitamin D supplements can reduce synovial fluid volume progression in patients with KOA (SMD = − 0.20, 95% CI − 0.39 to − 0.02). There was no statistical significance in improving tibia cartilage volume (SMD = 0.12, 95% CI − 0.05 to 0.29), joint space width (SMD = − 0.10, 95% CI − 0.26 to 0.05) and bone marrow lesions (SMD = 0.03, 95% CI − 0.26 to 0.31).
Conclusion
Vitamin D supplements can improve WOMAC pain and function in patients with KOA. But there is a lack of strong evidence that vitamin D supplementation can prevent structural progression in patients with KOA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis of the knee (KOA) is a chronic degenerative disease [1]. The most common cause of musculoskeletal disability and pain worldwide is osteoarthritis (OA) [2]. KOA accounts for 83% of all types of osteoarthritis [3]. Degradation of articular cartilage is a major feature of KOA, including cartilage degeneration, joint space changes, synovial inflammation and osteophyte formation [4]. Patients with KOA not only lack quality of life security, but also have a considerable economic and mental burden. Early changes in unhealthy lifestyles and exercise patterns, as well as enhanced functional training of the surrounding muscles, are the main treatments for KOA [5,6,7,8,9]. Studies have shown that patients often do not pay so much attention to the joint replacement that is usually required in late stages [10].
Vitamin D has been recognized as having a biological effect on bone and cartilage [11, 12]. It is known to increase bone mass or prevent bone loss [13]. Studies have shown that vitamin D deficiency is associated with a higher risk of KOA progression [14, 15]. A number of studies have shown that vitamin D supplementation can reduce pain and improve knee function in patients with KOA [2, 16]. Other studies have shown that vitamin supplementation has no significant effect on the prevention of tibia cartilage loss or improvement in Western Ontario and McMaster Universities Osteoarthritic Index (WOMAC) knee pain [2]. Furthermore, there is evidence that vitamin D supplementation is associated with a smaller increase in synovial fluid compared with placebo [17]. However, data from some randomized clinical trials suggest that vitamin D supplementation have no effect on the assessment of pain and joint space changes [12, 18, 19]. The previous research results are controversial and no uniform conclusion has been reached. Therefore, we conducted a meta-analysis of qualified randomized controlled trials (RCTs) to assess the efficacy of vitamin D supplementation in KOA.
Materials and methods
Literature search and selection
The data in the PubMed, Embase, Web of Science, Cochrane Library, Medline, China National Knowledge Infrastructure database(CNKI), Wanfang database, China Biological Medicine Database (CBM) and SinoMed databases were retrieved for our study (up to 15 November 2020). We searched the clinical RCTs of the effect of vitamin D supplementation on KOA. The search strategy used was the combination of subject words and free word retrieval. The English search terms: {“osteoarthritis, knee” (MeSH Terms) OR [“osteoarthritis” (All Fields) OR “knee” (All Fields)] OR “knee osteoarthritis” (All Fields) OR [“knee” (All Fields) OR “osteoarthritis” (All Fields)]} AND [“vitamin D” (MeSH Terms) OR “vitamin D” (All Fields) OR “Cholecalciferol” (MeSH Terms) OR “Cholecalciferol” (All Fields) OR “ergocalciferols” (MeSH Terms) OR “Ergocalciferols” (All Fields)]. The Chinese search terms: “osteoarthritis”, “OA” “knee osteoarthritis”, “KOA”, “vitamin D”, “ergocalciferol”. The languages were limited to English and Chinese.
Inclusion and exclusion criteria
Inclusion criteria: (1) study design: RCTs; (2) population: patients diagnosed with KOA with associated symptoms and older than 45; (3) intervention: vitamin D supplements; (4) comparison: placebo; (5) outcome index: the main outcome indicators were WOMAC scores, volume of tibia cartilage, bone marrow lesions, joint space width and synovial fluid volume. Serous vitamin D levels as the secondary outcome indicators.
Exclusion criteria: (1) observational study follow-up time less than 12 months; (2) summarize, summary of meeting, cross-sectional study, case report, cohort study, editors and letters; (3) the experiment of animals.
Data extraction
We used a pre-designed data table for data extraction. The extracted information includes: first author, year of publication, country, number of patients per group, patient characteristics, serum vitamin D baseline level, intervention dose, follow-up time and main results. When the same study included different follow-up times, we used the results of the study with the longest follow-up time. For those studies that did not report standard deviations related to baseline changes, we used correlation coefficients based on the methods described in the Cochrane manual. Correlation coefficients can be calculated from studies reporting detailed results.
Risk of bias assessment
The risk of study bias was assessed using the Cochrane Handbook for Systematic Reviews. The evaluation criteria include: random sequence generation, allocation scheme hiding, blinding method, the integrity of the resulting data, selective reporting and other bias. There were three risk choices that were “high”, “low”, or “unclear” risk of bias for each article. Funnel diagrams were used to detect publication bias.
Statistical analysis
The main outcome indicator of this statistical analysis was WOMAC score, synovial fluid volume, tibia cartilage volume, bone marrow lesions and joint space width. We used Review manager 5.3 software for meta-analysis. Standardized mean difference (SMD) and 95% confidence interval (CI) were used for the amount of effect that represents the same result for different units. We used weighted mean difference (WMD) for the same unit. We calculated the I2 and P values to test the heterogeneity between different studies. The fixed effect model was used for statistical analysis, if there was no clinical and statistical heterogeneity (P > 0.1, I2 < 50%) among the studies. If there was moderate or more statistical heterogeneity among the results of the study, but there was no clinical heterogeneity (P < 0.1, I2 > 50%), then subgroup analysis was performed.
Results
Research selection
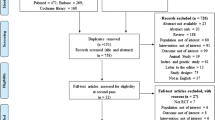
The screening process for inclusion in the literature is shown in Fig. 1. A total of 1128 articles were retrieved, including 615 duplicate articles. There were 26 articles retained after viewing the title and eliminating the type of article (summarize, summary of meeting, cross-sectional study, case report, cohort study, editors and letters). Finally, there were six RCTs left for meta-analysis by looking at the abstract and the full text [12, 16,17,18,19,20].
Study characteristics
Tables 1 and 2 listed the basic features and information of all subjects studied. All the studies were grouped according to the diagnostic criteria of the American College of Rheumatology (ACR). Country distribution in these RCTs included USA [20], UK [12, 19], Australia [17], India [16] and Australia [18]. These studies were published from 2013 to 2019. Sample sizes included in the study ranged from 103 to 474. Daily vitamin D supplements included in the study ranged from 800 to 6000 IU. There was no difference in age, sex ratio, body mass index and serum vitamin D levels between the experimental and control groups.
Assessment of risk bias
The results of the quality evaluation included in the study are shown in Fig. 2. The evaluation included WOMAC score, synovial fluid volume, tibia cartilage volume changes, joint space width, bone marrow lesions, serum vitamin D levels and adverse reactions. Each study included was high-quality studies with low risk of bias.
Impact of interventions
WOMAC score
All the studies included used WOMAC scores for pain assessment, but the scale was different, so we used SMD to evaluate the impact of the results. Effects of vitamin D supplementation on KOA WOMAC score are shown in Figs. 3 and 6. We can see from Fig. 3 that vitamin D supplementation is statistically significant for pain relief in patients with KOA (SMD = − 0.32, 95% CI − 0.63 to − 0.02). But there is obvious heterogeneity in the subjects (P = 0.0007, I2 = 82%). Therefore, we conducted a sensitivity analysis. At the same time, we conducted subgroup analysis according to the supplemental dose of vitamin D. In subgroup analysis, the pain score of knee joint patients was not affected by low vitamin D (SMD = − 0.12, 95% CI − 0.28 to 0.04) or high vitamin D (SMD = − 0.57, 95% CI − 1.14 to 0.27). Figure 4 shows that vitamin supplementation D has statistical significance for the WOMAC stiffness score of KOA (SMD = − 0.13, 95% CI − 0.26 to − 0.01), and there is no significant heterogeneity between studies (P = 0.28, I2 = 21%). Subgroup analysis showed that vitamin D less than 2000 IU reduced WOMAC stiffness score (SMD = − 0.22, 95% CI − 0.40 to − 0.04). The estimates summarized in Fig. 5 suggest that vitamin D supplementation is associated with WOMAC function scores in patients with knee osteoarthritis (SMD = − 0.34, 95% CI − 0.60 to − 0.08). Figure 6 is a summary estimate of the effect of vitamin supplementation D on the WOMAC total score. The results show that vitamin D is statistically significant in reducing the WOMAC total score (SMD = − 0.67, 95% CI − 1.23 to − 0.12) but there is significant heterogeneity between studies (P < 0.00001, I2 = 94%).
Synovial fluid volume
Figure 7 shows the effect of vitamin D supplementation on the volume of synovial fluid volume. Since the two studies included used different measurement methods and units, we used SMD to evaluate the impact of the results. We can see that vitamin D supplements are statistically significant for relieving synovial fluid volume progression in patients with KOA (SMD = − 0.20, 95% CI − 0.39 to − 0.02). There was no heterogeneity in the studies (P = 0.38, I2 = 0%).
Tibia cartilage volume
Effects of vitamin D supplements on volume of tibia cartilage are shown in Fig. 8. In terms of statistical results, vitamin D supplementation was not statistically significant in improving the volume of tibia cartilage (SMD = 0.12, 95% CI − 0.05 to 0.29). Similarly, the two articles included were not heterogeneous (P = 0.66, I2 = 0%).
Joint space width
The effect of vitamin D supplements on the joint space width is shown in Fig. 9. We used SMD and random effect models for statistical analysis. The effect of vitamin D supplementation on joint space was not statistically significant (SMD = − 0.10, 95% CI − 0.26 to 0.05). What’s more, there were moderately heterogeneous in included studies (P = 0.12, I2 = 59%).
Bone marrow lesions
From Fig. 10, we can see the assessment of the effect of vitamin D supplementation on bone marrow lesions. The improvement of bone marrow lesions by vitamin D supplementation was not statistically significant (SMD = 0.03, 95% CI − 0.26 to 0.31), and the included studies were not heterogeneous (P = 0.12, I2 = 59%).
Sensibility analysis
We used one-by-one method to exclude individual studies for sensitivity analysis. The results showed that the heterogeneity did not change, and the combined effect was still statistically significant, which indicated that the results were robust.
Publication bias
Because of the small number of studies included, which did not reflect the publication bias of the results, we did not conduct a statistical test of publication bias.
Discussions
In this meta-analysis, we evaluated the effect of vitamin D supplementation on pain and joint structure in patients with KOA. The results showed that vitamin D supplementation was statistically significant for pain and function improvement in patients with KOA. From the results, we can see that vitamin D supplementation could effectively delay the progression of synovial fluid volume, but there was no beneficial effect on the changes of tibia cartilage volume, bone marrow lesion and joint space.
Our study suggests that vitamin D supplementation is beneficial for WOMAC pain and functional improvement in patients with KOA. In addition, subgroup analysis showed that vitamin D supplementation with less than 2000 IU doses was statistically significant in reducing stiffness and total WOMAC score in KOA patients. However, the results of subgroup analysis have some heterogeneity. To this end, we performed sensitivity analysis. The sensitivity analysis showed that the heterogeneity did not change after the removal of individual studies. Therefore, we consider that the reason for this result may be related to the follow-up time and sample size. At the same time, we cannot rule out the impact of the results on the statistical capacity of the system. Due to the limited number of literatures included in this meta-analysis, no regression analysis was carried out.
In previous high-quality epidemiological studies, low-serum vitamin D levels are closely related to the occurrence and development of knee osteoarthritis [21, 22]. But some studies have come to the opposite conclusion [23,24,25,26]. The latest cohort study in India showed that vitamin D supplements significantly improved pain scores in OA patients [27]. The reasons for the different results may be related to differences in research methods, inclusion and exclusion criteria, and sample size. In addition, observational studies are subject to inherent bias and confounding factors such as physical activity and sun exposure [2, 15, 21, 27].
Based on the controversial role of vitamin D in the pathogenesis of patients with KOA. Some randomized controlled trials have also been designed to demonstrate the effect of vitamin D supplementation on knee bone and joint patients. The reason for this may be the difference in statistical methods and the possibility that the article considers statistical significance without mentioning clinical benefits. Our study included a high-quality, double-blind, placebo-controlled randomized controlled trial for analysis. In four studies on the effects of vitamin D supplementation on pain in patients with KOA, the majority of studies have shown that vitamin D supplementation has no effect on pain and function improvement in patients with KOA [2, 18, 19]. Only one study has shown that vitamin D supplementation is statistically significant for pain and function improvement in patients with KOA [16]. This positive result may be affected by sample size and follow-up time. Small incremental benefits could take more time to accrue into a measurable outcome. At the same time, it is possible to be affected by human diversity. In addition, most of the findings were negative, probably because everyone had a different response index to vitamin D and the study did not have sufficient follow-up time. Additional possible explanations for negative outcomes may be vitamin D rich in individuals in source populations [19].
For the effect of vitamin D on the structure of KOA, there is evidence from prospective studies that low-serum vitamin D may be linked with structural change in the knee [12]. Data from the Framingham study suggest that low-serum vitamin D is associated with increased odds of radiographic KOA progression [15]. Osteoarthritis Initiative (OAI) study also supports an association between low-serum vitamin D and an increased risk of radiographic progression in symptomatic KOA [25]. However, data from some RCTs showed that vitamin D supplementation had no effect on the assessment of narrowing of the joint space in patients with osteoarthritis by ordinary radiographs [18, 19]. An epidemiological statistical analysis of vitamin D and osteoarthritis suggests that studies do not provide evidence of an independent association between 25(OH) vitamin D serum levels with hip or hand OA. When analyzing subgroups of KOA, significant associations of low-vitamin D levels with prevalent knee JSN were observed [28]. Most of the literatures included in the meta-analysis were of low quality and were observational studies. Another statistical analysis of the association between vitamin D and osteoarthritis was included in the adult RCTs, cohort study, case–control study and cross-sectional study. The result displays that 25-(OH)-D appears to be implicated in structural changes of KOA rather than the symptoms [29]. The results were inconsistent, probably because of differences in design. Our combined effects are consistent with previous randomized controlled experiments and statistical analysis that vitamin D did not work on changes in the volume of tibia cartilage and the joint space width [2, 18, 19]. However, this result may be influenced by the measurement method, and the best method of cartilage measurement is still the topic of research and discussion [19, 25]. In addition, we also performed statistical analysis of bone marrow lesions and synovial fluid volume. The statistical results for bone marrow lesions were consistent with previous RCTs [2, 12, 19]. In terms of synovial fluid volume, our results are consistent with previous randomized controlled experiments that Vitamin D supplementation is ineffective for synovial fluid volume [17, 30, 31]. But both the studies were measured using magnetic resonance imaging (MRI). The data, however, were obtained using non-contrast images—such images are less sensitive in distinguishing effusion from underlying synovitis. Research from UK VIDEO shows the opposite [12]. Although the study used a more accurate measurement tool that was contrast-enhanced magnetic resonance imaging (CE MRI), the sample size was too small (n = 24) and fewer patients with KOA who lacked vitamin D in the study itself. Therefore, we cannot obtain sufficient evidence to demonstrate the effect of vitamin D supplementation on the volume of synovial fluid.
On the management treatment of KOA, the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) issued management advice on knee OA in the form of treatment algorithm. Among the latest algorithms, the use of background pharmacological therapy for OA is recommended background drug therapy for slow-acting drugs [32]. ESCEO especially recommended the use of pharmaceutical-grade prescription glucosamine sulfate (pCGS) and chondroitin sulfate, for which the evidence base is unequivocal and there are no safety issues [33,34,35]. They advanced pharmacological treatment of oral NSAID as step 2 is recommended. Another study suggests that drug-grade chondroitin sulfate can be used as a therapeutic option for patients with mild to moderate KOA [36]. Fuggle et al. reviewed alternative therapies for osteoarthritis. They recommend vitamin D supplements for KOA patients with evidence of vitamin D deficiency. According to their study, the problem of study design limits the extent to which symptomatic OA clinical outcomes can be inferred, so further design of appropriate, large, blind randomized controlled trials is needed [37]. The latest systematic review and network meta-analysis of 80 RCT mentioned that vitamin D can relieve pain and improve function in patients with knee OA [38], which is consistent with our results. The lack of endogenous vitamin D, which can be compensated for by circulating low levels of 25 OH of vitamin D, is associated with the onset and progression of knee OA, the literature also mentions.
Of course, our meta-analysis also has some flaws. For example, the failure to obtain the raw data of the experiment can only summarize the results of the study, and the time of follow-up of the intervention included in the study is inconsistent, which will make the results of the experiment different. Furthermore, because of the limited inclusion studies, no further regression analysis was carried out, and the studies on the volume of tibia cartilage, joint space width, bone marrow lesions and synovial effusion were limited, which had a certain effect on statistical efficacy.
In conclusion, our statistical analysis shows that vitamin D supplements are beneficial for WOMAC pain and functional improvement in patients with KOA. But there is a lack of strong evidence that vitamin D supplementation can prevent structural progression in patients with KOA.
References
Das SK, Farooqi A (2008) Osteoarthritis. Best Pract Res Cln Rheumatol. https://doi.org/10.1016/j.berh.2008.07.002
Manoy P, Yuktanandana P, Tanavalee A et al (2017) Vitamin D supplementation improves quality of life and physical performance in osteoarthritis patients. Nutrients. https://doi.org/10.3390/nu9080799
Arendt-Nielsen L (2017) Pain sensitisation in osteoarthritis. Clin Exp Rheumatol 35 (Suppl. 107): 68–74
Castaneda S, Roman-Blas JA, Largo R et al (2012) Subchondral bone as a key target for osteoarthritis treatment. Biochem Pharmacol. https://doi.org/10.1016/j.bcp.2011.09.018
Brown GA, AAOS (2013) Clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline 2nd edition. Am Acad Orthop Surg. https://doi.org/10.5435/JAAOS-21-09-577
Hochberg MC, Altman RD, April KT et al (2012) American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). https://doi.org/10.1002/acr.21596
Raman R, Henrotin Y, Chevalier X et al (2018) Decision algorithms for the retreatment with viscosupplementation in patients suffering from knee osteoarthritis: recommendations from the EUROpean VIScosupplementation COnsensus Group (EUROVISCO). Cartilage. https://doi.org/10.1177/1947603517693043
Zhang W, Doherty M, Peat G et al (2010) EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. https://doi.org/10.1136/ard.2009.113100
Bannuru RR, Osani MC, Vaysbrot EE et al (2019) OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. https://doi.org/10.1016/j.joca.2019.06.011
Feng X, Tian M, Zhang W et al (2018) Gastrointestinal safety of etoricoxib in osteoarthritis and rheumatoid arthritis: a meta-analysis. PLoS ONE 13:e0190798. https://doi.org/10.1371/journal.pone.0190798
Mabey T, Honsawek S (2015) Role of Vitamin D in osteoarthritis: molecular, cellular, and clinical perspectives. Int J Endocrinol. https://doi.org/10.1155/2015/383918
Perry TA, Parkes MJ, Hodgson R et al (2019) Effect of Vitamin D supplementation on synovial tissue volume and subchondral bone marrow lesion volume in symptomatic knee osteoarthritis. BMC Musculoskelet Disord. https://doi.org/10.1186/s12891-019-2424-4
Dietary Refence Intakes for Calcium and Vitamin D (2011) The National Academies Press, Washington
Lane NE, Gore LR, Cummings SR et al (1999) Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: a longitudinal study. Study of Osteoporotic Fractures Research Group. Arthritis Rheum. https://doi.org/10.1002/1529-0131(199905)42:5%3c854::AID-ANR3%3e3.0.CO;2-I
McAlindon TE, Felson DT, Zhang Y et al (1996) Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. https://doi.org/10.7326/0003-4819-125-5-199609010-00001
Sanghi D, Mishra A, Sharma AC et al (2013) Does vitamin D improve osteoarthritis of the knee: a randomized controlled pilot trial. Clin Orthop Relat Res. https://doi.org/10.1007/s11999-013-3201-6
Jin X, Jones G, Cicuttini F et al (2016) Effect of vitamin D supplementation on tibial cartilage volume and knee pain among patients with symptomatic knee osteoarthritis: a randomized clinical trial. JAMA. https://doi.org/10.1001/jama.2016.1961
Wang X, Cicuttini F, Jin X et al (2017) Knee effusion-synovitis volume measurement and effects of vitamin D supplementation in patients with knee osteoarthritis. Osteoarthr Cartil. https://doi.org/10.1016/j.joca.2017.02.804
Arden NK, Cro S, Sheard S et al (2016) The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: a randomised controlled trial. Osteoarthr Cartil. https://doi.org/10.1016/j.joca.2016.05.020
McAlindon T, LaValley M, Schneider E et al (2013) Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. JAMA. https://doi.org/10.1001/jama.2012.164487
Ding C, Cicuttini F, Parameswaran V et al (2009) Serum levels of vitamin D, sunlight exposure, and knee cartilage loss in older adults: the Tasmanian older adult cohort study. Arthritis Rheum. https://doi.org/10.1002/art.24486
Bergink AP, Uitterlinden AG, Van Leeuwen JP et al (2009) Vitamin D status, bone mineral density, and the development of radiographic osteoarthritis of the knee: the Rotterdam Study. J Clin Rheumatol. https://doi.org/10.1097/RHU.0b013e3181b08f20
Heidari B, Heidari P, Hajian-Tilaki K (2011) Association between serum vitamin D deficiency and knee osteoarthritis. Int Orthop. https://doi.org/10.1007/s00264-010-1186-2
Muraki S, Dennison E, Jameson K et al (2011) Association of vitamin D status with knee pain and radiographic knee osteoarthritis. Osteoarthritis Cartilage. https://doi.org/10.1016/j.joca.2011.07.017
Felson DT, Niu J, Clancy M et al (2007) Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum. https://doi.org/10.1002/art.22292
Zhang FF, Driban JB, Lo GH et al (2014) Vitamin D deficiency is associated with progression of knee osteoarthritis. J Nutr. https://doi.org/10.3945/jn.114.193227
Thomas JE, Bhat AK, Rao M et al (2019) Use of Vitamin D supplements in osteoarthritis: an observational study in a tertiary health care facility. J Am Coll Nutr. https://doi.org/10.1080/07315724.2018.1494641
Konstari S, Paananen M, Heliövaara M et al (2012) Association of 25-hydroxyvitamin D with the incidence of knee and hip osteoarthritis: a 22-year follow-up study. Scand J Rheumatol. https://doi.org/10.3109/03009742.2011.617314
Bergink AP, Zillikens MC, Van Leeuwen JP et al (2016) 25-Hydroxyvitamin D and osteoarthritis: a meta-analysis including new data. Semin Arthritis Rheum. https://doi.org/10.1016/j.semarthrit.2015.09.010
Conaghan PG, Hunter DJ, Maillefert JF et al (2011) Summary and recommendations of the OARSI FDA osteoarthritis Assessment of Structural Change Working Group. Osteoarthritis Cartilage. https://doi.org/10.1016/j.joca.2011.02.018
Zheng S, Jin X, Cicuttini F et al (2017) Maintaining Vitamin D sufficiency is associated with improved structural and symptomatic outcomes in knee osteoarthritis. Am J Med. https://doi.org/10.1016/j.amjmed.2017.04.038
Xia W, Cooper C, Li M et al (2019) East meets West: current practices and policies in the management of musculoskeletal aging. Aging Clin Exp Res 31:1351–1373. https://doi.org/10.1007/s40520-019-01282-8
Bruyere O, Honvo G, Veronese N et al (2019) An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO). Semin Arthritis Rheum. https://doi.org/10.1016/j.semarthrit.2019.04.008
Honvo G, Reginster J-Y, Rabenda V et al (2019) Safety of symptomatic slow-acting drugs for osteoarthritis: outcomes of a systematic review and meta-analysis. Drugs Aging 36:65–99. https://doi.org/10.1007/s40266-40019-00662-z
Bruyere O, Cooper C, Al-Daghri NM et al (2017) Inappropriate claims from non-equivalent medications in osteoarthritis: a position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Muscu-loskeletal Diseases (ESCEO). Aging Clin Exp Res 30:111–117. https://doi.org/10.1007/s40520-017-0861-1
Honvo G, Bruyère O, Reginster JY (2019) Update on the role of pharmaceutical-grade chondroitin sulfate in the symptomatic management of knee osteoarthritis. Aging Clin Exp Res 31:1163–1167. https://doi.org/10.1007/s40520-019-01253-z
Fuggle NR, Cooper C, Oreffo ROC et al (2020) Alternative and complementary therapies in osteoarthritis and cartilage repair. Aging Clin Exp Res 32:547–560. https://doi.org/10.1007/s40520-020-01515-1
Beaudart C, Lengelé L, Leclercq V et al (2020) Symptomatic efficacy of pharmacological treatments for knee osteoarthritis: a systematic review and a network meta-analysis with a 6-month time horizon. Drugs. https://doi.org/10.1007/s40265-020-01423-8
Funding
This study was supported by the Project of Youth Innovation in Medical Research in Sichuan Province (Q15027), the Project of Sichuan Education Department (18ZB0640) and the Project of Health Department in Sichuan Province (150078).
Author information
Authors and Affiliations
Contributions
Z-XZ conceived and designed the study. L-HP collected and collated the data. XL and ML analyzed data, and all authors interpreted the results. Z-XZ drafted and revised the paper. All authors revised the draft paper. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, ZX., He, Y., Peng, LH. et al. Does vitamin D improve symptomatic and structural outcomes in knee osteoarthritis? A systematic review and meta-analysis. Aging Clin Exp Res 33, 2393–2403 (2021). https://doi.org/10.1007/s40520-020-01778-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-020-01778-8