Abstract
Gluten ataxia (GA) has customarily been considered to be the main neurological manifestation of celiac disease (CD). In recent years, the condition of non-celiac gluten sensitivity (NCGS) has been defined, which includes some patients who are not considered “true celiacs.” We performed a comparative clinicopathological study of these three entities. We studied 31 GA, 48 CD and 37 NCGS patients, prospectively in the same center for a period of 7 years. The protocol study included two serological determinations for gluten sensitivity [anti-gliadin IgA and IgG (AGA) and anti-tissue transglutaminase IgA (TG) antibodies], HLA-DQ2 typing, and duodenal histological assessment. Demographics and investigative findings were compared. Females were 55 % in GA, 75 % in CD (p < 0.001), and 47 % in NCGS (N.S.). GA patients were older (59 ± 14 years) than CD (43 ± 13 years) and NCGS (41 ± 8 years) groups (p < 0.001). AGA positivity was higher in GA (100 %) than in CD (48 %) groups (p < 0.001), but similar to NCGS patients (89 %; N.S.); TG positivity was lower in GA (3.2 %) than in CD (33.3 %; p < 0.001), but similar to NCGS (2.7 %; N.S.). DQ2 (+) was lower in GA (32.2 %) than in CD (89.6 %; p < 0.001), but similar to NCGS (29.7 %; N.S.). Lymphocytic enteritis (Marsh type 1) was lower in GA (9.6 %) than in CD (66.7 %; p < 0.001), but similar to NCGS (10.8 %; N.S.). The other gluten sensitivity-related characteristics measured were different to CD patients, but very close to NCGS. We conclude that GA patients are better classified within the NCGS group, than within CD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gluten intolerance has a variety of clinical manifestations that can occur irrespective of the presence or absence of the classic small bowel lesions, characteristics of celiac disease (CD). Gluten ataxia (GA) is one of a number of different neurological manifestations attributed to CD and was originally defined as otherwise idiopathic sporadic ataxia, with positive serological markers for gluten sensitization. Like CD, GA is considered to be an autoimmune disease characterized by distinctive neurological features such as other types of ataxia. Dermatitis herpetiformis, CD, and GA are considered to be the three main types of gluten-related autoimmune diseases [1].
Defining criteria for GA include otherwise idiopathic sporadic ataxia in association with positive anti-gliadin antibodies (AGAs), with or without enteropathy revealed by duodenal biopsy. The finding most common to many GA studies is the consistently greater prevalence of AGA positivity in sporadic ataxias than in healthy controls. AGA (+) is present in an average of 40 % of GA patients, while in the so-called healthy population it varies from 5 to 12 %, depending on the assay used and the local prevalence of CD [2–6].
The condition of non-celiac gluten sensitivity (NCGS) relates to one or more of a variety of immunological, morphological, and symptomatic manifestations precipitated by the ingestion of gluten by people for whom CD has been ruled out [7]. NCGS is a condition in which gluten ingestion leads to morphological or symptomatic manifestations despite the absence of CD. It was first described in the early 1980s, but over the past decade the amount of patients diagnosed with NCGS and of publications on this topic have increased considerably [8, 9]. However, it is still not clear how to diagnose or manage the condition, and its pathophysiological mechanisms remain unclear. Unlike CD, NCGS may show signs of an activated innate immune response but without the finding of small bowel lesions, elevated levels of known serological markers of CD, and the increased mucosal permeability that are characteristic of CD. However, the patient response to a gluten-free diet (GFD) is similar to that found in patients with CD [10–14].
In this paper, we describe a series of GA, with their clinical characteristics all of them following a GFD.
Methods
Patients
We conducted a prospective observational study of a consecutive series of 31 patients suffering from GA, based on the original definition, i.e., presenting idiopathic cerebellar ataxia with positive anti-gliadin IgA and/or IgG antibodies, irrespective of the positivity for tissue transglutaminase antibodies (TG) and/or anti-endomysial antibodies (EMA) [15].
A cohort of 48 CD patients who had been diagnosed based on positive serologic testing (generally for IgA TG, first) followed by an upper endoscopy with several duodenal biopsies for confirmation and a positive clinical response to a strict gluten-free diet were followed up at least along one year [16].
We included also a third group of 37 NCGS patients according to international consensus diagnostic criteria, which was defined as self-reported gluten intolerance, negative celiac serology and absence of villous atrophy [17].
This clinical study was performed in a tertiary urban hospital in Northern Spain, serving an area with a population of 250,000. Patients were consecutively enrolled during a seven-year study period (January 2007–December 2013).
The positive criteria for the diagnosis of sporadic cerebellar ataxia included: (a) presence of progressive cerebellar ataxia with no symptomatic cause; (b) absence of a family history of neurodegenerative disorder; and (c) negative genetic diagnosis for Friedrich’s ataxia and spinocerebellar ataxias [2].
The exclusion criteria included: (a) clinical or magnetic resonance imaging (MRI) features of multiple system atrophy, progressive myoclonic ataxia, fragile-X-associated syndrome, or mitochondrial encephalomyopathy; (b) exposure to alcohol or other toxins; (c) vitamin B12 or vitamin E deficiency; and (d) evidence of HIV, syphilis, prion disease, or Whipple disease [3].
All GA patients underwent at baseline a complete neurological examination, including a broad neurophysiological study, determination of the presence of oligoclonal bands in the CSF, and an MRI brain scan with repeated visits along the study period, including routine analytical determinations and continuous clinical assessment every 6–12 months.
The gastrointestinal symptoms in these individuals were mild to moderate in severity, featuring a chronic recurrent pattern, ranging from flatulence to diarrhea or constipation, and fluctuating abdominal pain.
The study was approved by the Research and Ethics Committee of the HUCA, following the principles of the modified Declaration of Helsinki.
All the patients accepted an invitation to participate on a voluntary basis in a screening test for CD associated with their GA diagnosis. They were referred to a gastroenterology outpatient clinic specializing in the study of disease of the small intestine, which was located in the same hospital. All patients were evaluated by the same gastroenterologist and then underwent a series of analytical assessments that included a battery of serological and genetic markers, and an upper-GI endoscopy taking several (4–6) duodenal biopsies for analysis.
Analytical studies
Anti-IgA and anti-IgG gliadin (AGA) and anti-IgA tissue transglutaminase subtype 2 (TG) antibodies were measured with an ELISA kit (Phadia Diagnostics, Uppsala, Sweden). In these patients is better to use mainly the TG determination only.
Major histocompatibility complex class II (HLA-DQ2) genetic markers (DQA1*0501 and DQB1*0201 alleles) were determined by a polymerase chain reaction (PCR) with a Protrans HLA Celiac Disease Domino System kit (Protrans, Ketsch, Germany), and the HLA-DQ8 haplotype was measured in DQ2 negatives.
We quantified all classes of immunoglobulins (IgA, IgM, IgD) and carried out specific dosage of serum IgE and skin-prick tests to rule out wheat allergy in any of the patients.
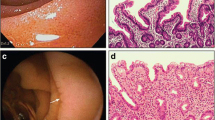
An upper-GI endoscopy with at least four duodenal biopsies was performed on all patients using the customary methods. Samples were routinely stained with hematoxylin–eosin (HE) and with anti-CD3 Dako immunohistochemical monoclonal antibodies, to verify the presence and determine the frequency of intraepithelial lymphocytes (IELs). These were quantified in turn in relation to 100 epithelial cells. Samples were studied by two expert pathologists from the HUCA and classified into the following types: stage 0, normal duodenum; stage 1, increased IEL infiltration with a total count of ≥25 %; stage 2, crypt hyperplasia and presence of diffuse chronic inflammatory infiltration of the lamina propria; stage 3, villous atrophy, subdivided into three categories (mild, moderate and severe), according to the histological classification of CD described by Marsh [18] with the subsequent modifications of Oberhüber et al. [19].
GA patients were compared with an adult group of 48 CD patients and a group of 37 NCGS patients. All three groups were studied in our clinic over the same period in an ambulatory context [20].
The criteria used to diagnose NCGS in our study were, besides the exclusion of CD from a determination of normal histology and negative CD-specific serology, exclusion of wheat allergy by normal specific IgE and a negative skin-prick test, a 6- to 12-month trial of a GFD with disappearance of symptoms, and relapse of symptoms upon a 1-month open gluten challenge.
Statistics
Descriptive statistics (mean, standard deviation and range) were calculated for continuous variables. Qualitative variables were summarized as percentages. Differences between group means of normally distributed continuous variables were assessed by Student’s unpaired-samples t test. Contingency tables of categorical variables were analyzed by Fisher’s exact test, and the odds ratio (OR) and 95 % confidence interval (CI) were estimated. All statistical tests were two-sided, with significance concluded for values of p < 0.05. They were carried out with SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Of the 31 GA patients included in the study, 17 were male and 14 female, giving a gender ratio M/F of 1.2/1. The mean age of onset of ataxia was 51 years, with a broad range, going from 30 to 80 years in the extreme cases. Gait ataxia was the main sign, present in 100 % of patients. Lower limb ataxia was found in 92 % being more often affected than upper limbs (68 %). Gaze-evoked nystagmus and other ocular signs of cerebellar dysfunction were seen in 77 % of the cases. Additional movement disorders including myoclonus, palatal tremor were found sporadically in only 2 patients. Peripheral neuropathy, mainly sensitive in type, was found in 56 % of cases. We did not find any autonomic dysfunction present in these patients.
AGA positivity was found in all patients, but TG was positive in only one case (3 %). HLA-DQ2 positivity was found in 10 patients (32 %), and lymphocytic enteropathy on duodenal biopsies >25 % (Marsh 1 stage) was present in only three cases (10 %).
We found the presence of oligoclonal bands in the cerebrospinal fluid in the 46 % of patients. MRI cerebral studies showed mild to moderate degrees of cerebellar lesions in 60 % of cases, with the presence of an atrophy at the cerebellar vermis (Fig. 1).
There were some associated diseases of autoimmune origin. The most frequently present was dermatitis herpetiformis in 7 cases (22.6 %) and 2 cases of thrombocytosis (6.5 %). The disease was very disabling at diagnosis, and 6 patients (19.3 %) needed a wheelchair for daily life activities. Finally, 3 patients (10 %) died by associated diseases, most of them not directly related to the main neurological disease. The demographic data, clinical characteristics, and the serological and histological findings of GA patients are summarized (Table 1).
The patients in the CD group were more likely to be female (gender ratio of 3:1), and were younger on average (mean age 43 ± 13 years; range 27–56 years) than those in the GA group (p = 0.007). With respect to the serological markers, AGA and TG positivity were present in significantly lower and higher proportions, respectively, in the CD group, compared with the GA group (p < 0.001).
The genetic marker of HLA-DQ2, was very predominant in CD patients (89.6 %), contrasting with the lower percentage found in GA (30 %; p < 0.001). Lymphocytic enteritis was the predominant lesion in celiacs (32 cases, 66.7 %), which was about twice as common as in GA patients (32.2 %; p < 0.001). These results, including the ORs and 95 % CIs, are shown in Table 2.
The comparative analysis with the NCGS group revealed no differences between genders, but the mean age of the GA patients was significantly older on average (59 ± 14 years) than that of the NCGS group (41 ± 8 years; p < 0.001).
AGA positivity was slightly but not significantly lower (89 %) in the NCGS group (p = 0.100). Likewise, analysis of TG positivity revealed a mean of 2.7 % in NCGS patients compared with 3.2 % in the GA group (p = 1.000). There was no significant difference in HLA-DQ2 between the two groups (32.2 vs. 29.7 %) (p = 1.000). The histological examination of duodenal biopsies also failed to identify any significant differences between the two groups (9.6 vs. 10.8 %; p = 1.000). These results, including the ORs and 95 % CIs, are shown in Table 3.
Discussion
Our results indicate that patients in the GA group are more accurately classified within the NCGS than within the CD group. The only significant difference between the first two groups was that the mean age of patients was greater in the GA group than in the CD group (p < 0.001). The other serological, genetic, and histological characteristics related to gluten intolerance were very similar, with no significant differences between the groups.
In contrast, we found several statistically significant differences with p values <0.001, between the GA and CD groups, with a lower proportion of women in the former group (55 vs. 75 %; OR 3.64; 95 % CI 1.39–9.54), older age, more frequent AGA positivity (p < 0.001), less frequent TG positivity (3.2 vs. 33.3 %; OR 14.9; 95 % CI 1.87–125), a lower percentage of DQ2 (+) (32.2 vs. 89.6 %; OR 23.25; 95 % CI 6.49–83.33), and less frequent Marsh 1 lesions in GA (9.6 vs. 66.7 %; OR 18.52; 95 % CI 4.92–71.4).These results lead us to conclude that, in relation to all the characteristics analyzed, GA seems to more closely resemble NCGS than CD.
GA was originally defined in 1968 by Hadjivassiliou et al. [2] as a variety of otherwise idiopathic sporadic ataxia with positive serological markers for gluten sensitization. Like CD, it is an autoimmune disease characterized by damage to the cerebellum that results in ataxia. There is some evidence to suggest that there is antibody cross-reactivity between antigenic epitopes on Purkinje cells and gluten proteins [21, 22].
However, GA is not the sole neurological manifestation occurring in CD, since there have been several reports linking CD and/or gluten sensitivity with mental health manifestations, including isolated psychosis and full-blown schizophrenia [23, 24]. Complete symptom resolution was reported in these cases when gluten was removed from the diet.
There is also evidence of frequent gluten sensitivity (but not CD) in some schizophrenia patients. Furthermore, similar reports have been published concerning various other neurological manifestations in response to gluten exposure, including the so-called idiopathic neuropathies, some forms of epilepsy, and autism [25–27].
The prevalence of CD is estimated to be close to 1 in 100 in Western countries [28]. However, a higher percentage of the general population consider themselves to be suffering from wheat sensitivity to wheat sensitivity and exclude wheat from their diet on the basis of their negative experience after eating wheat-containing foods. These wheat-reactive patients often present symptoms similar to those of CD, but have negative CD serology and histopathology.
In most cases, these patients consult several physicians, seeking to obtain a diagnosis of CD, but, very often, they are considered to be suffering “simply” from irritable bowel syndrome [29]. The diagnostic gold standard for wheat sensitivity ought to be the double-blind placebo-controlled (DBPC) challenge, but this is a cumbersome and time-consuming method and is therefore very rarely used.
A precise and widely agreed definition of NCGS does not yet exist. It is currently understood to be a condition associated with experiencing various symptoms in response to ingestion of foods containing wheat, rye and barley, and the resolution of these symptoms on withdrawal of those foods from the diet of individuals in whom CD and wheat allergy have been ruled out. The symptoms may be accompanied by an increase in levels of gluten antibody. The majority of symptoms associated with NCGS are subjective, including abdominal pain, headache, “brain fog,” tingling and/or numbness in the hands and feet, fatigue, and musculoskeletal pain. However, other symptoms, such as rash and diarrhea, as well as more severe neurological and psychiatric conditions including schizophrenia and cerebellar ataxia, have also been reported to be associated with NCGS.
Although NCGS has received greater attention in recent years, earlier reports had already suggested that the condition is a distinct clinical entity. A study in 1980 described eight female subjects with abdominal pain and chronic diarrhea who experienced dramatic relief when on a GFD and a return of symptoms when exposed to a gluten challenge. CD was ruled out due to the lack of villous atrophy on a gluten-containing diet, but it was noted that the gluten challenge induced a jejunal cellular infiltrate. The clinical description of these patients is similar to that of patients who are now frequently found in clinical practice and are thought to have NCGS [9].
A connection between CD and certain specific neurological and psychiatric disorders has been proposed in recent decades, based primarily on findings solely of elevated antibodies to gliadin in affected patients [30]. Some of the most entities frequently associated include schizophrenia, peripheral neuropathy, cerebellar ataxia, and autism. Recently published reports of large patient cohorts have described increased circulating levels of antibody to gluten in about a quarter of individuals with schizophrenia [31]. However, new data indicate that the anti-gluten immune response differs significantly from that in CD, whereby these patients display a unique antigenic specificity that is apparently independent of the action of TG enzyme and presentation by HLA-DQ2 and HLA-DQ8 molecules. As such, the majority of such patients may belong to the NCGS category, rather than to the CD group.
Similarly, elevated antibody reactivity to gluten has been reported in up to 40 % of patients with idiopathic sporadic ataxia. Other investigators have shown a similarly greater prevalence of increased antibody reactivity to gliadin in both sporadic and hereditary ataxias. Most of these patients do not appear to have the specific serological markers of CD and may again fit more comfortably within the NCGS spectrum [6]. We did not determine the TG subtype 6 (TG6), because this test is not available for routine clinical use. Also we did not include the deaminated peptides of gliadin (DGP) antibodies, because they are very sensitive in young children, but not in adults.
A study of a cohort of patients with ataxia and elevated AGA has shown a positive response to GFD in some individuals. However, the pathogenic relevance of the increased antibody response to gliadin in different forms of ataxia and the potential effect of GFD on disease remain matters of debate [15]. All patients were good compliant following a strict gluten-free diet from their inclusion into the study and most of them achieving a stopping or a clear improvement in their neurological clinical manifestations.
The clinical workup for diagnosis of NCGS usually focuses on the exclusion of CD and wheat allergy. Serological testing is particularly useful in this regard, including testing for IgA antibodies to TG2, as well as IgG and IgA antibodies to deamidated gliadin in children for CD, and IgE antibodies to wheat proteins for wheat allergy. HLA typing may also be very useful, as negativity for HLA-DQ2 and HLA-DQ8 has an excellent negative predictive value for CD [32]. Skin-prick testing is an additional tool for ruling out wheat allergy. The task of exclusion is difficult in those patients who are already on a strict GFD. If the patient does indeed have CD, the mucosa may have recovered and the serology could also be negative, and thus a short gluten challenge may be necessary. At present, there are no known specific serological markers that can confirm the clinical suspicion of NCGS.
No reliable epidemiological studies of NCGS have been published so far. According to an article published in The Wall Street Journal, some experts think as many as 6 % of North American people may have some form of NCGS [33], although it is not clear what data this estimate is based upon. It is also estimated that in 2010 the market for gluten-free food was worth $2.6 billion, implying that a considerable proportion of the US population is consuming gluten-free foods. Data from both scientific and lay literature suggest that there is greater public recognition of gluten sensitivity than of CD [34]. Although the “low-carb diet” was widely adopted from 2000 onwards, it has shown a steady decline in adherents since 2005. On the other hand, the GFD has become increasingly popular since 2008 and this trend is expected to continue. Although the prevalence of CD in the USA, like in all western countries, is around 1 %, most patients are undiagnosed and known celiacs make up a relatively small fraction of the consumers of gluten-free products. So far, there have not been any high-quality genetic studies of the NCGS population. There is no evidence to suggest that the condition has the same pattern of association with HLA-DQ2/-DQ8, as does CD.
Several studies have shown that NCGS patients have an increased density of intraepithelial lymphocytes (IELs), although not to the same extent as untreated CD patients. The IELs expressed the T cell receptor α/β, but not the γ/δ subtype. The investigators concluded that NCGS patients showed signs of increased innate, rather than adaptive, immune activation. Although the pathophysiology of NCGS is currently far from clear, the available data support the existence of an immune activation process that is a common factor in CD and NCGS [35–37]. This may be associated also with the so-called sporadic adult onset cerebellar ataxia [6, 38]. A good information to the patient and their families about the strict adherence to the GFD is crucial to achieve a better response not only in CD but also in NCGS and of course in GA patients [39].
In conclusion, our clinical study found that GA patients fits more with the NCGS patients, than with CD group, on the grounds that the serological, genetic, and histological findings from their duodenal biopsies are much more similar to those in the former clinical category. Therefore, the recognition of GA as part of NCGS does not simply mean a semantic issue, but allows the clinicians to think out of the box of CD and to consider more widely the spectrum of gluten sensitivity.
References
Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, Kaukinen K, Rostami K, Sanders DS, Schumann M, Ullrich R, Villalta D, Volta U, Catassi C, Fasano A. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13.
Hadjivassiliou M, Grünewald RA, Chattopadhyay AK, Davies-Jones GA, Gibson A, Jarratt JA, Kandler RH, Lobo A, Powell T, Smith CM. Clinical, radiological, neurophysiological and neuropathological characteristics of gluten ataxia. Lancet. 1998;352:1582–5.
Hadjivassiliou M, Grünewald RA, Sharrack B, Sanders D, Lobo A, Williamson C, Woodroofe N, Wood N, Davies-Jones A. Gluten ataxia in perspective: epidemiology, genetic susceptibility and clinical characteristics. Brain. 2003;126:685–91.
Pellecchia MT, Scala R, Filla A, De Michele G, Ciacci C, Barone P. Idiopathic cerebellar ataxia associated with celiac disease: lack of distinctive neurological features. J Neurol Neurosurg Psychiatry. 1999;66:32–5.
Luostarinen LK, Collin PO, Peräaho MJ, Mäki MJ, Pirttilä TA. Coeliac disease in patients with cerebellar ataxia of unknown origin. Ann Med. 2001;33:445–9.
Bürk K, Bösch S, Müller CA, Melms A, Zühlke C, Stern M, Besenthal I, Skalej M, Ruck P, Ferber S, Klockgether T, Dichgans J. Sporadic cerebellar ataxia associated with gluten sensitivity. Brain. 2001;124:1013–9.
Ludvigsson JL, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, Hadjivassiliou M, Kaukinen K, Kelly CP, Leonard JN, Lundin KE, Murray JA, Sanders DS, Walker MM, Zingone F, Ciacci C. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52.
Ellis A, Linaker BD. Non-coeliac gluten sensitivity? Lancet. 1978;1:1358–9.
Cooper BT, Holmes GK, Ferguson R, Thompson RA, Allan RN, Cooke WT. Gluten sensitive diarrhea without evidence of celiac disease. Gastroenterology. 1980;79:801–6.
Catassi C, Bai JC, Bonaz B, Bouma G, Calabrò A, Carroccio A, Castillejo G, Ciacci C, Cristofori F, Dolinsek J, Francavilla R, Elli L, Green P, Holtmeier W, Koehler P, Koletzko S, Meinhold C, Sanders D, Schumann M, Schuppan D, Ullrich R, Vécsei A, Volta U, Zevallos V, Sapone A, Fasano A. Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5:3839–53.
Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145:320–8.
Campanella JI, Biagi F, Bianchi PI, Zanellati G, Marchese A, Corazza GR. Clinical response to gluten withdrawal is not an indicator of coeliac disease. Scand J Gastroenterol. 2008;43:1311–4.
Ciccocioppo R, Cangemi GC, Roselli EA, Kruzliak P. Are stem cells a potential therapeutic tool in coeliac disease? Cell Mol Life Sci. 2015;72:1317–29.
Makovicky P, Rimarova K, Boor A, Makovicky P, Vodicka P, Samasca G, Kruzliak P. Correlation between antibodies and histology in celiac disease: incidence of celiac disease is higher than expected in the pediatric population. Mol Med Rep. 2013;8:1079–83.
Hadjivassiliou M, Davies-Jones GA, Sanders DS, Grunewald R. Dietary treatment of gluten ataxia. J Neurol Neurosurg Psychiatry. 2003;74:1221–4.
Fasano A, Catassi C. Celiac disease. N Engl J Med. 2012;367:2419–26.
Carroccio A, Mansueto P, Iacono G, Soresi M, D’Alcamo A, Cavataio F, Brusca I, Florena AM, Ambrosiano G, Seidita A, Pirrone G, Rini GB. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol. 2012;107:1898–906.
Marsh MN. Gluten, major histocompatibility complex and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology. 1992;102:330–54.
Oberhüber G, Granditsch G, Vogelsang H. The histopathology of celiac disease. Time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;1:1185–94.
Lucendo AJ, Garcia-Manzanares A, Arias A, Fuentes D, Alvarez N, Perez I, Guagnozzi D, Rodrigo L. Coeliac disease in the 21st century: no longer “kids stuff”. Gastroenterol Res. 2011;4:268–76.
Abele M, Bürk K, Schöls L, Schwartz S, Besenthal I, Dichgans J, Zühlke C, Riess O, Klockgether T. The aetiology of sporadic adult-onset ataxia. Brain. 2002;125:961–8.
Boscolo S, Lorenzon A, Sblattero D, Florian F, Stebel M, Marzari R, Not T, Aeschlimann D, Ventura A, Hadjivassiliou M, Tongiorgi E. Anti transglutaminase antibodies cause ataxia in mice. PLoS ONE. 2010;5:e9698.
Kalaydjian AE, Eaton W, Cascella N, Fasano A. The gluten connection: the association between schizophrenia and celiac disease. Acta Psychiatr Scand. 2006;113:82–90.
Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, Yang S, Krivogorsky B, Alaedini A, Yolken R. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry. 2010;68:100–4.
Chin RL, Latov N. Peripheral neuropathy and celiac disease. Curr Treat Options Neurol. 2005;7:43–8.
Canales P, Mery VP, Larrondo FJ, Bravo FL, Godoy J. Epilepsy and celiac disease: favorable outcome with a gluten-free diet in a patient refractory to antiepileptic drugs. Neurologist. 2006;12:318–21.
Genuis SJ, Bouchard TP. Celiac disease presenting as autism. J Child Neurol. 2010;25:114–9.
Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43.
Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the no man’s land of gluten sensitivity. Am J Gastroenterol. 2009;104:1587–94.
Bushara KO. Neurologic presentation of celiac disease. Gastroenterology. 2005;128:S92–7.
Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, Yang S, Krivogorsky B, Alaedini A, Yolken R. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry. 2010;68:100–4.
Sollid LM, Lie BA. Celiac disease genetics: current concepts and practical applications. Clin Gastroenterol Hepatol. 2005;3:843–51.
Beck M. Clues to gluten sensitivity. Wall Street J. http://online.wsj.com/article/SB10001424052748704893604576200393522456636.html. Accessed 8 Aug 2012.
Aggarwal S, Lebwohl B, Green PH. Screening for celiac disease in average-risk and high-risk populations. Therap Adv Gastroenterol. 2012;5:37–47.
Sapone A, Lammers KM, Casolaro V, Cammarota M, Giuliano MT, De Rosa M, Stefanile R, Mazzarella G, Tolone C, Russo MI, Esposito P, Ferraraccio F, Cartenì M, Riegler G, de Magistris L, Fasano A. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 2011;9:23.
Di Sabatino A, Corazza GR. Nonceliac gluten sensitivity: sense or sensibility? Ann Intern Med. 2012;156:309–11.
Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the “no man’s land” of gluten sensitivity. Am J Gastroenterol. 2009;104:1587–94.
Sivera R, Martín N, Boscá I, Sevilla T, Muelas N, Azorín I, Vílchez JJ, Bolonio M, Donat E, Ribes-Koninckx C, Bataller L. Autoimmunity as a prognostic factor in sporadic adult onset cerebellar ataxia. J Neurol. 2012;259:851–4.
Ludvigsson JF, Card T, Ciclitira PJ, Swift GL, Nasr I, Sanders DS, Ciacci C. Support for patients with celiac disease: a literature review. U Eur Gastroenterol J. 2015;3:146–59.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rodrigo, L., Hernández-Lahoz, C., Lauret, E. et al. Gluten ataxia is better classified as non-celiac gluten sensitivity than as celiac disease: a comparative clinical study. Immunol Res 64, 558–564 (2016). https://doi.org/10.1007/s12026-015-8750-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-015-8750-1