Abstract
Iso-α-acids (IAA) and reduced IAA can be used as beer-specific ingredient congeners to confirm beer consumption when detected in blood and other specimens using a UHPLC–MS/MS method. Recent analysis of postmortem casework demonstrated a high prevalence of beer consumption and the possibility of providing the source of alcohol in forensic casework. Research outlined in this manuscript has examined the degree to which the interval after death and quality of blood affects the concentration of IAA in postmortem cases. Postmortem whole blood and serum were analyzed in cases where natural or reduced IAA groups were detected. The trans-IAA, cis-IAA, and tetrahydro-IAA (TIAA) groups were subject to postmortem redistribution, although only weakly associated with the length of time from death to collection of specimens. Serum had threefold higher concentrations than blood for trans-IAA, cis-IAA, and TIAA. These studies confirm that although postmortem concentrations cannot be easily compared to concentrations found in living persons the presented findings do provide some understanding to assist in interpretation where the confirmation of beer consumption is required in forensic casework.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcohol consumption results in a significant increase in deaths, hospitalizations, and alcohol-related crimes and is reflected domestically with the recent findings that spotlight the dangers of alcohol-fueled violence [1, 2]. Beer is the most commonly consumed alcoholic beverage and third most consumed beverage overall, following water and tea; responsible for approximately 4.7 L of pure alcohol ingested per capita in Australia annually [3, 4].
Iso-α-acids (IAA) and three structurally similar but chemically-altered IAA known as reduced IAA provide the bitter properties in beer. However they can also be used as beer-specific ingredient congeners to confirm beer consumption. In the laboratory, a protein precipitation extraction and ESI–UHPLC–MS/MS method was developed and validated for the detection of these compounds in blood [5]. The long-term stabilities of these analytes in stored blood specimens was assessed over 8 weeks with freezing (−20 °C) and refrigeration (4 °C) conditions determined as acceptable [6]. The analysis of the blood and urine from volunteers consuming a range of brown and clear bottled beers in small amounts in controlled drinking studies demonstrated successful bioavailability of these compounds [7, 8]. Additional pharmacokinetic data demonstrated small inter-variable differences in concentration–time profile, absorption occurring within an hour, and half-lives ranging between approximately 30–46 min.
Recently this methodology was applied to the human tissue from forensic postmortem cases to determine the ability to detect natural IAA and reduced IAA groups and ultimately to demonstrate the prevalence of beer consumption [9]. Nearly 90 % of all cases that had “beer” mentioned in the circumstances or autopsy report of the cases, contained an IAA beer marker. It was further shown in a separate cohort that 57 % of cases that had no mention of beer ingestion but contained a positive alcohol concentration also demonstrated beer consumption prior to death. Such data demonstrates the high prevalence of beer consumption in Australia, in agreement with market data from the alcohol industry [3, 4].
Further investigation of postmortem casework is necessary in order to obtain information such as postmortem redistribution (PMR) and the comparison between serum and whole blood concentrations. This study aims to examine such postmortem phenomena to provide the relevant toxicology data required in order to interpret and confirm beer consumption when IAA type compounds are detected in casework.
Materials and methods
Case and specimen selection
The Victorian Institute of Forensic Medicine performs medico-legal investigations of deceased cases reported to the Coroners Court of Victoria. The toxicology laboratory within the Institute receives specimens on admission of the body to the mortuary (generally femoral whole blood and serum) and also following an autopsy when it is conducted with a larger range of body specimens including another whole blood and serum. Coronial, autopsy, and toxicological data (e.g., demographics, cause of death, drug exposure, BAC, etc.) were obtained using the Institute’s case management system. No decomposed cases were considered for analysis.
Ethical approvals
Permission for this research was obtained by the Research Advisory Committee (RAC 013/13) and Human Research Ethics Committee (EC 07/2013) of the Victorian Institute of Forensic Medicine.
Specimens
For mortuary admission specimens, blood (and often serum) was collected as soon as practicable after a body was admitted to the mortuary. At autopsy, additional blood and serum specimens from the same deceased person were collected using the same blood drawing technique. Mortuary admission blood was refrigerated (4 °C) prior to analysis, all other specimens were stored frozen (−20 °C). All specimens were collected in polypropylene tubes with all whole blood specimens containing 1 % sodium fluoride/potassium oxalate preservative. Serum was obtained by centrifugation for 10 min at 2,400×g. Blood was collected from the femoral region unless otherwise indicated.
Drug-free specimens were collected for instrument calibration and quality control purposes. Preserved blank blood (10 mL samples containing 200 mg sodium fluoride and 30 mg potassium oxalate) was obtained from a local blood bank (Melbourne, Australia). A blank postmortem serum was obtained from a case previously analyzed that showed no IAA analytes. All blank specimens underwent additional screening to ensure there were no IAA analytes or other interferences. All blank specimens were collected in polypropylene tubes and immediately frozen (−20 °C).
Chemicals and reagents
The details of the reference standards for the natural IAA and reduced IAA obtained from Labor Veritas (Zurich, Switzerland) are: IAA, so called “DCHA-Iso, ICS-I3,” (containing 62.3 % w/w of trans-IAA); rho-IAA (RIAA), so called “DCHA-Rho, ICS-R2” (containing 65.3 % w/w of cis-RIAA); tetrahydro-IAA (TIAA), so called “Tetra, ICS-T2” (containing 99.4 % w/w of TIAA), and; hexahydro-IAA (HIAA), so called “DCHA-Hexa, ICS-H1” (containing 65.7 % w/w of cis-HIAA).
The isotope labeled internal standard nimodipine-d7 was purchased from PM Separations (Brisbane, Australia). Acetonitrile (ACN), methanol, and formic acid were purchased from Merck (Melbourne, Australia). Ammonium formate was purchased from Sigma Aldrich (Sydney, Australia). All chemicals were of analytical grade or better and water was purified using a Milli-Q Ultrapure Water System from Waters (Sydney, Australia).
IAA and reduced IAA analytical methodology
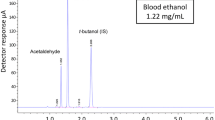
Natural IAA (trans-IAA and cis-IAA) and reduced IAA (RIAA, TIAA, and HIAA) determination was performed using a previously published UHPLC–MS/MS method that was validated for blood analysis [5]. Briefly, the extraction consisted of a protein precipitation of 200 µL of whole blood using −20 °C ACN with the resulting supernatant dried under nitrogen and the residue reconstituted with 50 µL of a mixture of eluent A and eluent B (60:40, v:v). Preparation of stock solutions, calibration standards, quality controls, and extraction procedures were performed as published previously [5]. Blank serum replaced whole blood in the calibration and quality control models for the analysis of serum casework. The availability of IAA reference standards allowed for the quantification of trans-IAA, RIAA, TIAA, and HIAA groups. Residual cis-IAA in the trans-IAA reference standard was used to allow for qualitative cis-IAA results.
The cis-IAA group was unable to be quantified due to lack of specific reference standard. However to allow for comparisons to be made, the internal standard/area ratios of each cis-IAA analyte were summed to provide a total are ratio for the cis-IAA group.
The UHPLC–MS/MS system comprised of a Shimadzu MS 8030 quadrupole mass spectrometer (Melbourne, Australia) operated in electrospray ionization negative mode. A Shimadzu Nexera UHPLC system (Melbourne, Australia) consisted of a degasser, two eluent pumps, a column oven (30 °C) with a Kinetex C18 column (3.0 × 150 mm, 2.6 μm from Phenomenex, Melbourne, Australia), and a chilled autosampler (4 °C). The mobile phases consisted of 50 mmol/L aqueous:ACN (90:10) ammonium formate pH 2.8 (eluent A) and ACN containing 0.1 % formic acid (eluent B). The flow rate of the mobile phase was 0.5 mL/min and was degassed by the integrated Shimadzu Nexera degasser during use. The gradient was programmed as follows: 0–0.5 min hold at 50 % eluent B; 0.5–6.0 min eluent B increasing to 60 %; 6.0–9.5 min eluent B increasing to 75 %; 9.5–10 min eluent B hold at 75 %. Before the start of batch analysis and before each injection, the UHPLC system was flushed for 2 min (90 % eluent B) and equilibrated at starting conditions (50 % eluent B) for 5 min.
For MS data evaluation, Shimadzu Postrun and Shimadzu Browser Analysis (Melbourne, Australia) software were used.
Statistical analysis
All statistical analysis was performed using GraphPad Prism 5.04 from GraphPad Software (San Diego, USA). The median, range, and population size (n) was reported and p < 0.05 was considered statistically significant for all analyses.
Postmortem redistribution
To demonstrate PMR any changes in natural and reduced IAA median concentrations between mortuary admission (ADM) and autopsy (AUT) bloods was determined. The median postmortem interval (time of death–time of collection, PMI), median concentrations and their ranges, and the AUT/ADM ratio were examined. The Wilcoxon matched-pairs signed rank test (p < 0.05) was performed on the concentrations of natural and reduced IAA in femoral whole bloods from cases that had five or more paired mortuary admission and autopsy specimens.
Additionally any correlation between the pre-autopsy interval (collection time difference between mortuary admission and autopsy specimens) and changes in IAA concentrations for the same cases were plotted to demonstrate if PMR was influenced by a prolonged or delayed specimen collection. The following formula was used to evaluate the change in concentration [%] between ADM and AUT specimens:
If ∆Conc [%] > 0 an increase in concentration was observed between ADM and AUT blood specimens, if ∆Conc [%] < 0 a decrease in concentration was observed between ADM and AUT blood specimens.
Finally, any influence of different blood collection sites on the concentrations of natural and reduced IAA were assessed. Femoral and non-femoral (heart, cavity, and subclavian) specimens were compared in paired specimens with positive IAA groups.
Serum/blood ratios
The serum to blood ratios (S/B) for the natural and reduced IAA groups were estimated. Comparison of whole blood and serum specimens collected only from the femoral region at admission to the mortuary admission were considered in order to determine if a significant difference exists in the IAA median concentrations by utilizing a Wilcoxon two-tailed matched-pairs test.
Results
The analysis of this authentic casework cohort provided for the statistical examination for trans-IAA, cis-IAA, and TIAA in most test parameters. Unfortunately, the low prevalence of the RIAA and HIAA (n < 5) did not allow these IAAs to be considered. However, these three common IAA groups were largely similar in results between each of the test parameters.
Table 1 shows the trans-IAA, cis-IAA, and TIAA median concentrations for ADM and AUT bloods. There was a significant difference between the specimens for trans- and cis-IAA which had AUT/ADM ratios of 1.6 and 1.7, respectively. The median PMI (time of specimen collection–time of death) for the natural IAA (trans- and cis-IAA) were similar for the ADM and AUT specimens at 0.2–0.3 and 4.7–4.9 days, respectively. However, TIAA showed no difference in median concentrations and had a median AUT collection time approximately a day earlier than the natural IAA group.
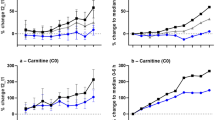
The influence of the pre-autopsy interval was also examined by comparing it against the change in IAA concentrations in the same case. This was assessed in 18, 22, and 9 cases for trans-IAA, cis-IAA, and TIAA, respectively (Fig. 1). The pre-autopsy interval ranged up to approximately 8.5 days with each case plotted in sequence from lowest to highest pre-autopsy interval. The largest ΔConc was approximately 1,000 %, while there were also some cases where concentrations were considerably lower than at mortuary admission. Although there appears to be a slight relationship for AUT concentrations to rise with prolonged collection times, there were also cases where all IAA concentrations decreased, particularly for cases collected between the 3-5 days pre-autopsy interval region.
There were insufficient non-femoral (heart, cavity, and subclavian) whole blood specimens that were positive for IAA groups to suitably compare to matched femoral blood concentrations. However, a single case study describes the difference in natural IAA concentrations from multiple bloods taken at mortuary admission and at autopsy, where three autopsy bloods were collected from different sites (Table 2). Redistribution was observed between bloods collected at the same time at autopsy with 0.010, 0.010, and 0.026 mg/L trans-IAA concentrations in the femoral, subclavian, and cavity bloods, respectively. Similar redistribution of analytes was observed with the cis-IAA area ratios. This analysis demonstrates the potential fluctuations in IAA redistribution between specimens collected from femoral and non-femoral blood collection sites. Following a pre-autopsy interval length of approximately 8.5 days, there was also a slight increase in natural IAA in the autopsy femoral blood, when compared to the mortuary admission.
An assessment of the trans-IAA, cis-IAA, and TIAA median concentrations, S/B ratios and p values of cases with both serum and blood specimens are given in Table 3. A significant and marked difference was demonstrated between the matched serum and whole bloods for the three IAA concentrations and resulted in an average S/B ratio of consistently around 3 for the analyzed IAA groups. In addition, linear regression analysis (serum vs. blood concentrations) was performed on the plotted S/B ratios (data not shown) that demonstrated a uniform trend between matched pairs. This demonstrated that the increase in concentrations did not show any changes in ratios over the concentration range for the IAA groups under investigation. Moreover, there was no evidence of an inacceptable number of outliers for any IAA group.
Discussion
This current study examines key forensic toxicology parameters that provide a greater understanding when interpreting postmortem IAA results to confirm beer consumption. In 2014, 78 % of Australians believe the country has an alcohol problem with over 36 % of drinkers claiming they “drink to get drunk” [10–12]. A previous study has demonstrated the high prevalence of beer consumption in forensic casework with over half of all alcohol positive cases confirming beer intake prior to death [9]. This research provides additional information when interpreting such results.
The PMR processes during the postmortem interval can lead to variations in drug concentrations which can affect the way in which case results are interpreted [13–18]. Comparison of mortuary admission and autopsy blood IAA concentrations can assist in determining the PMR phenomena [19]. Generally, this study showed that the natural IAA groups were subject to significant changes in concentration when femoral blood was collected on admission to the mortuary and compared to blood taken at autopsy, approximately 4 days later. Such fluctuations in blood drug concentrations have been shown when bodies are stored for long periods of time, hence the timely collection of specimens is preferable [20, 21]. Furthermore, it has been shown that PMR may occur in the early hours following death and therefore the extent to which PMR may have already taken place prior to the mortuary admission specimen is unknown [19]. Nonetheless, specimens collected on admission to the mortuary that are closer to the time of death may help to minimize these changes [22, 23]. The comparison of the pre-autopsy interval and change in IAA concentration for each case reflected this. It appeared that there were smaller fluctuations closer to the time of death. However this comparison of individual cases also demonstrated the non-uniform trend for IAA concentrations to increase, and also decrease, throughout the PMI.
Although the bodies were refrigerated immediately upon admission to the mortuary, generally aiding compound stability; studies investigating the stability of drugs in a decomposing body simulating the influence of PMI have shown that many drugs can quickly degrade following death [21, 24]. Long-term stability studies demonstrated that IAA and reduced IAA at low concentrations in blood underwent minimal degradation under refrigerated storage temperatures [6]. While this stability study provides information on the changes which can occur to IAA stability in a controlled environment; they cannot mimic the considerable influence that bacteria, fungi, body trauma, and other factors, are known to influence analyte concentrations following death [24–27]. Even with the possibility of losses, the natural IAA concentrations were shown to generally increase during body storage and exhibit PMR with AUT/ADM ratios 1.6–1.7.
Fat tissue and skeletal muscle are possible body compartments which allows for substance accumulation and therefore for the redistribution of IAA into the blood after death [15, 16, 28]. As this is most significant in central blood, the effects of PMR can possibly be minimalized with the collection of peripheral (e.g., femoral) blood [17, 29, 30]. This exaggerated increase in PMR from centrally located blood was demonstrated in one case where heart blood natural IAA concentrations were significantly higher than the peripheral specimens. These matched blood specimens demonstrated the time and site collection dependence of PMR.
Although there is no technique to measure the degree of redistribution, it is largely accepted that PMR is magnified for substances that collect in high concentrations in body compartments, which are commonly lipophilic, have an appropriate pKA, or have high volumes of distribution (Vd), commonly greater than 3 L/kg [17, 19, 30]. A quantitative structure–activity relationship (QSAR) model predicted a relatively small Vd range of 0.56–0.58 L/kg for the IAA groups [31]. However the distribution-coefficient (logD) for IAA groups was estimated to range from approximately 2.5–4 at pH 7.4 using a QSAR model [32] (with equal weighting [33, 34]). These logD properties may demonstrate the ability for IAA to collect in body compartments. Furthermore, the IAA groups have been shown to diffuse across lipophilic bilayers dependent on partition-coefficients (logP), pKa, and molecular size [35]. The acidic IAA groups possess a pKa of approximately 3–4 and have logP properties ranging from about 2.5–4.5 demonstrating good lipophilicity (i.e. logP) capable of transport across cell membranes [36, 37].
Such properties of the IAA groups demonstrate the lipophilicity of these compounds, sufficient to explain the elevation of the natural IAA concentrations in the heart blood of the case study following a prolonged PMI. Further investigation that provides in vivo data may further assist in the interpretation of IAA PMR in casework.
This paper also compared the serum-to-blood distribution (S/B ratio) of trans-IAA, cis-IAA, and TIAA in postmortem specimens. Whole blood and serum (or plasma) are commonly obtained and analyzed in forensic investigations [38]. It is well known that drugs are unevenly distributed between the fluid and cellular phases of blood [39]. Although whole blood has recently been shown suitable for the analysis of postmortem and pharmacokinetic controlled studies [7–9], detection off IAA in serum was also found to be potentially more useful with higher concentrations of IAA in serum compared to blood [9]. Acidic and neutral compounds will primarily bind to albumin that if saturated, may bind to lipoprotein [39]. Serum concentrations for IAA are also dependent on the hematocrit value [40]. As it will not be possible to measure hematocrit in some of the circumstances in which whole-blood samples have been analyzed, the comparison between serum and whole blood presented here can be used to compare results obtained in different blood specimens.
A consistent S/B ratio of approximately 3 was shown without any obvious outliers throughout the concentration range observed. Serum (and plasma) to blood ratios are commonly measured in antemortem specimens where the integrity of the erythrocyte cell membranes are relatively maintained. However the hemolysis of the erythrocytes in postmortem whole blood and therefore the liberation of intracellular water can commonly occur [40]. This may explain the considerably high IAA and reduced IAA S/B values. Nonetheless, such a difference in serum and blood concentrations in postmortem casework demonstrates that specimen matched calibration and quality control matrices should be used when analyzing natural and reduced IAA.
In summary, this study showed that the IAA and reduced IAA groups detected in postmortem blood and serum are subject to postmortem phenomena such as redistribution. These studies confirm that although postmortem concentrations cannot be easily compared to clinical concentrations, the presented findings do provide a greater understanding to assist interpretation of forensic casework where the confirmation of beer is needed.
Key Points
-
1.
IAA are subject to considerable PMR
-
2.
There is an association between the postmortem interval and the extent of PMR
-
3.
There is evidence that different sites of blood collection can influence IAA concentrations
-
4.
The serum to whole blood ratio of IAA concentration was approximately 3:1 in postmortem blood specimens
References
Global status report on alcohol and health. Switzerland: World Health Organization; 2011.
Pilgrim JL, Gerostamoulos D, Drummer OH. “King hit” fatalities in Australia, 2000–2012: the role of alcohol and other drugs. Drug Alcohol Depend. 2014;135:119–32.
Australian Bureau of Statistics. 4307.0.55.001—apparent consumption of alcohol, Australia, 2012–13. 2014. www.abs.gov.au/ausstats/abs@.nsf/Lookup/4307.0.55.001main+features12012-13. Accessed May 2014.
Nelson M. The Barbarian’s beverage: a history of beer in ancient Europe. New York: Taylor & Francis e-Library; 2005.
Rodda LN, Gerostamoulos D, Drummer OH. The rapid identification and quantification of Iso-α-acids and reduced Iso-α-acids in blood using UHPLC-MS/MS: validation of a novel marker for beer consumption. Anal Bioanal Chem. 2013;405(30):9755–67.
Rodda LN, Gerostamoulos D, Drummer OH. The stability of Iso-α-acids and reduced Iso-α-acids in stored blood specimens. Forensic Sci Int. 2014;239:44–9.
Rodda LN, Gerostamoulos D, Drummer OH. Pharmacokinetics of Iso-α-acids in volunteers following the consumption of beer. J Anal Toxicol. 2014;38:354–9.
Rodda LN, Gerostamoulos D, Drummer OH. Pharmacokinetics of reduced Iso-α-acids in volunteers following clear bottled beer consumption (accepted). Forensic Sci Int. 2014.
Rodda LN, Gerostamoulos D, Drummer OH. Detection of Iso-α-Acids to confirm beer consumption in postmortem specimens (under Review). Drug Test Anal. 2014.
Annual Alcohol Poll: Attitudes and behaviours. Australian Capital Territory, Australia: Foundation for Alcohol Research and Education (FARE); 2014.
Byrnes JM, Doran CM, Shakeshaft AP. Cost per incident of alcohol-related crime in New South Wales. Drug Alcohol Rev. 2012;31(7):854–60.
Collins DJ, Lapsley HM. The costs of tobacco, alcohol and illicit drug abuse to Australian society in 2004–05. Canberra: Commonwealth of Australia; 2008.
Drummer O, Forrest AR, Goldberger B, Karch SB. Forensic science in the dock. BMJ. 2004;329(7467):636–7.
Rodda KE, Drummer OH. The redistribution of selected psychiatric drugs in post-mortem cases. Forensic Sci Int. 2006;164(2–3):235–9.
McIntyre IM. Identification of a postmortem redistribution factor (F) for forensic toxicology. J Anal Sci Tech. 2014;5(24):1–3.
Pounder DJ, Jones GR. Post-mortem drug redistribution—a toxicological nightmare. Forensic Sci Int. 1990;45(3):253–63.
Prouty RW, Anderson WH. The forensic science implications of site and temporal influences on postmortem blood-drug concentrations. J Forensic Sci. 1990;35(2):243–70.
Robertson MD, Drummer OH. Postmortem distribution and redistribution of nitrobenzodiazepines in man. J Forensic Sci. 1998;43(1):9–13.
Gerostamoulos D, Beyer J, Staikos V, Tayler P, Woodford N, Drummer OH. The effect of the postmortem interval on the redistribution of drugs: a comparison of mortuary admission and autopsy blood specimens. Forensic Sci Med Pathol. 2012;8(4):373–9.
Skopp G. Preanalytic aspects in postmortem toxicology. Forensic Sci Int. 2004;142(2–3):75–100.
Saar E, Beyer J, Gerostamoulos D, Drummer OH. The time-dependant post-mortem redistribution of antipsychotic drugs. Forensic Sci Int. 2012;222(1–3):223–7.
Flanagan RJ, Connally G. Interpretation of analytical toxicology results in life and at postmortem. Toxicol Rev. 2005;24(1):51–62.
Drummer OH. Post-mortem toxicology. Forensic Sci Int. 2007;165(2–3):199–203.
Butzbach DM. The influence of putrefaction and sample storage on post-mortem toxicology results. Forensic Sci Med Pathol. 2010;6(1):35–45.
Carroll FT, Marraccini JV, Lewis S, Wright W. Morphine-3-D glucuronide stability in postmortem specimens exposed to bacterial enzymatic hydrolysis. Am J Forensic Med Pathol. 2000;21(4):323–9.
Martinez-Ramirez JA, Walther G, Peters FT. Studies on drug metabolism by fungi colonizing decomposing human cadavers. Part II: biotransformation of five model drugs by fungi isolated from post-mortem material. Drug Test Anal. 2014; doi:10.1002/dta.1669.
Robertson MD, Drummer OH. Postmortem drug metabolism by bacteria. J Forensic Sci. 1995;40(3):382–6.
Kennedy MC. Post-mortem drug concentrations. Intern Med J. 2010;40(3):183–7.
Hilberg T, Rogde S, Morland J. Postmortem drug redistribution-human cases related to results in experimental animals. J Forensic Sci. 1999;44(1):3–9.
Yarema MC, Becker CE. Key concepts in postmortem drug redistribution. Clin Toxicol. 2005;43(4):235–41.
Demir-Kavuk O, Bentzien J, Muegge I, Knapp EW. DemQSAR: predicting human volume of distribution and clearance of drugs. J Comput Aided Mol Des. 2011;25(12):1121–33.
ChemAxon MarvinSketch. 6.3.1,201. 2014. www.chemaxon.com.
Viswanadhan VN, Ghose AK, Revankar GR, Robins RK. Atomic physicochemical parameters for three dimensional structure directed quantitative structure-activity relationships. 4. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally occurring nucleoside antibiotics. J Chem Inf Comput Sci. 1989;29(3):163–72.
Klopman G, Li J-Y, Wang S, Dimayuga M. Computer automated log P calculations based on an extended group contribution approach. J Chem Inf Comput Sci. 1994;34(4):752–81.
Cattoor KO, Bracke M, Deforce D, De Keukeleire D, Heyerick A. Transport of hop bitter acids across intestinal Caco-2 cell monolayers. J Agric Food Chem. 2010;58(7):4132–40.
Blanco CA, Rojas A, Nimubona D. Effects of acidity and molecular size on bacteriostatic properties of beer hop derivates. Trends Food Sci Technol. 2007;18(3):144–9.
Kappler S, Schönberger C, Krottenthaler M, Becker T. Isohumulones—a review. Brew Sci. 2010;63:105–11.
Skopp G, Potsch L, Ganssmann B, Aderjan R, Mattern R. A preliminary study on the distribution of morphine and its glucuronides in the subcompartments of blood. J Anal Toxicol. 1998;22(4):261–4.
Skopp G, Potsch L, Mauden M, Richter B. Partition coefficient, blood to plasma ratio, protein binding and short-term stability of 11-nor-delta(9)-carboxy tetrahydrocannabinol glucuronide. Forensic Sci Int. 2002;126(1):17–23.
Drummer OH. Pharmacokinetics and metabolism. In: Moffat AC, Osselton MD, Widdop B, Watts J, editors. Clarke’s analysis of drugs and poisons. 4th ed. London: Pharmaceutical Press; 2010.
Acknowledgments
The authors would like to acknowledge Mr Gavin Reichel in obtaining the mortality data and the assistance of forensic pathologists, mortuary technicians, and toxicologists at the Victorian Institute of Forensic Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodda, L.N., Gerostamoulos, D. & Drummer, O.H. The postmortem redistribution of iso-α-acids in postmortem specimens. Forensic Sci Med Pathol 10, 550–556 (2014). https://doi.org/10.1007/s12024-014-9609-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12024-014-9609-9