Abstract
Purpose
Thyroidectomy is the treatment of choice for malignant thyroid diseases as well as for benign conditions who cannot be treated medically. The most common complication following thyroidectomy is hypocalcaemia and hypoparathyroidism that usually results from accidental damage or removal of one or more parathyroid glands. Parathyroid gland autotransplantation has been one of the most common intraoperative strategies applied to tackle this problem. The aim of this study is to assess whether parathyroid auto trasnplantation is associated with a decrease in postoperative hypoparathyroidism following thyroidectomy.
Methods
We conducted a thorough systematic review and meta-analysis of relevant studies published up to February 2024 in MEDLINE, Scopus, Embase and Cochrane Library databases. We compared the incidence of postoperative hypoparathyroidism between the group of patients who underwent autotransplantation and the patients were the parathyroid glands were preserved in situ. A trial sequential analysis was performed subsequently to confirm the findings.
Results
Eighteen studies fulfilled all the inclusion criteria and were ultimately included in our study. The total number of patients was 8,182 with 4,029 receiving parathyroid gland autotransplantation. Autotransplantation was associated with a higher incidence of immediate (within 24 h) and transient hypoparathyroidism (RR 1.58, 1.45–1.73, CI 95%, p < 0.00 and RR 1.60, 1.47–1.76, CI 95%, p < 0.001, respectively). However, it did not affect the rate of permanent postoperative hypoparathyroidism (RR 0.85, 0.51–1.41, CI 95%, p = 0.54). The subsequent trial sequential analysis confirmed these findings.
Conclusion
Parathyroid autotransplantation does not lead to a decrease in the rate of permanent post-thyroidectomy hypoparathyroidism. The most important factor to decrease its incidence remains the accurate identification and preservation of the parathyroid glands intraoperatively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid nodules are commonly encountered during everyday clinical practice [1]. Their overall prevalence in the general population ranges from 34% to 66% depending on ultrasound or histology findings [2]. Risk factors for the development of thyroid nodules include female sex, advanced age, positive family history, neck radiation exposure and higher body mass index (BMI) [1, 2]. The clinical question that is always raised in the presence of thyroid nodules is exclusion of malignancy, which has a steady increase in its incidence, mainly because of the increased detection rate of differentiated thyroid cancer [1, 3, 4]. While active surveillance still remains as a choice for the management of unifocal papillary microcarcinoma, surgery is still considered the primary tumour management for thyroid cancer [3, 4]. The extent of surgery is still a matter of debate, with hemithyroidectomy recommended for small (≤4 cm), low-risk thyroid cancers as it has similar outcomes to total thyroidectomy in terms of recurrence rate and cancer-specific survival, while it also carries a smaller overall complication rate and the advantage of remaining euthyroid postoperatively [5]. Nonetheless, this approach is still controversial with many recent studies comparing its outcomes to total thyroidectomy, which still remains the procedure of choice for larger or multifocal tumours with poor prognostic factors [6,7,8,9,10]. Surgery is also a valid treatment for benign thyroid disease in cases of compression symptoms, hyperthyroidism and cosmetic problems, as it has shown benefits in treating Hashimoto’s thyroiditis, improving Grave’s ophthalmopathy, eradicating multinodular goitres and decreasing the likelihood for a future operation as a result of recurrence [11].
Nowadays, thyroidectomy can be performed via a variety of surgical approaches, included traditional thyroidectomy via a neck incision, transaxillary thyroidectomy, facelift thyroidectomy and transoral thyroidectomy [12]. As any other procedure and irrespectively of the approach utilised, thyroidectomy carries the risk of significant post-operative complications, such as haematoma, recurrent laryngeal nerve palsy and hypoparathyroidism [13]. Post-operative hypoparathyroidism is the most common complication following thyroidectomy and can either be transient or permanent, with a respective incidence of 29.05% and 4.08% [14]. The most common cause of post-operative hypoparathyroidism is accidental damage to the blood supply of the parathyroid glands or inadvertent excision during the operation [15]. Selective parathyroid gland autotrasnplantation is expected to provide biochemically functional grafts that should counteract the detrimental factors to parathyroid function [15]. Nonetheless, there are multiple other factors that affect the incidence of post-operative hypoparathyroidism and the success rate of parathyroid autotransplantation, such as the number and function of parathyroid glands left intact, as well as the technique and site of autotransplantation [15]. In general, parathyroid autotransplantation has been associated with an increase in the transient hypoparathyroidism rates, but is also effective against permanent hypoparathyroidism [15]. However, there is still conflicting evidence on the matter with some studies indicating no significant difference in the rates of permanent hypoparathyroidism [16], while other indicate that parathyroid autotransplantation is associated with a decrease in transient hypoparathyroidism rates [17].
The aim of this study is to evaluate the efficiency of parathyroid gland autotransplantation in preserving parathyroid gland function in patients who undergo thyroid surgery.
Materials and methods
This study is a systematic review, meta-analysis and trial sequential analysis that was conducted without a pre-existing registered protocol. The preparation of the manuscript followed strictly the PRISMA 2020 checklist. The authors conducted a thorough and systematic literature search until February 2024 in the following electronic databases: MEDLINE (via PubMed), Scopus, EMBASE and Cochrane Library. The search of the MEDLINE database was carried out using the following search string: (((autotransplant*[Title]/Abstract) OR (auto-transplant*[Title/Abstract]) OR (transplant*[Title/Abstract] OR (graft*[Title/Abstract]))) AND (parathyroid*[Title/Abstract]). Similar search strategies were utilised to perform the literature search in the rest of the databases searched. No restrictions were applied, while the reference lists of the generated articles were reviewed, as well. Also, an additional search to look for any potentially available grey literature was conducted on the websites of international endocrine associations and on the abstract books of endocrine surgery and endocrinology conferences.
At first, two independent researchers (G.K. and G.G.) performed a thorough literature search of the selected databases for relevant articles applying the following inclusion criteria: (1) studies performed on human patients, (2) patients were subjected to surgery for thyroid pathology with total or near total thyroidectomy as the initial operation, (3) parathyroid gland autotransplantation was the intervention investigated, (4) the incidence of post-operative hypoparathyroidism or hypocalcaemia was mentioned with enough available data to calculate the respective relative risk (RR) with a confidence interval (CI) 95%, (5) articles written in English and (6) articles published after 2004. Articles published before 2004 were considered outdated and superseded by more recent literature. The following exclusion criteria were also applied: (1) surgery for parathyroid pathology and (2) studies where full-text was not available online or following communication with the authors. In cases of disagreement between the two reviewers, the matter was resolved by a third experienced reviewer (T.P.) and ultimately, the decision on these studies was made either by reaching a consensus or by utilising the majority opinion.
The data extraction from the selected articles was carried out by two independent members of the research team (L.S. and G.G.) using a standardized data-collection form, and their findings were corroborated by a third researcher (G.K.). The data that were collected from each selected study were the following: name of the first author, year of publication, type of study, study period, number of patients including number of controls and cases, patients’ demographics (age and sex), surgical intervention, number and site of autotransplanted parathyroid glands, assessment criteria of post-operative hypoparathyroidism and/or hypocalcaemia, incidence of post-operative hypoparathyroidism/hypocalcaemia and corresponding RR with 95% CI and duration of follow-up. The quality of the included studies was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS).
In this meta-analysis and trial sequential analysis, the statistical analyses were carried out by utilizing Reviewer Manager 5.4.1 software [Review Manager (RevMan) (computer program) version 5.4.1, Copenhagen: The Nordic Cochrane Centre, Denmark, the Cochrane Collaboration, 2020] and STATA version 16.1. In this study the dichotomous data were compared by calculating the combined risk ratio with a 95% CI. Continuous data are presented as mean ± standard deviation or median (min, max). The level of statistical significance was set at a p-value of less than 0.05. When large heterogeneity was encountered during the statistical analysis (I2 ≥ 50%), a random effects model was applied and further sensitivity analysis at I2 = 50% and I2 = 25 was carried out to assess the influence of this large heterogeneity on the outcome of the statistical analysis. Otherwise, in cases of low heterogeneity a fixed effects model was applied for the statistical analysis. The potential publication bias was evaluated by designing the respective Begg’s funnel plot. Trial sequential analysis (TSA) was also performed to assess if the sample size in each analysis was enough to generate valid results or if further studies were required. The software used to conduct TSA was Trial Sequential Analysis (TSA) (computer program) version 0.9.5.10 beta, Copenhagen Trial Unit, Centre for Clinical Intervention Research, Capital Region, Copenhagen University Hospital—Rigshospitalet, 2021.
The potential risk of bias across the studies that were included in this study was evaluated using the ‘Cochrane Collaboration tool for assessing the risk of bias’ as integrated into the Review Manager 5.4.1 software, and the outcomes are demonstrated in a risk of bias graph and a risk of bias summary.
Results
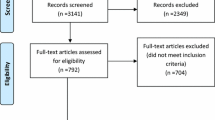
The initial search of the selected online databases resulted in a total number of 1416 articles, while 12 more records were identified through additional sources. Following exclusion of duplicates, 849 articles were screened based on their title and abstract. This process resulted in 53 articles eligible for full-text analysis, with 18 articles accepted for qualitative and quantitative analysis [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. This selection process is portrayed in Fig. 1.
The articles that were included in the final analysis were published between 2005 and 2024 with data collected from 1993 to 2021. Only three of these studies were prospective while the rest were retrospective. In total, 8182 patients were enlisted in these studies, with 4029 receiving autotransplantation (cases) of one or more parathyroid glands and 4149 parathyroid gland preservation in situ (controls). Eight of the included studies mention the number of the autotransplanted glands in each case. Eleven studies used a cut-off of six months to diagnose permanent hypoparathyroidism based on low parathyroid hormone (PTH) levels, five studies used a cut-off of twelve months for the diagnosis, one study did not mention any cut-off and another one based the diagnosis on the presence of symptoms of hypocalcaemia. All studies mentioned the rate of permanent hypoparathyroidism, while the incidence of transient hypoparathyroidism was not mentioned in two of them. Moreover, six studies mentioned the rate of immediate (within 24 h) post-operative hypoparathyroidism. The basic characteristics of the included studies are shown in Tables 1 and 2. All the included studies were of relatively high quality as they scored between six and eight on NOS.
Primary outcomes
The meta-analysis of data on immediate (within 24 h) hypoparathyroidism provided by six of the included studies revealed that parathyroid autotransplantation leads to a higher incidence of hypoparathyroidism (RR 1.58, 1.45–1.73, CI 95%, p < 0.001). A fixed effect model was used for this analysis, as the studies were homogeneous (I2 = 36%). This outcome is shown in Fig. 2. Parathyroid autotransplantation was also associated with a higher risk of transient hypoparathyroidism (RR 1.60, 1.47–1.76, CI 95%, p < 0.001). This analysis was also performed with a fixed effects model as the studies were homogeneous (I2 = 17%) and the outcome is shown in Fig. 3. Finally, the meta-analysis on the rate of permanent hypoparathyroidism revealed that there is no statistically significant difference between autotransplantation and preservation of parathyroid glands (RR 0.85, 0.51–1.41, CI 95%, p = 0.54). This outcome is shown in Fig. 4. Due to the large heterogeneity of the studies (I2 = 68%) a random effects model was again applied and further sensitivity analysis was carried out at I2 = 50% and I2 = 25% levels. The p-value generated after this sensitivity analysis was 0.62 and 0.87 respectively, indicating that the heterogeneity of the studies did not affect the outcome and the difference in the RR was indeed not statistically significant.
The potential publication bias of the included studies in each of the above analysis was assessed by designing the respective Funnel plots as provided in the supplementary material. According to their visual interpretation, no publication bias was introduced in the meta-analysis performed in each case. Also, a TSA was performed for each one of the above analyses to assess if the number of the included studies was sufficient to draw valid conclusions or whether further studies are required. These outcomes are shown in Figs. 5–7 and confirm the findings of the primary meta-analysis performed for immediate, transient and permanent hypoparathyroidism as well as that the number of patients and studies included in each case was sufficient to draw valid conclusions.
Subgroup analysis
Due to the large heterogeneity of the include studies in the meta-analysis of the data on permanent hypoparathyroidism, which was our main primary outcome, a further subgroup analysis was carried out. The first factor to be assessed was the location of the autotransplanted glands (sternocleidomastoid or brachioradialis muscle).). The second factor to be assessed was the timing of the diagnosis of permanent hypoparathyroidism (6 or 12 months postoperatively) and the last one was the number of the autotransplanted glands (one or more). This subgroup analysis showed that autotransplantation of the parathyroid glands in the sternocleidomastoid muscle is not associated with a decreased risk of permanent hypoparathyroidism (RR 1.18, 0.75–1.87, CI 95%, p = 0.48), while autotransplantation to the brachioradialis muscle is associated with a decrease in the incidence of permanent hypoparathyroidism (RR 0.16, 0.07–0.37, CI 95%, p < 0.001). Also, when permanent hypoparathyroidism is diagnosed at 6 months post-operatively, autotransplantation is not associated with a decreased incidence (RR 0.53, 0.24–1.17, CI 95%, p = 0.12) and the same applies when the diagnosis is made 12 months postoperatively (RR 1.20, 0.56–2.58, CI 95%, p = 0.64). Finally, a subgroup analysis comparing the outcomes of autotransplanting a single parathyroid gland to autotransplanting two or more showed that the number of autotransplanted parathyroid glands is not associated with a difference in the incidence of permanent postoperative hypoparathyroidism (RR 2.36, 0.95–5.83, CI 95%, p = 0.06).
The potential bias of the included studies is demonstrated in the risk of bias graph and summary figures (Figs. 8 and 9). According to this, the included studies had a high risk of selection and performance bias as they were all non-randomised studies but there was low risk of detection, attrition, reporting or other form of bias.
Discussion
In this study we conducted a systematic review of the literature with meta-analysis and trial sequential analysis of the included studies to determine if parathyroid gland autotransplantation during thyroid surgery compared to parathyroid gland preservation is associated with a decreased risk of hypoparathyroidism postoperatively. According to the findings of our meta-analysis, when parathyroid autotransplantation is performed, the risk of immediate (within 24 h) and transient hypoparathyroidism is increased, but there is no difference in the incidence of permanent hypoparathyroidism. The trial sequential analysis that we performed subsequently corroborated these findings and also confirmed that the number of studies included was sufficient to draw valid conclusions. Moreover, the subgroup analysis that we performed revealed that the difference in the diagnostic criteria of permanent hypoparathyroidism (diagnosis at 6 or 12 months post-operatively) did not affect the outcome of our meta-analysis. The risk of permanent hypoparathyroidism is also not affected by the number of parathyroid glands that are autotransplanted. Nonetheless, when a subgroup analysis was carried out including only the studies where the parathyroid gland was autotransplanted in the brachioradialis muscle, the risk of permanent hypoparathyroidism decreased significantly. This can potentially be explained by the fact that in all these studies thyroidectomy was carried out endoscopically, which might have affected all the parathyroid glands due to extensive dissection. As a result, in these cases the autotransplantation might have protected the only functional parathyroid gland left.
The outcomes of our study are similar to the ones published by Wang et al. in [36]. In this meta-analysis 25 studies were included with a total number of 10,531 patients. Although, the authors use different definitions for immediate and transient hypoparathyroidism (postoperative and protracted, respectively), the respective RRs were 1.75 (95% CI: 1.51–2.02, p < 0.001) and 1.72 (95% CI: 1.45–2.05, p < 0.001). Also, the authors of this study conclude that parathyroid autotransplantation does not affect the risk of permanent hypoparathyroidism (RR = 0.95, 95% CI: 0.62–1.45, p = 0.801). These findings are also in agreement with a study by Edafe et al. [37] on the predicting factors of post-thyroidectomy hypocalcaemia. In this study parathyroid gland autotransplantation was again associated with a higher risk of transient hypocalcaemia but no effect on the risk of permanent hypocalcaemia was identified. Another systematic review by Antakia et al. [38] also concluded that the majority of the included studies reported that autotransplantation does not have any significant effect on permanent hypocalcaemia. Moreover, in the systematic review by Iorio et al [39] the majority of the included studies suggest that parathyroid gland autotrasnplantation should only be carried out in selected cases such as devascularisation or intrathyroid position of the gland, while only three of the included studies reported lower values of permanent hypoparathyroidism in reimplanted patients. Another meta-analysis on post thyroidectomy risk factors of transient and permanent hypocalcaemia identified parathyroid gland autotransplantation as a risk factor for transient hypocalcaemia (Odds ratio 1.62, CI 95% 1.35–1.95, p < 0.001) but no significant effect was reported on the incidence of permanent hypocalcaemia (Odds ratio 1.25, CI 95% 0.72–2.18, p = 0.42) [40]. The systematic review and meta-analysis by Ning et al. also comes to the same conclusions [41].
Post-operative hypocalcaemia is the most common complication of thyroid surgery and can be attributed to multiple factors. A systematic review and meta-analysis by Chen et al. identified twelve different factors that increase the risk for that: total thyroidectomy, thyroid malignancy, preoperative hypoparathyroidism, hypomagnesaemia, female sex, vitamin D deficiency, substernal multinodular goitre, thyroiditis, central compartment neck dissection, modified radical neck dissection, central neck dissection and parathyroidectomy [42]. Incidental parathyroidectomy occurs in 6–28% of thyroidectomies [43]. The risk factors for incidental parathyroidectomy include thyroid malignancy, total thyroidectomy, central neck dissection and reoperation [43]. So far, various techniques and intraoperative strategies have been implemented to identify and protect the parathyroid glands during thyroidectomies. According to a systematic review and network meta-analysis by Lu et al. [44], there are three main imaging modalities for intraoperative identification of the parathyroid glands: autofluorescence, indocyanine green autofluorescence and carbon nanoparticles. Based on the outcomes of this study, carbon nanoparticles have the greater advantage in reducing postoperative hypoparathyroidism, while autofluorescence has the highest rate of parathyroid gland identification and leads to the greatest decrease in inadvertent resection. On the contrary, indocyanine green fluorescence leads to a greater rate of autotransplantation as it assesses the viability of the glands. The role of carbon nanoparticles in the protection of parathyroid glands and decrease of postoperative hypoparathyroidism has been confirmed by other studies, as well [45,46,47]. Nonetheless, in a meta-analysis by Wang et al. [48] autofluorescence was associated only with a decrease in the rate of postoperative day 1 hypocalcaemia, while it had no effect on the rate of hypocalcaemia at six months after surgery.
The management of transient and permanent post-thyroidectomy hypoparathyroidism is still a matter of controversy [49]. Nonetheless, most patients are usually treated with regular calcium replacement therapy and vitamin D analogue administrations [50]. Chronic hypoparathyroidism has been associated with a decreased quality of life, independently of other comorbidities [51]. Moreover, post-thyroidectomy hypoparathyroidism is associated with a significant increase in health expenditures, leading to an additional economic burden to national health systems [52]. Novel techniques are currently emerging, such as parathyroid allotransplantation [50] and parathyroid cryopreservation [53] that could potentially provide new ways for permanent resolution of the issue. However, despite the lack of universal guidelines operative strategies, it is still evident that the most effective way of dealing with postoperative hypoparathyroidism is the accurate intraoperative identification and protection of the parathyroid glands.
This study suffers from certain limitations. The studies included in the analysis were not randomized clinical trials, and there was no blinding of the researchers involved. As a result, the risk for selection bias is high, as in such cases patients tend to be allocated to the treatment that seems more beneficial to them. However, across all of the included studies the risk of detection and reporting bias was low, as the outcomes were assessed objectively and there was no obvious missing data. Also, the trial sequential analysis reveal that the total number of patients and studies included was sufficient to draw robust conclusions.
Conclusion
In this study we performed a meta-analysis on the effect of parathyroid gland autotransplantation on postoperative hypoparathyroidism. Autotransplantation was associated with a higher risk of immediate and transient hypoparathyroidism, while it did not affect the rate of permanent hypoparathyroidism. This can potentially be attribute to autotransplantation being performed in cases where a parathyroid gland’s viability is compromised intraoperatively and autotransplantation is the only means of salvaging it. However, since the rate of permanent hypoparathyroidism is not affected, the main operative strategy to avoid it is the identification and preservation of all parathyroid glands when possible.
References
B.R. Haugen et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26(no. 1), 1–133 (2016). https://doi.org/10.1089/thy.2015.0020
N. Uppal, R. Collins, B. James, Thyroid nodules: Global, economic, and personal burdens. Front. Endocrinol. 14, 1113977 (2023). https://doi.org/10.3389/fendo.2023.1113977
S. Filetti et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 30(no. 12), 1856–1883 (2019). https://doi.org/10.1093/annonc/mdz400
K.A. Araque, S. Gubbi, J. Klubo-Gwiezdzinska, Updates on the Management of Thyroid Cancer. Horm. Metab. Res. Horm. Stoffwechselforschung Horm. Metab 52(no. 8), 562–577 (2020). https://doi.org/10.1055/a-1089-7870
F. Nabhan, P.H. Dedhia, M.D. Ringel, Thyroid cancer, recent advances in diagnosis and therapy. Int. J. Cancer 149(no. 5), 984–992 (2021). https://doi.org/10.1002/ijc.33690
L. Han, W. Li, Y. Li, W. Wen, Y. Yao, Y. Wang, Total thyroidectomy is superior for initial treatment of thyroid cancer. Asia Pac. J. Clin. Oncol. 17(no. 5), e170 (2021). https://doi.org/10.1111/ajco.13379
G. Li, L. Wu, Total or Near-total Thyroidectomy in treatment of Thyroid Cancer. Pak. J. Med. Sci. 38(no. 6), 1662–1667 (2022). https://doi.org/10.12669/pjms.38.6.5765
S. Xu et al. Comparison of Lobectomy vs Total Thyroidectomy for Intermediate-Risk Papillary Thyroid Carcinoma With Lymph Node Metastasis. JAMA Surg 158(no. 1), 73–79 (2023). https://doi.org/10.1001/jamasurg.2022.5781
M.G. Rugiu, C. Miani, L.G. Locatello, Total or partial thyroidectomy for low-risk differentiated thyroid cancer: that is the question! Acta Otorhinolaryngol. Ital. 42(no. 5), 487 (2022). https://doi.org/10.14639/0392-100X-N2247
Q. Yuan et al. Total thyroidectomy versus hemithyroidectomy with intraoperative radiofrequency ablation for unilateral thyroid cancer with contralateral nodules: A propensity score matching study. J. Otolaryngol. - Head Neck Surg. 51, 2022, https://doi.org/10.1186/s40463-022-00578-6
R.B. Gangappa, M.B. Kenchannavar, P.B. Chowdary, A.M. Patanki, M. Ishwar, Total Thyroidectomy for Benign Thyroid Diseases: What is the Price to be Paid? J. Clin. Diagn. Res. JCDR 10(no. 6), PC04–PC07 (2016). https://doi.org/10.7860/JCDR/2016/18733.7991
G.D. Koimtzis, T.S. Papavramidis, Proper handling of the pyramidal lobe in minimal access thyroid procedures. Endocrine 65(no. 3), 520–523 (2019). https://doi.org/10.1007/s12020-019-01961-6
B. Ludwig et al. Modern Surgical Techniques of Thyroidectomy and Advances in the Prevention and Treatment of Perioperative Complications. Cancers 15(no. 11), 2931 (2023). https://doi.org/10.3390/cancers15112931
G.D. Koimtzis, L. Stefanopoulos, K. Giannoulis, T.S. Papavramidis, What are the real rates of temporary hypoparathyroidism following thyroidectomy? It is a matter of definition: a systematic review. Endocrine 73(no. 1), 1–7 (2021). https://doi.org/10.1007/s12020-021-02663-8
G. Hicks, R. George, M. Sywak, Short and long-term impact of parathyroid autotransplantation on parathyroid function after total thyroidectomy. Gland Surg 6(Suppl 1), S75–S85 (2017). https://doi.org/10.21037/gs.2017.09.15
P. Hallgrimsson, E. Nordenström, M. Almquist, A.O.J. Bergenfelz, Risk factors for medically treated hypocalcemia after surgery for Graves’ disease: a Swedish multicenter study of 1,157 patients. World J. Surg. 36(no. 8), 1933–1942 (2012). https://doi.org/10.1007/s00268-012-1574-4
M. Testini et al. The impact of single parathyroid gland autotransplantation during thyroid surgery on postoperative hypoparathyroidism: a multicenter study. Transplant. Proc. 39(no. 1), 225–230 (2007). https://doi.org/10.1016/j.transproceed.2006.10.192
F. Famà et al. Parathyroid Autotransplantation During Thyroid Surgery: A Novel Technique Using a Cell Culture Nutrient Solution. World J. Surg. 41(no. 2), 457–463 (2017). https://doi.org/10.1007/s00268-016-3754-0
F.F. Palazzo, M.S. Sywak, S.B. Sidhu, B.H. Barraclough, L.W. Delbridge, Parathyroid Autotransplantation during Total Thyroidectomy—Does the Number of Glands Transplanted Affect Outcome? World J. Surg. 29(no. 5), 629–631 (2005). https://doi.org/10.1007/s00268-005-7729-9
N. Ahmed, M. Aurangzeb, M. Muslim, M. Zarin, Routine parathyroid autotransplantation during total thyroidectomy: A procedure with predictable outcome’. J. Pak. Med. Assoc. 63, 190–193 (2013)
S.H. Paek, Y.M. Lee, S.Y. Min, S.W. Kim, K.W. Chung, Y.K. Youn, Risk Factors of Hypoparathyroidism Following Total Thyroidectomy for Thyroid Cancer. World J. Surg. 37(no. 1), 94–101 (2013). https://doi.org/10.1007/s00268-012-1809-4
B.H.-H. Lang, D.T.Y. Chan, F.C.-L. Chow, Visualizing fewer parathyroid glands may be associated with lower hypoparathyroidism following total thyroidectomy. Langenbecks Arch. Surg. 401(no. 2), 231–238 (2016). https://doi.org/10.1007/s00423-016-1386-3
T. Kirdak, H.Z. Dundar, E. Uysal, G. Ocakoglu, N. Korun, Outcomes of Parathyroid Autotransplantation During Total Thyroidectomy: A Comparison with Age- and Sex-Matched Controls. J. Invest. Surg. 30(no. 3), 201–209 (2017). https://doi.org/10.1080/08941939.2016.1232768
M. Teshima et al. Postoperative hypoparathyroidism after total thyroidectomy for thyroid cancer. Auris. Nasus. Larynx 45(no. 6), 1233–1238 (2018). https://doi.org/10.1016/j.anl.2018.04.008
A. Sitges-Serra, S. Ruiz, M. Girvent, H. Manjón, J.P. Dueñas, J.J. Sancho, Outcome of protracted hypoparathyroidism after total thyroidectomy. Br. J. Surg. 97(no. 11), 1687–1695 (2010). https://doi.org/10.1002/bjs.7219
T. Wei et al. Autotransplantation of Inferior Parathyroid glands during central neck dissection for papillary thyroid carcinoma: A retrospective cohort study. Int. J. Surg. 12(no. 12), 1286–1290 (2014). https://doi.org/10.1016/j.ijsu.2014.11.001
L. Lorente-Poch, J.J. Sancho, S. Ruiz, A. Sitges-Serra, Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br. J. Surg. 102(no. 4), 359–367 (2015). https://doi.org/10.1002/bjs.9676
F. Tartaglia et al. Parathyroid autotransplantation during total thyroidectomy. Results of a retrospective study. Int. J. Surg. 28, S79–S83 (2016). https://doi.org/10.1016/j.ijsu.2015.05.059
E. Sonne-Holm, C. Holst Hahn, Prolonged Duration of Surgery Predicts Postoperative Hypoparathyroidism among Patients Undergoing Total Thyroidectomy in a Tertiary Referral Centre. Eur. Thyroid J. 6(no. 5), 255–262 (2017). https://doi.org/10.1159/000470840
A. Su, Y. Gong, T. Wei, R. Gong, Z. Li, J. Zhu, A new classification of parathyroid glands to evaluate in situ preservation or autotransplantation during thyroid surgery. Medicine (Baltimore) 97(no. 48), e13231 (2018). https://doi.org/10.1097/MD.0000000000013231
Z. Dong et al. Single inferior parathyroid autotransplantation during total thyroidectomy with bilateral central lymph node dissection for papillary thyroid carcinoma: a retrospective cohort study. World J. Surg. Oncol. 21, 102 (2023). https://doi.org/10.1186/s12957-023-02886-1
Z. Wang, Q. Zhang, J. Gao, T. Cao, Y. Zhang, K. Qu, Investigating the optimal parathyroid autotransplantation strategy in transareolar endoscopic thyroidectomy: A retrospective cohort study. Asian J. Surg. 47(no. 2), 886–892 (2024). https://doi.org/10.1016/j.asjsur.2023.10.036
B. Wang, C.-R. Zhu, X.-M. Yao, J. Wu, The Effect of Parathyroid Gland Autotransplantation on Hypoparathyroidism After Thyroid Surgery for Papillary Thyroid Carcinoma. Cancer Manag. Res. 13, 6641–6650 (2021). https://doi.org/10.2147/CMAR.S323742
X. Cheng, Y. Li, L. Chen, Efficacy of parathyroid autotransplantation in endoscopic total thyroidectomy with CLND. Front. Endocrinol. 14, 1193851 (2023). https://doi.org/10.3389/fendo.2023.1193851
Q. Zhang, K.-P. Qu, Z.-S. Wang, J.-W. Gao, Y.-P. Zhang, W.-J. Cao, Clinical application of parathyroid autotransplantation in endoscopic radical resection of thyroid carcinoma. Front. Oncol. 12, 942488 (2022). https://doi.org/10.3389/fonc.2022.942488
B. Wang, C.-R. Zhu, H. Liu, J. Wu, The effectiveness of parathyroid gland autotransplantation in preserving parathyroid function during thyroid surgery for thyroid neoplasms: A meta-analysis. PloS One 14(no. 8), e0221173 (2019). https://doi.org/10.1371/journal.pone.0221173
O. Edafe, R. Antakia, N. Laskar, L. Uttley, S.P. Balasubramanian, Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br. J. Surg. 101(no. 4), 307–320 (2014). https://doi.org/10.1002/bjs.9384
R. Antakia, O. Edafe, L. Uttley, S.P. Balasubramanian, Effectiveness of Preventative and Other Surgical Measures on Hypocalcemia Following Bilateral Thyroid Surgery: A Systematic Review and Meta-Analysis. Thyroid 25(no. 1), 95–106 (2015). https://doi.org/10.1089/thy.2014.0101
O. Iorio et al. Parathyroid Autotransplantation During thyroid Surgery. Where we are? A Systematic Review on Indications and Results. J. Invest. Surg. 32(no. 7), 594–601 (2019). https://doi.org/10.1080/08941939.2018.1441344
Y. Qin et al. A Meta-Analysis of Risk Factors for Transient and Permanent Hypocalcemia After Total Thyroidectomy. Front. Oncol. 10, 614089 (2021). https://doi.org/10.3389/fonc.2020.614089
K. Ning et al. Risk factors of transient and permanent hypoparathyroidism after thyroidectomy: a systematic review and meta-analysis. Int. J. Surg. Lond. Engl. Apr. 2024, https://doi.org/10.1097/JS9.0000000000001475
Z. Chen et al. Risk factors for postoperative hypocalcaemia after thyroidectomy: A systematic review and meta-analysis. J. Int. Med. Res. 49(no. 3), 0300060521996911 (2021). https://doi.org/10.1177/0300060521996911
B. Bai, Z. Chen, W. Chen, Risk factors and outcomes of incidental parathyroidectomy in thyroidectomy: A systematic review and meta-analysis. PLoS ONE 13(no. 11), e0207088 (2018). https://doi.org/10.1371/journal.pone.0207088
D. Lu et al. Intraoperative strategies in identification and functional protection of parathyroid glands for patients with thyroidectomy: a systematic review and network meta-analysis. Int. J. Surg. Lond. Engl. 110(no. 3), 1723 (2024). https://doi.org/10.1097/JS9.0000000000000991
S. Xu, Z. Li, M. Xu, H. Peng, The role of carbon nanoparticle in lymph node detection and parathyroid gland protection during thyroidectomy for non-anaplastic thyroid carcinoma- a meta-analysis. PloS One 15(no. 11), e0223627 (2020). https://doi.org/10.1371/journal.pone.0223627
L. Wang, D. Yang, J.-Y. Lv, D. Yu, S.-J. Xin, Application of carbon nanoparticles in lymph node dissection and parathyroid protection during thyroid cancer surgeries: a systematic review and meta-analysis. ’, OncoTargets Ther. 10, 1247–1260 (2017). https://doi.org/10.2147/OTT.S131012
G. Koimtzis et al. The Role of Carbon Nanoparticles in Lymph Node Dissection and Parathyroid Gland Preservation during Surgery for Thyroid Cancer: A Systematic Review and Meta-Analysis. Cancers 14(no. 16), 4016 (2022). https://doi.org/10.3390/cancers14164016
B. Wang, C.-R. Zhu, H. Liu, X.-M. Yao, J. Wu, The Ability of Near-Infrared Autofluorescence to Protect Parathyroid Gland Function During Thyroid Surgery: A Meta-Analysis. Front. Endocrinol. 12, 714691 (2021). https://doi.org/10.3389/fendo.2021.714691
O. Edafe, C.E. Mech, S.P. Balasubramanian, Calcium, vitamin D or recombinant parathyroid hormone for managing post‐thyroidectomy hypoparathyroidism. Cochrane Database Syst. Rev. 2019(no. 5), CD012845 (2019). https://doi.org/10.1002/14651858.CD012845.pub2
R. Mihai, R.V. Thakker, MANAGEMENT OF ENDOCRINE DISEASE: Postsurgical hypoparathyroidism: current treatments and future prospects for parathyroid allotransplantation. Eur. J. Endocrinol. 184(no. 5), R165–R175 (2021). https://doi.org/10.1530/EJE-20-1367
C.U. Jørgensen, P. Homøe, M. Dahl, M.F. Hitz, Postoperative Chronic Hypoparathyroidism and Quality of Life After Total Thyroidectomy. JBMR Plus 5(no. 4), e10479 (2021). https://doi.org/10.1002/jbm4.10479
F. Benmiloud, C. Le Bihan, S. Rebaudet, P. Marino, P.-J. Bousquet, E. Bouée-Benhamiche, Hypoparathyroidism-related health care utilization and expenditure during the first postoperative year after total thyroidectomy for cancer: a comprehensive national cohort study. Front. Endocrinol. 14, 1193290 (2023). https://doi.org/10.3389/fendo.2023.1193290
M.A. Guerrero, Cryopreservation of parathyroid glands. Int. J. Endocrinol. 2010, 829540 (2010). https://doi.org/10.1155/2010/829540
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by G.K. and L.S. The first draft of the manuscript was written by G.G. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This study is a systematic review and meta-analysis. No ethical approval is required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koimtzis, G., Stefanopoulos, L., Geropoulos, G. et al. The outcomes of parathyroid gland autotransplantation during thyroid surgery: a systematic review, meta-analysis and trial sequential analysis. Endocrine (2024). https://doi.org/10.1007/s12020-024-04011-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12020-024-04011-y