Abstract
Background
Careful parathyroid gland dissection and in situ preservation was the time-honored approach to prevent parathyroid failure after total thyroidectomy. The relative success of parathyroid autotransplantation of hyperplastic parathyroid tissue in patients with renal or hereditary hyperparathyroidism did popularize the use of normal parathyroid tissue autografts during thyroidectomy to prevent permanent hypoparathyroidism. Proof of autograft function in this setting, however, is controversial.
Purpose
This narrative review aims at reviewing critically the current status of parathyroid autotransplantation during total thyroidectomy. It is also meant to analyze from the historical, methodological, and clinical points of view the claimed benefit of normal parathyroid gland autotransplantation. A focus is placed on the prevention of permanent hypoparathyroidism by parathyroid autotransplantation.
Conclusions
Liberal parathyroid autotransplantation was proposed in the mid 1970s but evidence of function is scarce. Proofs are accumulating that parathyroid autografts not only increase the rate of postoperative hypocalcemia, but may be also contribute to permanent hypoparathyroidism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Parathyroid autotransplantation should not be used as a means of avoiding dissection no matter how long or tedious.
Henry JF, Ann Chir 1990
Introduction
Since total thyroidectomy was adopted widely at the end of the last century as a preferred surgical technique for most patients with thyroid cancer or bilateral benign disease, endocrine surgeons have become progressively aware of the fact that it carries a substantial risk of injury to the parathyroid glands, often translating into acute or chronic postoperative parathyroid failure due to low or undetectable parathormone (iPTH) serum concentration. There are three main reasons why the iPTH serum concentration falls below reference values shortly after total thyroidectomy in a significant number of patients: inadvertent parathyroidectomy, autotransplantation (PAT), and parathyroid devascularization. Eventually, all three circumstances may occur in the same patient, particularly after extensive operations performed for advanced/recurrent thyroid malignancies or big goiters, even if thyroidectomy is performed by an experienced surgeon [1].
The prevalence of permanent hypoparathyroidism was consistently underestimated until around the year 2000. Multicenter and registry studies published during the last two decades have reported data closer to the day-to-day reality. As an example, in the collaborative study on thyroidectomy for cancer of the American College of Surgeon Commission on Cancer Patient Care evaluation and the German Thyroid Cancer Study Group, permanent hypoparathyroidism was diagnosed in 10% of the patients despite not all of them were treated with total thyroidectomy with or without lymphadenectomy [2]. A 25% rate of permanent hypoparathyroidism was reported in a prospective observational multicenter European study on surgery for differentiated thyroid cancer [3], similar to that observed in a random sample of total thyroidectomy patients, cared for in a Polish endocrinological clinic [4]. Rates of permanent hypoparathyroidism of 19 and 8% after total thyroidectomy for papillary cancer, with or without prophylactic central neck dissection, have been published from a high-volume Italian institution [5]. Unfortunately, none of these reports document the PAT and inadvertent parathyroidectomy rates which are the main reasons underlying postoperative hypocalcemia and permanent parathyroid failure [6].
The functionality of PAT has received little attention despite it is being used liberally and even routinely in many endocrine surgery units. It has been assumed widely and rather uncritically that the transplantation of normal parathyroid tissue may not only be active but also afford protection against permanent hypoparathyroidism. This is why this narrative review focuses on the need for a reappraisal of the supposed value of the autotransplantation of normal parathyroid tissue to prevent or reduce the permanent hypoparathyroidism rates after total thyroidectomy.
Historical notes
The autotransplantation of 1-2 parathyroids did not provide protection against permanent hypoparathyroidism, while autotransplantation of 3-4 glands could do this.
Alveryd A, Acta Chir Scand 1968
The classical conservative approach
Traditional teaching in thyroid surgery during the 1960s and 1970s emphasized the importance of preserving the parathyroid gland blood flow and leaving the glands in situ. In his seminal paper on the surgical technique of thyroidectomy, Norman Thompson wrote Preservation of parathyroid gland supply is extremely important during total lobectomy [7]. Of the same opinion was Charles Proye who, a few years later, wrote that to prevent postoperative hypoparathyroidism, a painstaking dissection of parathyroid blood supply seems more promising and effective than routine autotransplantation of the glands [8]. These recommendations were meant also to avoid inadvertent resection of the parathyroid glands that, even today, continues to be a major challenge, particularly after node dissection in compartment VI [9,10,11].
The pioneers of PAT
Frank Lahey is commonly cited as the pioneer of PAT during thyroidectomy in humans [12] in the years when total thyroidectomy was seldom, if ever, performed. Alveryd [13] performed careful anatomical studies of the parathyroid glands in the late 1960s and did advocate PAT using the whole gland intramuscular implantation technique. In his initial report, however, 7/32 patients undergoing PAT (one or more glands) developed permanent hypoparathyroidism. Four-gland autotransplantation afforded some benefit compared to fewer transplanted glands.
The modern era of PAT was initiated by the seminal work of Wells et al. [14, 15] describing normal parathyroid function after total parathyroidectomy for renal or multigland primary hyperparathyroidism, with forearm implantation of parathyroid tissue fragments. It is worth noting that in these reports, diseased parathyroid tissue was implanted. It is difficult to understand why the relative functional success of hyperplastic tissue grafting was uncritically and enthusiastically extrapolated to PAT of normal parathyroid glands. Whatever the reasons, several groups adopted a policy of (very) liberal and even routine use of PAT [16,17,18,19] and this technique was incorporated to the armamentarium of thyroidectomy despite hard evidence for its ability to prevent permanent hypoparathyroidism was and is still lacking. In fact, the same groups that advocated a liberal PAT policy have published recently data suggesting that they are no longer in favor of such radical approach [20,21,22].
The crisis and our contributions
Despite several methodological flaws, the 1998 paper by the Lille group on factors predicting permanent hypoparathyroidism [23] initiated a critical review of PAT outcomes. These authors wrote … patients carried a high risk for permanent hypoparathyroidism if <3 parathyroid glands were preserved in situ during surgery or the early serum parathyroid hormone level was ≤12 pg/ml and the delayed serum calcium levels ≤ 8 mg/dl.
We did approach the issue of the clinical significance of PAT using the fragmented Wells’ technique from two angles: (1) investigating the impact of the number parathyroid glands remaining in situ (PGRIS), according to the formula 4—(excised PGs + autotransplanted PGs), on short- and long-term parathyroid function and (2) comparing the rate of parathyroid failure syndromes in PGRIS 3 patients in whom the fourth gland had been either autotransplanted or accidentally excised.
A first study was focused on patients with protracted hypoparathyroidism requiring replacement therapy for more than 1 month after total thyroidectomy [24]. Previous findings from the Lille group were confirmed: patients with PAT had a worse postoperative and 1-month parathyroid function outcome than those not autografted. In situ gland preservation and delayed (1-month) high-normal serum calcium concentration were key factors predicting recovery of the parathyroid function. The latter was associated with higher calcium and calcitriol, dosages at the time of hospital discharge.
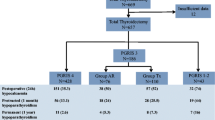
In a second study [6], total thyroidectomy patients were classified in three groups (PGRIS 1–2, PGRIS 3, and PGRIS 4). Patients were followed up in our unit until parathyroid function was restored or the diagnosis of permanent hypoparathyroidism was made after at least 1-year follow-up using standardized definitions [25]. Those patients with fewer glands remaining in situ had worse short- and long-term parathyroid function outcomes (Table 1). Specifically, the rate of permanent hypoparathyroidism in 143 patients undergoing PAT was higher than that observed in 514 patients not autografted (9.8 vs. 3.1%; P < 0.002).
In a third study [26], PGRIS 3 patients were divided retrospectively in two clinically and surgically similar groups: one in which the fourth gland had been autotransplanted into the ipsilateral sternocleidomastoid muscle and another in which the fourth gland had been inadvertently resected. As can be seen in Fig. 1, the rates of immediate, protracted (1-month), and permanent parathyroid failure were the same independently of whether the fourth gland was autotransplanted or inadvertently sent to the pathology lab.
Absence of proof of clinically significant graft function
It remains hard to prove that autotransplanted normal parathyroid tissue takes well into a muscle or subcutaneous pocket and that is functional and able to reduce the permanent hypoparathyroidism rates. The presence of other potentially functioning glands remaining in situ after thyroidectomy and the absence of a clear-cut proof of function due to impossibility of venous sampling close to the sternocleidomastoid muscle have obscured the benefits of PAT and have been a serious theoretical obstacle for those claiming a benefit.
Most initial studies on PAT function were based on serum calcium and iPTH concentrations in peripheral blood [17, 27,28,29]. This is, however, a rather unreliable method since in these reports inadvertent parathyroidectomy, parathyroid identification, or the number of glands remaining in situ were not considered as relevant factors influencing iPTH concentrations and permanent hypoparathyroidism rates. Lo et al. [27] claimed a reduction of permanent hypoparathyroidism rate in PAT patients but their non-PAT patients had a threefold rate of inadvertent parathyroidectomy compared to that of PAT cases. According to their paper’s abstract, Kikumori et al. [29] performed total parathyroidectomy plus four-gland PAT and total thyroidectomy for thyroid cancer in 86 patients. Careful reading of this report, however, reveals that only 33/86 patients received four-gland PAT and 20/86 no PAT at all (incidentally found cancer in multinodular goiters). The best iPTH serum concentrations were observed in patients in whom the parathyroid glands were preserved in situ. PAT of four glands resulted in no case of permanent hypoparathyroidism. Patients with two or three glands transplanted had a 17 and 3% hypoparathyroidism rates respectively. As already suggested by Alveryd [13], only four-gland PAT afforded protection against permanent hypoparathyroidism. This would also fit with the initial proposal of Sam Wells’ group to add total parathyroidectomy and four-gland PAT to preventive total thyroidectomy for MEN2, a policy that has now been abandoned in favor of in situ preservation [20].
Supporters of PAT assume that normal parathyroid tissue takes well on a muscular bed [30] but solid proof for that is lacking. Fibrosis replacing much of the autografted parathyroid tissue was already reported in one out of three cases that were studied histologically by Alveryd [13]. El-Sharaki et al. [31] performed graft biopsies on three patients with forearm PAT for electron microscopy studies after subtotal thyroidectomy. Their findings of “reversible degenerative insult at 1 week”, however, are difficult to interpret as well as those at 2 weeks when “parathyroid cells showed features of regeneration and activity.” No functional correlation was reported.
A first attempt to demonstrate function of forearm PAT was made by Sierra et al. [32]. They performed a forearm PAT in 12 patients undergoing different types of thyroidectomies and reported as functioning those grafts resulting in a > 1.5 gradient of iPTH serum concentration between the grafted and the non-grafted forearm. In two patients, there was no iPTH gradient. In functioning grafts, a peak gradient of 12.7:1 was observed at 4 weeks falling thereafter to a 4:1 gradient at 6 months. The authors omit giving the actual iPTH concentrations and claim positive sestamibi scans on the autograft sites that most probably were nonspecific. Lo et al. [30] compared iPTH gradient between grafted and non-grafted forearms after PAT in seven patients. Results are difficult to interpret since iPTH reference values were not properly stated due to some confusion between pmol/L, pg/mL, and ng/L. An iPTH gradient was observed > 1.5 in six out of seven patients, but we suspect that iPTH concentrations in the non-grafted arm at 3 months were below normal in some patients. Using subcutaneous forearm PAT, Cavallaro et al. [33] documented a significant iPTH gradient (1.5–5) between grafted and non-grafted forearms in 48% of patients 1 week after surgery, 88% after 1 month, and 96% (24 out of 25 patients) 3 months after surgery, but failed to properly document long-term parathyroid function. The Casanova test [34] was not performed in neither of these two gradient studies; therefore, it remains difficult to quantify the contribution of PAT to peripheral blood iPTH concentration.
A sensible interpretation of most reports claiming functionality of PAT is that although some endocrine function from grafted parathyroid fragments may be detectable, it does not follow that it is enough to prevent permanent hypoparathyroidism.
Technical variations
The most commonly used technique for PAT is implantation of the fragmented gland into pockets on the ipsilateral sternocleidomastoid muscle. This can be done immediately after gland excision or after a period of cooling in cold saline. A European survey revealed that this technique is currently used by over 80% of the endocrine surgeons [35] and it seems highly unlikely that differences in specific phases of the PAT process could substantially modify its long-term function [26].
Gauger et al. [36] explored an alternate PAT technique comparing the injection of dispersed parathyroid tissue into the ipsilateral sternocleidomastoid muscle with the standard fragmented technique. They found no differences in terms of long-term iPTH concentrations. The study, however, is flawed by the lack of a control non-autografted group and failure to obtain iPTH measurements in 25% of their patients.
The psychological variables: color change, angiography, and surgeon’s fees
The threshold for PAT is highly subjective except in cases where glands have been completely isolated from surrounding tissues or inadvertently excised and found in the specimen before it is sent to the pathology lab. Other than this, the indication of PAT lays on a gray zone. We suspect that, independently of the appearance or the more or less firm attachment of the glands to surrounding structures, those surgeons convinced of the efficacy of PAT adopt a more liberal (even routine) transplantation policy compared to more skeptical surgeons. As an example, PAT of discolored glands (or of the most discolored one) has been widely adopted. A study by Promberger et al. [37], however, suggests that transplanting discolored glands gives worse results that leaving them in situ. This would support the concept that PAT based on color changes does not afford a functional advantage and, in fact, may result in worse long-term parathyroid function. Furthermore, change in color cannot be used as a reliable marker of parathyroid dysfunction. Lang et al. [38] reported a higher rate of permanent hypoparathyroidism in cases in which no color change was detected in any of the four parathyroid glands than in cases where discoloration of one or two glands was noticed. Most probably, this is due to the more detrimental effects of parathyroid ischemia (not resulting in color change) than venous congestion.
Reduced or absence of vascular supply as determined by green indocyanine fluorescence angiography [39] has been proposed to determine when a (non-perfused) parathyroid gland should be transplanted. This implies however that the authors rely on PAT rather than on meticulous dissection. It also implies that the possibility of parathyroid gland revascularization after the wound is closed is not considered. This is not consistent with the fact that recovery of the parathyroid function is a dynamic, long-standing process [40]. In addition, interpretation of the parathyroid function data in this study is hampered because four glands were identified in only 4/36 patients and all subjects received routine calcium and vitamin D supplementation after thyroidectomy. Another study on parathyroid gland angiography found a poor (in fact, non-existent) correlation between impaired parathyroid blood flow and postoperative parathyroid function [41]. Our guess is that intraoperative angiography will never match iPTH measurements 4–6 h following thyroidectomy as predictor of parathyroid failure. In addition, selecting the less-perfused glands for PAT may not be the appropriate solution.
Last but not least, it should be mentioned that in the USA (perhaps in other countries too) surgeons and/or the institutions they work in, charge a fee of up to 1000$ if PAT is performed during total thyroidectomy [42]. This conflict of interest probably influences PAT rates favoring a liberal—if not routine—and uncritical PAT policies. It may also interfere with scientific publishing. In addition, PAT may be further encouraged by pathologists if a frozen biopsy is taken before autotransplantation for histological verification.
Conclusion
Normal parathyroid gland autotransplantation is currently regarded more critically than in the past two decades. Detailed scrutiny of the available literature reveals that hard evidence supporting its role in preventing permanent hypoparathyroidism is lacking. On the other hand, more data are accumulating on the benefits of leaving the parathyroid glands well vascularized in situ. Endocrine surgeons are urged to improve their technical skills and anatomical knowledge to better identify and preserve the parathyroid normal glands when performing thyroidectomy.
References
Duclos A, Peix JL, Colin C, Kraimps JL, Menegaux F, Pattou F, Sebag F, Touzet S, Bourdy S, Voirin N, Lifante JC, CATHY Study Group (2012) Influence of experience on performance of individual surgeons in thyroid surgery: prospective cross sectional multicentre study. BMJ 344(jan10 2):d8041. https://doi.org/10.1136/bmj.d8041
Hundahl SA, Cady B, Cunningham MP, Mazzaferri E, McKee RF, Rosai J, Shah JP, Fremgen AM, Stewart AK, Hölzer S (2000) Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. U.S. and German Thyroid Cancer Study Group. An American College of Surgeons Commission on Cancer Patient Care Evaluation study. Cancer 89(1):202–217. https://doi.org/10.1002/1097-0142(20000701)89:1<202::AID-CNCR27>3.0.CO;2-A
Vrachimis A, Wenning C, Gerß J, Dralle H, Vaez Tabassi M, Schober O, Riemann B, MSDS study group (2015) Not all DTC patients with N positive disease deserve the attribution “high risk”. Contribution of the MSDS trial. J Surg Oncol 112(1):9–14. https://doi.org/10.1002/jso.23948
Nawrot I, Pragacz A, Pragacz K, Grzesiuk W, Barczyński M (2014) Total thyroidectomy is associated with increased prevalence of permanent hypoparathyroidism. Med Sci Monit 20:1675–1681. https://doi.org/10.12659/MSM.890988
Viola D, Materazzi G, Valerio L, Molinaro E, Agate L, Faviana P, Seccia V, Sensi E, Romei C, Piaggi P, Torregrossa L, Sellari-Franceschini S, Basolo F, Vitti P, Elisei R, Miccoli P (2015) Randomized, prospective trial finds no clinical advantage to prophylactic central-neck dissection for papillary thyroid cancer. J Clin Endocrinol Metab 100(4):1316–1324. https://doi.org/10.1210/jc.2014-3825
Lorente-Poch L, Sancho JJ, Ruiz S, Sitges-Serra A (2015) Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg 102(4):359–367. https://doi.org/10.1002/bjs.9676
Thompson NW, Olsen WR, Hoffman GL (1973) The continuing development of the technique of thyroidectomy. Surgery 73(6):913–927
Proye C, Maes B, Bondil P, Vanseymortier L, Lagache G (1982) Parathyroid risk in thyroid surgery (in French). J Chir 119(8–9):491–498
Sitges-Serra A, Gallego-Otaegui L, Suárez S, Lorente-Poch L, Munné A, Sancho JJ (2017) Inadvertent parathyroidectomy during total thyroidectomy and central neck dissection for papillary thyroid carcinoma. Surgery 161(3):712–719. https://doi.org/10.1016/j.surg.2016.08.021
Ondik MP, McGinn J, Ruggiero F, Kim SW, Chung KW, Youn YK (2009) Unintentional parathyroidectomy and hypoparathyroidism in secondary central compartment surgery for thyroid cancer. Head Neck 32:462–466
Applewhite MK, White MG, Xiong M, Pasternak JD, Abdulrasool L, Ogawa L, Suh I, Gosnell JE, Kaplan EL, Duh QY, Angelos P, Shen WT, Grogan RH (2016) Incidence, risk factors, and clinical outcomes of incidental parathyroidectomy during thyroid surgery. Ann Surg Oncol 23(13):4310–4315. https://doi.org/10.1245/s10434-016-5439-1
Lahey FH (1926) Transplantation of the parathyroids in partial thyroidectomy. Surg Gynecol Obstet 62:508–509
Alveryd A (1968) Parathyroid glands in thyroid surgery I. Anatomy of parathyroid glands. II. Postoperative hypoparathyroidism—identification and autotransplantation of parathyroid glands. Acta Chir Scand 389:1–120
Wells SA, Ellis GJ, Gunnells JC, Schneider AB, Sherwood LM (1976) Parathyroid autotransplantation in primary parathyroid hyperplasia. N Engl J Med 295(2):57–62. https://doi.org/10.1056/NEJM197607082950201
Wells SA, Gunnells JC, Shelburne JD, Schneider AB, Sherwood LM (1975) Transplantation of the parathyroid glands in man: clinical indications and results. Surgery 78(1):34–44
Paloyan E, Lawrence AM, Paloyan D (1977) Successful autotransplantation of the parathyroid glands during total thyroidectomy for carcinoma. Surg Gynecol Obstet 145:364–368
Olson JA Jr, DeBenedetii MK, Baumann DS, Wells SA Jr (1996) Parathyroid autotransplantation during thyroidectomy. Ann Surg 223(5):472–480. https://doi.org/10.1097/00000658-199605000-00003
Zedenius J, Wadstrom C, Delbridge L (1999) Routine autotransplantation of at least one parathyroid gland during total thyroidectomy may reduce permanent hypoparathyroidism to zero. Aust N Z J Surg 69(11):794–797. https://doi.org/10.1046/j.1440-1622.1999.01697.x
Lo CY, Lam KY (2001) Routine parathyroid autotransplantation during thyroidectomy. Surgery 129(3):318–323. https://doi.org/10.1067/msy.2001.111125
Moley JF, Skinner M, Gillanders WE, Lairmore TC, Rowland KJ, Traugott AL, Jin LX, Wells SA Jr (2015) Management of the parathyroid glands during preventive thyroidectomy in patients with multiple endocrine neoplasia type 2. Ann Surg 262(4):641–646. https://doi.org/10.1097/SLA.0000000000001464
Prichard RS, Edhouse PJ, Sidhu SB, Sywak MS, Delbridge L (2011) Post-operative partial hypoparathyroidism: an under-recognized disorder. ANZ J Surg 81(7–8):524–527. https://doi.org/10.1111/j.1445-2197.2010.05633.x
Lang BH, Chan DT, Chow FC (2016) Visualizing fewer parathyroid glands may be associated with lower hypoparathyroidism following total thyroidectomy. Langenbeck’s Arch Surg 401(2):231–238. https://doi.org/10.1007/s00423-016-1386-3
Pattou F, Combemale F, Fabre S, Carnaille B, Decoulx M, Wemeau JL, Racadot A, Proye C (1998) Hypocalcemia following thyroid surgery: incidence and prediction of outcome. World J Surg 22(7):718–724. https://doi.org/10.1007/s002689900459
Sitges-Serra A, Ruiz S, Girvent M, Manjón H, Dueñas JP, Sancho JJ (2010) Outcome of protracted hypoparathyroidism after total thyroidectomy. Br J Surg 97(11):1687–1695. https://doi.org/10.1002/bjs.7219
Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, Sánchez-Velázquez P, Sitges-Serra A (2015) Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg 4(1):82–90. https://doi.org/10.3978/j.issn.2227-684X.2014.12.04
Lorente-Poch L, Sancho J, Muñoz JL, Gallego-Otaegui L, Martínez-Ruiz C, Sitges-Serra A (2017) Failure of fragmented parathyroid gland autotransplantation to prevent permanent hypoparathyroidism after total thyroidectomy. Langenbeck's Arch Surg 402(2):281–287. https://doi.org/10.1007/s00423-016-1548-3
Lo CY, Lam KY, Weber CJ, Shaha AR, Davis O (1998) Postoperative hypocalcemia in patients who did or did not undergo parathyroid autotransplantation during thyroidectomy: a comparative study. Surgery 124(6):1081–1087. https://doi.org/10.1067/msy.1998.92560
Funahashi H, Satoh Y, Imai T, Ohno M, Narita T, Katoh M, Tanaka Y, Andoh H, Miyazaki K (1993) Our technique of parathyroid autotransplantation in operation for papillary thyroid carcinoma. Surgery 114(1):92–96
Kikumori T, Imai T, Tanaka Y, Oiwa M, Mase T, Funahashi H (1999) Parathyroid autotransplantation with total thyroidectomy for thyroid carcinoma: long-term follow-up of grafted parathyroid function. Surgery 125(5):504–508. https://doi.org/10.1016/S0039-6060(99)70201-1
Lo CY, Tam SC (2001) Parathyroid autotransplantation during thyroidectomy: documentation of graft function. Arch Surg 136(12):1381–1385. https://doi.org/10.1001/archsurg.136.12.1381
El-Sharaky MI, Kahalil MR, Sharaky O, Sakr MF, Fadaly GA, El-Hammadi H, Moussa MM (2003) Assessment of parathyroid autotransplantation for preservation of parathyroid function after total thyroidectomy. Head Neck 25(10):799–807. https://doi.org/10.1002/hed.10278
Sierra M, Herrera MF, Herrero B, Jiménez F, Sepúlveda J, Lozano RR, Gamino R, González O, Correa-Rotter R (1998) Prospective biochemical and scintigraphic evaluation of autografted normal parathyroid glands in patients undergoing thyroid operations. Surgery 124(6):1005–1011. https://doi.org/10.1067/msy.1998.92003
Cavallaro G, Iorio O, Centanni M, Porta N, Iossa A, Gargano L, Del Duca S, Gurrado A, Testini M, Petrozza V, Silecchia G (2015) Parathyroid reimplantation in forearm subcutaneous tissue during thyroidectomy: a simple and effective way to avoid hypoparathyroidism. World J Surg 39(8):1936–1942. https://doi.org/10.1007/s00268-015-3070-0
Casanova D, Sarfati E, De Francisco A, Amado JA, Arias M, Dubost C (1991) Secondary hyperparathyroidism: diagnosis of site of recurrence. World J Surg 15(4):546–549. https://doi.org/10.1007/BF01675660
Lorente L, Sancho JJ, Sitges-Serra A (2015) European survey of the indications and technique of parathyroid autotransplantation during thyroidectomy. Langenbeck’s Arch Surg 400:396 (Abstract)
Gauger P, Reeve TS, Wilkinson M, Delbridge LW (2000) Routine parathyroid autotransplantation during total thyroidectomy: the influence of technique. Eur J Surg 166(8):605–609. https://doi.org/10.1080/110241500750008240
Promberger R, Ott J, Kober F, Mikola B, Karik M, Freissmuth M, Hermann M (2010) Intra- and postoperative parathyroid hormone-kinetics do not advocate for autotransplantation of discolored parathyroid glands during thyroidectomy. Thyroid 20(12):1371–1375. https://doi.org/10.1089/thy.2010.0157
Lang BH, Chan DT, Chow FC, Wong KP, Chang RY (2016) The association of discolored parathyroid glands and hypoparathyroidism following total thyroidectomy. World J Surg 40(7):1611–1617. https://doi.org/10.1007/s00268-016-3462-9
Vidal Fortuny J, Belfontali V, Sadowski SM, Karenovics W, Guigard S, Triponez F (2016) Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg 103(5):537–543. https://doi.org/10.1002/bjs.10101
Villarroya-Marquina I, Sancho J, Lorente-Poch L, Gallego-Otaegui L, Sitges-Serra A (2018) Time to parathyroid function recovery in patients with protracted hypoparathyroidism after total thyroidectomy. Eur J Endocrinol 178(1):105–113. https://doi.org/10.1530/EJE-17-0589
Lang BH, Wong CK, Hung HT, Wong KP, Mak KL, Au KB (2017) Indocyanine green fluorescence angiography for quantitative evaluation of in situ parathyroid gland perfusion and function after total thyroidectomy. Surgery 161(1):87–95. https://doi.org/10.1016/j.surg.2016.03.037
Savarise M, Senkowski C (eds) (2017) Principles of coding and reimbursement for surgeons. Springer International Publishing, Switzerland, pp 330–331
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Sitges-Serra, A., Lorente-Poch, L. & Sancho, J. Parathyroid autotransplantation in thyroid surgery. Langenbecks Arch Surg 403, 309–315 (2018). https://doi.org/10.1007/s00423-018-1654-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-018-1654-5